Maysaloon A. AL-Sadoon1 , Muntaha A. H. Nasir2, Esraa T. Yasir3, Ahmed O. Khalaf3 and Saja J. Kadim3
, Muntaha A. H. Nasir2, Esraa T. Yasir3, Ahmed O. Khalaf3 and Saja J. Kadim3
1Department of Microbiology, University of Basra, College of Medicine.
2Department of Clinical Laboratory Sciences ,University of Basra, College of Pharmacy.
3University of Basra, College of Medicine.
Corresponding Author E-mail: hmaysloon@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1591
Abstract
Toxoplasma gondii is of great concern in public health because it affects a lot of people in the globe. However, in the clinical setting, it rarely causes serious disease. The currents research was performed on university students in Basra province to estimate the prevalence of toxoplasmosis and its risk factors. It is first the time research was done in Basra province. Venous samples of blood were obtained from 177 women in the higher institution of learning of Basra in 2018. The participants were aged between nineteen to twenty-four years. The samples were analyzed if they had anti-T. gondii IgM & IgG antibodies that would show evidence of T.gondii infection. The participants were also given questionnaires to determine risk factors. The mean age of the participants was 21.24 years, and a majority of them were aged between twenty-two to twenty-four years. The differences between contact with an animal and age that has examined positive for toxoplasmosis were not statistically significant. Among the 177 participants only two, who are about 1.13% tested positive for T. gondii IgM which is consider as recent infection while, 20 of them with positive IgG antibodies was detect as a past infection. The only variable that had a positive association with testing positive to T. gondii was contacting with soil (garden at the house) the level of significance for the association was less than 0.05.
Keywords
Antibodies; IgM, IgG Antibodies; Risk Factors; Toxoplasmosis; University Students
Download this article as:| Copy the following to cite this article: AL-Sadoon M. A, Nasir M. A. H, Yasir E. T, Khalaf A. O, Kadim S. J. Toxoplasmosis and Risk Factors Among Female Students of Medical Colleges at Basra University, Iraq. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: AL-Sadoon M. A, Nasir M. A. H, Yasir E. T, Khalaf A. O, Kadim S. J. Toxoplasmosis and Risk Factors Among Female Students of Medical Colleges at Basra University, Iraq. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=24295 |
Introduction
Toxoplasmosis is among the most famous parasitic zoonosis in the globe; it is due to apicomplexan protozoan Toxoplasma gondii.1 The definitive hosts for the parasite are cats; the warm-blooded creature is it intermediate host. It happens in three kinds, which are bradyzoites sporozoites and tachyzoites.2 The parasite is found in the lungs, brain, at most of the times in the lymph nodes and the heart.3,4 The sickness influences about 33% of the worldwide populace5 it is an opportunistic parasitic disease that effect people whose immune system is deficiency.6 It found that childbearing age ladies and women who is pregnant have a high rate of infection with the disease.7 The disease is wide, and variety relies upon social and cultural mores, geographic components, and mode of transmission. The pervasiveness of the disease is more in warm and humid areas,8 which is caused by an obligate intracellular protozoan parasite. Individuals can be infected after ingestion of raw or undercooked meat, by ingestion of oocysts shed from cat in the taint soil, water or food; or by trans-placental transmission of tachyzoites.9,10 Women infected with T. gondii at the pregnancy can result in neonatal death or different inborn imperfections, like nervous, sensory system anomalies, hydrocephalus, and chorioretinitis.9,10-11
After ingested, the parasite changes to a quick replicating structure referred to as the tachyzoite which invades host cells and produce three successive waves of proteins are secreted from parasite organelles.These proteins can alter host cell function and inhibit the immune response directed towards the parasite.12 By forming a parasitophorous vacuole [PV]. Which preventing lysosomal fusion and killing of the parasite.13 In immune competant individual the infection cleared from the host by the immune system.14,15 The parasite at that point changes to a stage that replicates slowly referred to as the bradyzoite that persists in the neural and muscle tissues of the host for the entire life.16 In parasitic attacks, the cytotoxic action of white blood cells is increased due to the effect