Manuscript accepted on :1-Oct-2018
Published online on: 10-10-2018
Plagiarism Check: Yes
Reviewed by: Arun Singh
Second Review by: Wael Mayah
Final Approval by: Dr Mohamed Abdel-Daim
Randa Mohamed M.A. Farag1 , Dujana Al-Ayobi2
, Dujana Al-Ayobi2 , Khalid A Alsaleh3
, Khalid A Alsaleh3 , Hye-Joo Kwon4
, Hye-Joo Kwon4 , Afaf EL-Ansary5
, Afaf EL-Ansary5 and Emad Anwar Dawoud6
and Emad Anwar Dawoud6
1Virology and Molecular biology, Health Sciences Research Center (HSCR), Princess Nourah bint Abdulrahman University (PNU), Kingdom Saudi Arabia, KSA.
2Genetic, Biology department, Princess Nourah bint Abdulrahman University (PNU), Kingdom Saudi Arabia, KSA.
3Oncology and Hematology, college of Medicine, King Saud University (KSU), Kingdom Saudi Arabia, KSA.
4Molecular biology, Princess Nourah bint Abdulrahman University (PNU), Kingdom Saudi Arabia, KSA.
5Biochemistry, Central Labe, King Saud University, KSU.
6Hepatopathology, Faculty of Medicine, EL-Azher University and Specialist Physician, Oncology Clinic-Medical Affaies, Tawam Hospital, AL Ain, UAE.
Corresponding Author E-mail: randa792006@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1550
Abstract
In Saudi Arabia AFP considered the main serum marker for diagnostic Hepatocellular carcinoma (HCC), due to the continuous detection of HCC in Saudi Arabia, using new biomarkers for early surveillance are essential to control in prevalence of HCC. The present study depend on compare the significant between serum and mRNA Glypican-3 (GPC-3) as newly identified diagnostic and prognostic biomarkers for HCC between study cases. And combined sensitivity of AFP and GPC-3. Three hundred study cases, divided into: 250 blood samples were 145 samples from HCC , 105 samples from chronic liver cirrhosis (CLC) and 50 normal controls were investigated for serum GPC-3 (sGPC-3) by Sandwich ELISA. Glypican-3 mRNA from whole blood cells was detected by quantitative RT-PCR. The comparison between two techniques was by sensitivity and specificity. The results of sGPC-3 showed higher significant in HCC group than CLC and normal controls (p<0.001). sGPC-3 sensitivity was 95% and specificity was 100%, while in GPC-3 mRNA were 100% and 94% respectively. The combination of sensitivity between AFP and sGPC-3 was 80% and 95% respectively. The data demonstrated that, can depend on sGPC-3 and Glypican-3 mRNA as tumor biomarkers for detection and surveillance of Hepatocellular carcinoma in Saudi patients. The sensitivity of Reverse Transcriptase-PCR is high accurate (100%) than estimating sGPC-3 by ELISA (95%).