of cytokines (TNF-α and IL-5).17 The lymphocytes Th2 produce some cytokines like (IL-4, IL-5, IL-6, and IL-10) that assume a major part in the formation of the pathogen for the disease,18,19 Disturbance of immune response associated with toxoplasmosis may explain the success of parasite in escaping from discrimination and elimination by the immune system then supporting its survival and replication.20 Treatment of this disease is often only recommended for people with serious health problems, such as people with HIV, because the disease is most serious when one’s immune system is weak Clinically, acute toxoplasmosis is usually treated with a combination pyrimethamine and sulfadiazine although sometimes may be replaced with trimethoprim, and the latter with the clindamycin.21 Other studies show that triple combination of PYR-SDZ-levamisole could be an alternative treatment option in case of infections caused by T. gondii,.22 The drug combinations also the usual choice for prophylaxis. However, because none of the inhibitors commonly used for the treatment of primary disease is able to penetrate tissue cysts and thus clear the bradyzoite form of the parasite, lifelong prophylaxis is essential if recrudescent disease is to be prevented in immunosuppressed patients.21 Female university students are close to childbearing age, and their status of T. gondii infection is important. The current research was structured to give recent data about the Toxoplasma infection of a female university student who was studying at Basra University, in Iraq.
Methods
Blood tests were gathered from 177 healthful female understudies at the college of medicine & college of pharmacy University of Basra, Iraq, in 2018. Ethical endorsement was acquired from the University of Basra; a questionnaire was organize to collect & analyze the importance factors influencing toxoplasmosis.
Three to five millilitres of blood were collected from female students. The blood samples were given time to clot totally before centrifugation at 2000 rpm for 10 minutes to obtain serum. Serum was isolated from the clot and stored in too tightly screwed tubes and stored at−20°C. This frozen serum was then tested for the availability of anti-Toxoplasma IgM & IgG antibodies, using linked Immunosorbent Assays (ELISA) kit (Rapid Labs, United Kingdom). The steps to test the samples were according to the manufacturer’s instructions. The questionnaires’ data along with the findings of the serological assay were entered in SPSS software (version 22) and analyzed.
Result
A total of 177 female understudies going to college amidst of 19 and 24years old; the mean age was 21.24 years. A majority of the participants were aged between 22 and 24 years; they were 78 accounting for 44.07%. Twelve of the participants, which is 6.78% had cats in their homes, and six of them, which is 3.39% indicated that they drunk unpasteurized milk. Only two who accounted for 1.13% consumed undercooked meat while 93.79% indicated to wash their hand after contact with raw meat. That appear in table (1), about 58.2% of the study populations lived in the urban area, and about 41.8% who is lived in rural areas.
Table 1: Characteristics features of study participants:
| Variable | Category | Number | (%) |
| Age years | > 20
20–22 22–24 |
38/177
61/177 78/177 |
21.47
34.46 44.07 |
| Place of residence | Rural
Urban |
74/177
103/177 |
41.8
58.2 |
| Ownership of cat | Yes
No |
12/177
165/177 |
6.78
93.22 |
| Changing cat litter | Yes
No |
5/12
7/12 |
41.67
58.33 |
| Ingestion raw or undercooked meat | Yes
No |
2/177
175/177 |
1.13
98.87 |
| Washing hand after contact with raw meat | Yes
No |
166/177
11/177 |
93.79
6.21 |
| Contacting with soil
Garden at house |
Yes
No |
65/177
112/177 |
36.72%
63.28% |
| Drinking unpasteurized milk | Yes
No |
6/177
171/177 |
3.39
96.61 |
Table 2: Toxoplasmosis in female of child-bearing age in Basra:
| ELISA test | Number of women tested | ||||||
| Positive | Negative | Total | |||||
| No. | % | No. | % | No. | % | ||
| IgM | 2 | 1.13 | 175 | 98.87 | 177 | 100 | |
| IgG | 20 | 11.3 | 157 | 88.7 | 177 | 100 | |
| IgM & IgG | 1 | 0.6 | 176 | 99.4 | 177 | 100 | |
| X2= 17.5 P<0.05 | |||||||
This table shows that two of the participants had positive T. gondii– IgM antibodies which show acute infection and 20 (11.3%) of them had a positive T. gondii-specific IgG antibodies which indicate previous infection. While 1(0.6) of samples had both acute and chronic infection which is indicated by the presence positive of both IgM and IgG antibodies. The statistical analysis showed significant differences between them (p<0.05).