Keywords
Glypican 3- Sandwich ELISA- Quantitative RT-PCR- Early surveillance- Liver Cancer- Saudi Arabia
Download this article as:| Copy the following to cite this article: Farag R. M. M. A, Al-Ayobi D, Alsaleh K. A, Kwon H. J, EL-Ansary A Dawoud E. A. Influence of Glypican-3 as a Newly Diagnostic Biomarker in Early Detection of Hepatocellular Carcinoma Among Saudi Patients. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Farag R. M. M. A, Al-Ayobi D, Alsaleh K. A, Kwon H. J, EL-Ansary A Dawoud E. A. Influence of Glypican-3 as a Newly Diagnostic Biomarker in Early Detection of Hepatocellular Carcinoma Among Saudi Patients. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=23580 |
Introduction
According to Saudi Arabia, cancer registry, hepatic malignancies was 4.8% of all newly detected cases of all other types of malignance. HCC ratio 2.1: 1 a male to female and it accounts for 83% and 80% of all Hepatocellular carcinoma cases in males and females, respectively1
The chronic liver diseases are play main role in development of liver cancer. So, many genetic changes is hypothesized in HCC patients during long time. Many studies demonstrate that the infection of HBV and HCV is the main etiology agent of Liver cancer.2,3 But in Saudi Arabia, Hepatitis C is also common with a prevalence rate of 1% to 3% of the population which further increases the risk of HCC.3,4
The main problems in most surveillance programs of hepatocellular carcinoma (HCC) that is highly cost. In fact low percent of patients with HCC are able to treatments and transplantation of liver, but most of them are poor and can’t follow-up the treatment. And many clinical diagnosis depend on serum alpha fetoprotein (s AFP) that is normal levels in about 30% of liver cancer. On other hand AFP is significant in cases tumor size is >3 to 5, But it was in significant in HCC with small size < 2.5,6 Therefore, depend on AFP as the famous biomarker for detection and follow-up of HCC isn’t accurate and for early surveillance of HCC needed to a new diagnostic markers.7
Glypican-3 (GPC-3) is type of heparin sulfate proteoglycans cell that bind to the cell membrane and interacts with many growth factors in the migration, proliferation, and modulation of cell survival in different tissues which contributing to development of Liver cancer.7,8 Recently viewed some studies reported that GPC-3 levels are elevated in in serum or liver tissues of Hepatocellular carcinoma patients but is negative in primary liver lesion, hepatic cirrhosis and hepatitis.9 In addition, GPC-3 detected in HCC cases AFP- negative.10 GPC-3 could use as new biomarker for early detection of Hepatocellular carcinoma.8,10
The present study advocate to compare between the sensitivity and specificity of sGPC-3 and GPC-3 mRNA by using ELISA and RT-PCR in diagnostic Hepatocellular carcinoma patients from cirrhotic patients and normal controls and compared them with AFP levels in the same study groups.
Material and Methods
In the current study conduct on 300 blood samples, 145 collected from HCC and 105 samples collected from chronic liver cirrhosis (CLC) patients, where 185 (74%) males and 65 (26%) females (age range from 23 -78 years). The control group included 50 healthy blood donors were 36 males and 14 females (age range between 22 -60 years).
All studied samples collected from King Faisal Specialist Hospital; Pathology Department & Research Center (KFSHRC, Riyadh & Jeddah), Pathology Laboratory at Saudi German Hospitals (Riyadh, Jeddah, and Medina), Ministry of National Guard Hospitals, Princess Nourah Oncology Center, Jeddah, Security Forces Hospital Riyadh, Armed Forces Hospitals (Riyadh, Western, South & Northern), Public and Privet Hospitals in different regions in Saudi Arabia from July 2014 to May 2017
This study was done after approval from institute Review Board (IRB), NO. (14/4180/IRB), College of Medicine, King Khalid University Hospital. All participants were approved before enrolling in the study.
Detection of Tumor Markers
Serum GPC-3 was investigated by using commercially available ELISA kit provided by LifeSpan (LifeSpan BioSciences, USA). This technique is quantitative sandwich enzyme-linked immunoassay. The procedure of the ELISA assay was described briefly by Sung et al., (2011).11 The concentration of Glypican-3 was evaluated from the curve. The normal range of serum Glypican-3 was 0-55 ng/mL and the abnormal malignant rang was detected in the case of Glypican-3 >100 ng/mL.11
AFP was detected by using the Cobas601 electrochemiluminescence immunoassay analyzer. It indicated positive in the case of serum AFP > 7 μg/L.
Detection of Glypican-3 mRNA by RT-PCR
PCR was performed using Dream Taq Green PCR Master Mix (ThermoScientific, Fermentas). The temperatures protocol of reaction was performed at 25˚C for 10 min then 120 min respectively, and 85˚C for 5 min and kept at 4˚C. The reaction for β actin was performed in 25 µl reaction containing 1 µl cDNA, 1x master mix, 25 pmole of each primer.
For amplified β actin, the cyclic condition consisted of initial denaturation at 94˚C for 5 min, followed by 34 cycles of denaturation at 94˚C for 1 min, annealing at 63˚C for 2 min and elongation at 72˚C for 3 min and the primers used were: forward, 5′-ACCCACACTGTGCCCATCTA-3′, and reverse, 5′-GCCACAGGATTCCATACCCA-3′.