Table 3: Toxoplasma seropositivity & Risk factors:
| Variable | Toxoplasmosis seropositivity | p-value | |
| Yes
n=22 (12.4%) |
No
N=155(87.6%) |
||
| Contacting with soil(Garden at house)
Yes No |
16(24.6%)
6 ( 5.4%) |
49(75.4 %)
106 (94.6%) |
P <0.05 |
This table demonstrates that just contact with soil (garden at the house) from chose factors had significant differences with seropositivity (P <0.05).
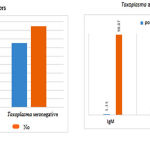 |
Figure 1
|
Discussion
Toxoplasmosis is a curable but potentially deadly sickness.23 The parasite have the ability to crosses the blood–brain barrier and establishes persistent infection in a drug-resistant bradyzoite stage.24 From the past, has been consider to be one of the most common parasitic disease of human and other blood warm animals(2). In this research, we sought to detect recent or past infection, by using antibodies against Toxoplasma gondii in the serum of females on childbearing age at Basra University in Iraq. In view of previous study done in Iraq which indicated that type II strains often associated with human toxoplasmosis and dominant among Iraqi female25 and this results was going well with the results of studies in other countries.26,27
Most investigations led on the seroprevalence of toxoplasmosis are centered around childbearing age, pregnant ladies and immunodeficient patients.28,29 Moreover, the seroprevalence rate of T. gondii IgG in the Basra pregnant women has been reported to be 43.07%. 30 Female university students are close to childbearing age, and their status of T. gondii infection is important. In the present investigation, among 177 female college understudies, 1.13% were seropositive for Toxoplasma IgM, & 11.3 % seropositive for Toxoplasma IgG which are less than the seroprevalence already found in different nations in or close to the Middle East, including Yemen (45.4%), Jordan (47.1%), Iran (75.7%), and Ethiopia (85.4%).31-34 It could be as a result of higher education, as shown to be a decreasing factor in T.gondii infection.35 Another reason for a lower prevalence rate of toxoplasmosis among the female students in the current study might be the lower age of the participants, which in turn lowered the exposure to T. gondii and the subsequent infection. A new systematic review of studies detection seroepidemiology and T. gondii increased with age.36,37 One probable explanation behind this finding is the extra-long periods of potential exposure with age. However, like to our detection, a few studies didn’t detect a significant connection between Toxoplasma infection and age.
In the present investigation, contact with animals for example cats was not related with Toxoplasma seropositivity. It likewise has been reported in some past studies.38,39
Among the different risk factors analyzed by our examination, contact with soil (garden at the house) was the only one positively associated with toxoplasmosis. This factor was also identified significantly by different analysts.40–42 Sporulated oocyst can hold viable for a considerable length of time in moist soil, and poor sanitation. Therefore, it is essential that awareness of how Toxoplasma infections are caused is raised so that ladies can find a way to avoid contracting this parasitic infection.
Acknowledgements
Authors are very grateful and want to thank all the students of medicine college & pharmacy college at the University of Basra who participated in this study for providing the blood samples, and readily filled the questionnaire.
References
- Tenter A. M., Heckeroth A. R., Weiss L. M. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–58.
CrossRef - Dubey J. P. Toxoplasmosis of animals and humans. 2nd ed. Beltsville: CRC Press. 2010.
- Evering T and Weiss L. M. The immunology of parasite infections in immuno compromised hosts. Parasite Immunology. 2006;28:549-565.