For amplified Glypican-3, the cyclic condition consisted of initial denaturation at 94˚C for 5 min, followed by 35 cycles of denaturation at 94˚C for 30 sec, annealing at 58˚C for 45 sec, elongation at 72˚C for1 min and final elongation at 72˚C for 10 min and the primer used was: forward, 5′-TTCTCAACAACGCCAATA-3′, and reverse, 5′-GATGTAGCCAGGCAAAGC-3′. The PCR products were visualized on 2% agarose and were 452 bp for β-actin and 256 bp for target gene of Glypican-3.11
Statistical Analysis
The statistical analysis in this study was done by using IBM SPSS version 20 (SPSS Inc., Chicago, IL). Where qualitative data were expressed as frequencies and percentages. Mann-Whitney test (non-parametric t-test) using in comparison between two groups for qualitative data. Kruskal-Wallis test (non-parametric ANOVA) then post-Hoc “Schefe test” on rank of variables was used for pair-wise comparison between three groups. Medians and ranges were using for Numerical data. Spearman-rho method was used to test correlation between numerical variables. For prediction of cut off values was used Receiver Operating Characteristic (ROC) curve. Sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) were calculated for all markers used. The p-value <0.05 was considered significant.
Results
Table 1: The demographic feature of HCC and CLC patients.
| Characteristics | Patients with HCC
(n 145) |
Patients without HCC (CLC)
(n = 105) |
| Gender (%)
Male/Female |
138/145 (95%)
7/145 (5%) |
M 75/105 (71.4%)
F 30/105 (28.6%) |
| Hepatitis virus (%)
HBV HCV HBV+HCV |
40/145 (27.6%)
105/145(72.4%) N/A |
37/105 (35.2%)
57/105 (54.3%) 11/105 (10.5%) |
| Chronic Hepatitis/LC (%)
Chronic non Hepatitis /L C (%) |
125/145 (86%)
20/145 (14%) |
75/105 (71%)
30/105 (29%) |
| Size of Tumor | ||
| < 2 cm
> 2 cm and ≤ 3 cm |
105/145
40/145 |
N/A
N/A |
*LC (Liver cirrhosis), N/A (not detected).
The data shown significant difference in HCC sGPC-3 and AFP results compared to CLC and normal (healthy) control group (p<0.001).
Detection of sGPC -3 by ELISA was showed high positivity rate in Hepatocellular carcinoma 95.2 % (138/145) patients. While in CLC it was 12.4% (/105) and it was negative in the normal group (p<0.001). GPC -3 mRNA was detected by RT-PCR in 100% Hepatocellular carcinoma group ( Table 2, Figure 2) and it was 10.5 % (11 /105) positivity in CLC group and negative in healthy group (p<0.001). While AFP was detected in HCC group (80%), in the cirrhosis group (25%), and was negative in the control group (p<0.001).
sGlypican -3 median levels were 125, 62, 0.89 ng/ml in the HCC , CLC, and health control group, and AFP were 147.5, 15, 3.2 respectively.
One hundred five out of 145 of Hepatocellular carcinoma patients (72.4 %) were positive Hepatitis C virus (HCV) and 40/145 (27.6%) were positive Hepatitis B virus (HBV). All hepatitis Hepatocellular carcinoma patients (HCC) were cirrhotic.
The highest results of sGPC-3 were detected in HCC with HBV and HCV positive compared to HBV and HCV negative patients. It was 96% (120/125), 90% (18/20) in positive and negative hepatitis virus HCC patients respectively. While for AFP, the highest results were measured in HBV and HCV positive patients.
In contrast, sGPC-3 was 10.5 % positivity in cirrhosis group (11/105); where 12 % (9/75) in positive hepatitis B and C patients and it was 6.7 % (2/30) in non-hepatitis viruses cases (negative HBV and HCV); also it was negative in healthy control group.
This results showed that level of sGPC-3 was higher and significant in HCC in positive hepatitis B and C patients than negative hepatitis viruses cases (p<0.001). While in CLC there is no significant difference between positive and negative HBV, HCV patients (p = 0. 612).
Table 2: The positivity rate and median levels of serum GPC-3, GPC-3mRNA and compare with AFP.
| Study groups | sGPC-3
Positivity rate |
sGPC-3 (ng/ml)
median levels |
GPC-3 mRNA
Positivity rate |
sAFP
Positivity rate |
sAFP
(ng/ml) median levels |
| HCC group | 92.2 % | 125 | 100 % | 80 % | 147.5 |
| CLC group | 12.4 % | 62 | 10.5% | 25 % | 15 |
| Healthy control group | 0 % | 0.89 | 0 % | 0 % | 3.2 |
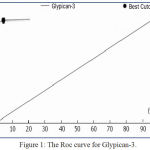 |
Figure 1: The Roc curve for Glypican-3.
|
According to the data shown in Figure 1, The area under curve of GPC-3 was 0.985 ± 0.024 and the Se:sensitivity, Sp: specificity, PPV :positive predictive value ,NPV: negative predictive value, and Da: diagnostic accuracy for sGlypican3 were (95%, 100%, 97.5%, 90.5% and 95%) respectively and it CI: confidence interval was 95%.
The detection value of GPC-3 was higher than that of AFP was 0.457 to 0.496, and could use these marker in early diagnosis of HCC. Moreover, GPC-3 level was elevated in the serum of individuals with HCC who had serum AFP level less than 400 ng/ml. So could use these markers in early diagnosis of early HCC in Saudi patients.
This result demonstrated that sensitivity and specificity of AFP is not accuracy in primary HCC compared to accuracy in new tumor marker (GPC-3), which not detected in healthy group and it can distinguish between primary HCC and CLC patients. Further we need more investigated the combination of the two markers (GPC-3, AFP) in continuous follow-up of primary HCC and CLC.
GPC-3mRNA measured by RT-PCR was positive in 100% (145/145) of all cases in the HCC group in both positive and negative Hepatitis virus’s patients. In the CLC group, GPC-3mRNA was 17% (18/105) (in Positive HBV, HCV was 20% (15/75) and 10% (3/30) in negative HBV, HCV) and not detected in healthy control group (p<0.001). The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for Glypican3 mRNA were 100%, 94%, 95%, 100% and 97% respectively and the optimal cut off was 4.5 ng/ml.
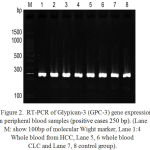 |
Figure 2: RT-PCR of Glypican-3 (GPC-3) gene expression in peripheral blood samples (positive cases 250 bp). (Lane M: show 100bp of molecular Wight marker, Lane 1:4 Whole blood from HCC, Lane 5, 6 whole blood CLC and Lane 7, 8 control group).
|
As shown in Figure 2. The columns 1-8 show GPC-3 positive cases 250 bp. The band density of Glypican-3 gene is constant in normal control while was higher in HCC than in CLC, so expression of GPC-3 mRNA was significantly difference in HCC than healthy control group.
We found sGPC-3 to have sensitivity of 95%, specificity was 100%, positive predictive value was 97.5%, negative predictive value was 90.5 and Diagnostic accuracy was 95% at cut off 4.5 ng/ml and for AFP were (80%, 80%, 91%, 70.8 and 80.3%) respectively at cut off 40.5 ng/ml. For GPC-3 mRNA, they were (100 %, 94%, 95%, 100% and 97%) respectively (Table 3).