CrossRef - Suzuki L. M., Rocha R. J and Rossi C. L. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. Journal of Medical Microbiology. 2001;50:62-70.
CrossRef - Montoya J. G., Liesenfeld O. Toxoplasmosis. Lancet. 2001;363:1965–76.
CrossRef - Ferreira S. M., Borges S. A. Some aspects of protozoan infections in immune compromised patients, a review. Bio Line International System. 2002;97(4):443–57.
- Pappas G., Roussos N., Falagas M. E. Toxoplasmosis snapshots global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–94.
CrossRef - Studeničová C., Benčaiová G., Holková R. Seroprevalence of Toxoplasma gondii antibodies in a healthy population from Slovakia. Eur J Intern Med. 2006;17:470–3.
CrossRef - Torgerson P. R., Macpherson C. N. L. The socioeconomic burden of parasitic zoonoses: Global trends. Vet Parasitol. 2011;182:79–95.
CrossRef - Montoya J. G., Rosso F. Diagnosis and management of Toxoplasmosis. Clin Perinatal. 2005;32:705–726.
CrossRef - Dubey J. P. Toxoplasmosis- a waterborne zoonosis. Vet Parasitol. 2004;126:57–72. 12. Lim D. C.,
Cooke B. M., Doerig C., Saeij J. P. Toxoplasma and Plasmodium protein kinases roles in invasion and host cell remodelling. International j. for parasitol. 2012;42(1):21– 32.
CrossRef - Cesbron-Delauw M. F., Gendrin C., Travier L., et al. Apicomplexa in mammalian cells trafficking to the parasitophorous vacuole. Traffic. 2008;9(5):657–664.
CrossRef - Weiss L. M., Dubey J. P. Toxoplasmosis: A history of clinical observations. International journal for parasitology. 2009;39(8):895–901.
CrossRef - Johnson L. L. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect Immun. 1992;60(9):3719–3724.
- Frenkel J. K. Toxoplasma in and around us. BioScience. 1973;23(6):343–352.
CrossRef - Nickdel M. B., Roberts F., Brombacher F., Alexander J and Roberts C. W. Counter-protective role for interleukin-5 during acute Toxoplasma gondii infection. Infection and Immunity. 2001;2:1044-1052.
CrossRef - Lang C., Gross U and Lüder C. G. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitolological Research. 2007;100:191-203.
CrossRef - Zaccone P., Fehervari Z., Phillips J. M., Dunne D. W and Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunolology. 2006;28(20):515-523. Aldabagh M. A., Hachim S. K., Qassim K. W., et al. Immune profile in aborted Iraqi women with toxoplasmosis. Med J Babylon. 2018;15:48-52.
CrossRef - Sims P. F. G. Drug Resistance in Toxoplasma gondii. In: Mayers D. L. (eds) Antimicrobial Drug Resistance. Infectious Disease. Humana Press. 2009.
CrossRef - Köksal Z. Ş., Yanik K.,B. K., Yılmaz E. M., Hokelek M. In Vivo Efficacy of Drugs against Toxoplasma gondii Combined with Immunomodulators. Jpn J Infect Dis. 2016;69(2):113-7.
CrossRef - Negash T., Tilahun G., Medhin G. Seroprevalence of Toxoplasma gondii in Nazareth town, Ethiopia. East Afr J Public Health. 2008;5:211–4.
- Alday P. H and Doggett J. S. Drugs in development for toxoplasmosis: advances challenges and current status. Drug Des Devel Ther. 2017;11: 273–293.
CrossRef - Mohammed S. N., Al-A’ssie A. H. A and Al-saqur I. M. Genotyping of Toxoplasma gondii Isolated from Aborted Iraqi Women. Diyala Journal of Medicine. 2015;9(1):44-52.
- Boothroyd, J. C Toxoplasma gondii: 25 years and 25 major advances for the Field. Int. J. Parasitol. 2009;39(8):935-946.