Table 3: Diagnostic performance of sGPC-3, GPC-3mRNA compared with alpha-fetoprotein.
| s Glypican-3
(cutoff 4.5 ng/ml) |
sAFP
(cutoff 40.5 ng/ml) |
Glypican-3mRNA | |
| Se % (95% CI)* | 95 (86.0-99.0) | 80 (70.1-90.7) | 100 (91.0-100.0) |
| Sp % (95% CI) | 100 (85.2-98.7) | 80 (70.9-90.5) | 94 (68.0-98.0) |
| P P value % (95% CI) | 97.5 (88.5-99.7) | 91 (80.9-94.9) | 95 (83.0-99.0) |
| N P value % (95% CI) | 90.5 (79.0-96.0) | 70.8 (57.5-81.5) | 100 (81.0-99.9) |
| D A % (95% CI) | 95 | 80 | 97 |
Se: Sensitivity, Sp: Specificity, PP value: Positive Predictive value, NP value: Negative Predictive value, DA: Diagnostic Accuracy; *95%CI: Confidence IntervalView it in a separate window
Discussion
In fact, the surveillance programs of HCC has evolved significantly over the past few decades14. Unfortunately, the most of its depend on ultrasound and alpha fetoprotein (AFP)15. However, in some reports recorded that the sensitivity for diagnosis of Hepatocellular carcinoma approximately 25%-60%, and its specificity is low and AFP was estimated in patients with CLC range between 11%-47% and 15%-58% in non -cirrhosis chronic hepatitis patients2.In addition, 30% HCC patients present with normal AFP. Thus, the needed for new tumor markers that can differentiated among Hepatocellular carcinoma and primary hepatic necrosis it is much important for specific diagnosis and early surveillance of HCC.16,17
The data in this study demonstrated that, Glypican-3 mRNA in HCC was higher sensitive between the studied markers for detection liver cancer in Saudi patients (100% positivity rate), followed by Serum Glypican-3 (95%), then AFP (80%). And also in cirrhosis group, GPC-3 mRNA showed the highest positivity (30%), then sGPC-3 (10.5%).
In contrast, by comparison of sGlypican-3 and AFP between the all studied groups showed significant difference between HCC, CLC and healthy control groups. This demonstrated that the continuous measurement of GPC-3 could provide a significant improved test for detection of HCC in both cases with/not hepatitis viruses, this is in accordance with other researchers.10,15,18,19,20,21 And the influence of Glypican-3 as a newly tumor biomarker for liver cancer and early detection of primary HCC has been recently confirmed by El-Shenawy et al.22 and Iman Attia et al.23
The present study documented the clinical utility of Glypican-3 mRNA as sensitive quantitative assay for screening HCC. Our results in agreements with youssef et al.24 Yan et al.25 Gomaa et al.26 Some studies reported that, in HCC patients, s GPC-3 and AFP levels increased and negative in other benign liver cases.10,18,19 The absence of serum GPC-3 in healthy individuals was reported by some other studies.15,20,21,22
The results in this study, demonstrated that sGlypican-3 in Hepatocellular carcinoma group was significantly differed from CLC, health control subject, that pointed to in Saudi patients, serum Glypican-3 was high significantly in HCC as is can considered as newly tumor biomarker for early diagnosis and surveillance of HCC. And that because release of GPC-3 protein by hepatoma cells in first stage HCC cases, sGPC-3 was significantly elevated in HCC. These results indicated that the hepatitis cirrhotic cases also had Glypican-3 elevated, that agreement with the study’s results of youssef et al.24 Yan et al.25 Gomaa et al.26 So, Glypican-3 have specific sensitive in Saudi patients with hepatic disorders.
Recently, several studies demonstrated that Glypican-3 is highly sensitive and specific and be favorite than AFP in diagnosis and early surveillance of HCC,21. The comparison of ROC curves for GPC-3 displayed that GPC-3 was high accurate than AFP in the diagnosis of HCC. The sensitivity of sGPC-3 was 95% and AFP was 80% in HCC consistent with the findings of by El-Shenawy et al.22 Iman Attia et al.23 and youssef et al.24
As regards Glypican-3mRNA level by RT-PCR, our results showed that GPC-3mRNA was estimated in all Hepatocellular carcinoma group (100%), in cirrhosis patients was (30%) and was absent completely in healthy controls subject. Our results were in constant with youssef et al.24Yan et al.25 Gomaa et al,26 Yongle et al27 and Sung et al28 these studies reported similar result reported that Glypican-3mRNA is significantly up regulated in Hepatocellular carcinoma compared to normal and benign liver samples, and hence Glypican-3 mRNA could be useful as diagnostic molecular tumor marker for early diagnosis of Hepatocellular carcinoma.
Regarding the one hundred five CLC cases, included in the present study, eighteen of them were positive for Glypican-3mRNA. These patients were surveillance for 12 months. The fifteen who were positive hepatitis viruses also having moderate elevation in serum Glypican-3 and AFP levels has been detected as HCC after 6 months of follow up. These results could support that Glypican-3 could be used for screening and early diagnosis of HCC among CLC patients. This suggestion was agreement with El-Saadany et al 15 and Iman Attia et al.23 where reported that sGPC-3 levels was estimated during the follow up of their patients with liver cirrhosis and Hepatocellular carcinoma developed during follow-up selected cases with detect a significant change of serum AFP levels.
The low positivity of Glypican-3 (10.5 % ) in cases of HBV and HCV without hepatic malignant (cirrhosis group (11/105) in this study was 12 % (9/75) in positive hepatitis B and C patients and it was 6.7 % (2/30) in non-hepatitis viruses cases (negative HBV and HCV) give evidence that the high specificity of Glypican-3 in HCC versus non HCC hepatitis cases, as positivity of HBV or HCV infection will not give false positive result especially in Saudi patients, where there is continuous prevalence of hepatitis viral infection. Liu et al., (2010) reported that GPC-3 was present in the serum of HCC patients, but was undetectable in all patients with hepatitis as well as healthy individuals.9 And Li et al., (2013) reported that Glypican-3 was highly expressed in acute and chronic hepatitis patients, indicating that such protein was less sensitive in detection of hepatic diseases but could be helpful as their serum biomarker.11 While Gao et al., (2015) demonstrated that serum Glypican-3 was evaluated in the chronic hepatitis patients, cirrhosis, and hepatocellular carcinoma.20
By comparing between two techniques used in this study, the sensitivity of GPC-3by RT-PCR proved to be more sensitive (100%) than ELISA (95%).
Glypican-3 play important role in tumor development and expressed by hepatocytes, the hepatitis viral infection induced the regulation expression of Glypican-3 in HCC hepatocytes, and this give strongly suggestion that Glypican-3 is a good molecular marker for diagnosis and early surveillance of HCC. Estimation of Glypican-3 diagnostics may be help in prevention and treatment of liver cancer (Tahon et al,10 El-Saadany et al,15 Yongle et al27 Sung et al 28 and Sun et al.29)
In conclusion, Glypican-3 is a highly sensitive and specificity diagnostic biomarker for Hepatocellular carcinoma which can used in early detection and screening of HCC and CLC among Saudi patients. There is no influence of hepatitis viruses on the diagnostic accuracy of Glypican-3 in detection of HCC among Saudi patients. Estimation of Glypican-3mRNA by RT-PCR proved to be more sensitive (100%) than measuring Glypican-3 by ELISA (95%), and it is more suitable for follow up of CLC cases. Further studies with larger sample sizes and long follow up of HCC and CLC patients are needed to estimate and study the effect of Glypican-3 in early detection and surveillance of HCC.
Acknowledgment
The authors would like to thank King Abdul Aziz City for Science and Technology (KACST) for supporting the present work as part of NTPC funded projects No. AT-34-208.
References
- Abdo A. A., Hassanain M., AlJumah A. R et al., Saudi guidelines for the diagnosis and management of hepatocellular carcinoma technical review and practice guidelines. Annals of Saudi Medicine. 2012;32(2):174–199.
CrossRef - Abdulaziz A and ALSalloom M. An update of biochemical markers of hepato cellular carcinoma. Int J Health Sci. 2016;10(1):121–136. (Qassim).
- Anand M., Harmin H., Timothy B., Glycosylation and Liver Cancer. Adv Cancer Res. 2015;126:257-279.
CrossRef - Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma (2014), http://apasl.info/apasl/wp- content/uploads/2014/02/ Guidelines-for-HCC- Management-2010. pdf.
- Block T. M., Comunale M. A., Lowman M., Steel L. F., Romano P. R., Fimmel C., Tennant B. C., London W. T., Evans A. A., Blumberg B. S., Dwek R. A., Mattu T. S., Mehta A. S. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci USA. 2005;102:779-784.