CrossRef - Ajzenberg D., Banuls A. L., Su C., Dumetre A., et al. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int.J .Parasitol. 2004;34:1185–96.
CrossRef - Babaie J., Amiri S., Mostafavi E., Hassan N., Lotfi P.,Rastaghi A. R. E ., et al. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Northeast of Iran. Clin Vaccine Immunol. 2013;20:1771-3.
CrossRef - Foroutan-Rad M., Khademvatan S., Majidiani H., Aryamand S., Rahim F., Malehi A. S. Seroprevalence of Toxoplasma gondii in the Iranian pregnant women: A systematic review and meta-analysis. Acta Trop. 2016;158:160-9.
CrossRef - Alsadoon M. A. The role of cytokines isolated from feto-placental tissue in women with spontaneous miscarriage. PhD. Thesis. College of Medicine, Basra University. 2015.
- Al-Eryani S. M., Al-Mekhlafi A. M., Al-Shibani L. A., Mahdy M. M., Azazy A. A. Toxoplasma gondii infection among pregnant women in Yemen: Factors associated with high seroprevalence. J Infect Dev Ctries. 2016;10(6):667-672.
CrossRef - Jumaian N. F. Seroprevalence and risk factors for Toxoplasma infection in pregnant women in Jordan. East Mediterr Health J. 2005;11(1-2):45-51.
- Rostami A., Seyyedtabaei S. J., Aghamolaie S., Behniafar H., Lasjerdi Z., Abdolrasouli A., et al. Seroprevalence and risk factors associated with Toxoplasma gondii infection among rural communities in northern Iran. Rev Inst Med Trop Sao Paulo. 2016;58:70.
- Gelaye W., Kebede T., Hailu A. High prevalence of anti-Toxoplasma antibodies and absence of Toxoplasma gondii infection risk factors among pregnant women attending routine antenatal care in two Hospitals of Addis Ababa, Ethiopia. Int J Infect Dis. 2015;34:41-45.
CrossRef - Hatam G., Shamseddin A., Nikouee F. Seroprevalence of toxoplasmosis in high school girls in Fasa district, Iran. Iranian J Immunol. 2005;2:177-81.
- Rahimi M. T., Mahdavi S. A., Javadian B., Rezaei R., Moosazadeh M., Khademlou M. et al. High seroprevalence of Toxoplasma gondii antibody in HIV/AIDS individuals from North of Iran. Iran J Parasitol. 2015;10:584-9.
- Mwambe B., Mshana S. E., Kidenya B. R., Massinde A. N., Mazigo H. D., Michael D., et al. Sero-prevalence and factors associated with Toxoplasma gondii infection among pregnant women attending antenatal care in Mwanza, Tanzania. Parasit Vectors Aug. 2013;6:222.
- El-Gozamy B. R., Mohamed S. A., Mansour H. A. Toxoplasmosis among pregnant women in Qualyobia Governorate. Egypt. J Egypt Soc Parasitol. 200;39:389-401.
- Ertug S., Okyay P., Turkmen M., Yuksel H. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health. 2005;5:66.
CrossRef - Wam E. C., Sama L. F., Ali I. M., Ebile W. A., Aghangu L. A., Tume C. B. Seroprevalence of Toxoplasma gondii IgG and IgM antibodies and associated risk factors in women of child-bearing age in Njinikom, NW Cameroon. BMC Res Notes. 2016;9(1):406.
CrossRef - Andiappan H., Nissapatorn V., Sawangjaroen N., Lau Y. l., Kumar T., Onichandran S., Suwanrath C, Chandeying V. Toxoplasma infection in pregnant women: a current status in Songklanagarind hospital, southern ailand. Parasit Vectors. 2014;22:239.
CrossRef - Alvarado-Esquivel C., Estrada-Martínez S., Liesenfeld O. Toxoplasma gondii infection in workers occupationally exposed to unwashed raw fruits and vegetables: a case control seroprevalence study. Parasit Vectors. 2011;4:235.
CrossRef