CrossRef - Cancer Incidence Report in Saudi Arabia Saudi Cancer Registry http://www.chs.gov.sa/Ar/mediacenter/NewsLetter/2010%20Report%pdf. 2010;20(1).
- Capurro M., Wanless I. R., Sherman M., et al: Glypican-3 a novel serum and histochemical marker for hepatocellular carcinom. Gastroenterology. 2003;125:89-97.
CrossRef - Wang X. Y., Degos F., Dubois S., eta. Glypican-3 expression in hepatocellular tumors diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 2006;37:1435-1441.
CrossRef - Liu H., Li P., Zhai Y., eta. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410-4415.
CrossRef - Tahon A. .M, El-Ghanam M. Z., Zaky .S, Emran T. M., Bersy A. M., El-Raey F. A. Z. E., El Kharsawy A. M., Johar D. Significance of Glypican-3 in Early Detection of Hepatocellular Carcinoma in Cirrhotic Patients. J Gastrointest Cancer. 2018. doi: 10.1007/s12029-018-0095-2.
CrossRef - Li B., Liu H., Shang H. W., Li P., Li N and Ding H. G. Diagnostic value of glypican-3 in alpha fetoprotein negative hepato cellular carcinoma patients. Afr Health Sci. 2013;13:703-709.
- Sung Y. K., Hwang S. Y., Park M. K et al. Glypican-3 is overexpressed in human hepa to cellular carcinoma. Cancer Sci. 2003;94:259-62.
CrossRef - Page A. J., Cosgrove D. C., Philosophe B., Pawlik T. M. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am. 2014;23:289–311.
CrossRef - Mustafa M. G., Petersen J. R., Ju H. Cicalese L.,Snyder N., Haidacher S. J., et al. Biomarker discovery for early detection of hepato cellular carcinoma in hepatitis C-infected patients. Mol Cell Proteomics. 2013;12(12):3640–52.
CrossRef - El-Saadany S., El-Demerdash T., Helmy A., Mayah W. W., El-Sayed H. B., Hassanien M., Elmashad N., Fouad M. A., Basha E. A. Diagnostic Value of Glypican-3 for Hepato cellular Carcinomas. Asian Pac J Cancer Prev. 2018;19(3):811-817.
- Salim E. I., Moore M. A., Al-Lawati J. A., Al-Sayyad J., Bazawir A., Bener A., Corbex M., El-Saghir N., Habib O. S., Maziak W., et al. Cancer epidemiology and control in the arabworld – past present and future. Asian Pac J Cancer Prev. 2009;10:3–16.
- See comment in PubMed Commons below Shaker M. K.,Fattah A .H. I., Sabbour G. S., Montasser I. F., et al. Annexin A2 as a biomarker for hepatocellular carcinoma in Egyptian patients. World J Hepatol. 2017;28;9(9):469-476.
- Liu H., Li P., Zhai Y., et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J. Gastroenterol. 2010;16:4410-4415.
CrossRef - Yasuda E., Kumada T., Toyoda H., et al. Evaluation for clinical utility of GPC-3, measured by a commericially available ELISA kit with GPC-3 antibody as a serological and histological marker for. Hepatol Res. 2010;40:477-85.
CrossRef - Gao G., Dong F.,Xu X., Hu A and Hu Y. Diagnostic value of serum Golgi protein 73 for HBV-related primary hepatic carcinoma. Int J Clin Exp Pathol. 2015;8:11379.
- Jeon Y., Jang E. .S, Choi Y. S., Kim J. W., Jeong S. H. Glypican-3 level assessed by the enzyme-linked immunosorbent assay is inferior to alpha-fetoprotein level for hepatocellular carcinoma diagnosis. Clin Mol Hepatol. 2016;2(3):359-365.
CrossRef - El-Shenawy S., El Sabawi M., Sheble N.,et al. Diagnotic Role of serum Glypican-3 as a tumor marker for hepatocellular carcinoma. Nature Sci. 2012;10:32-8.
- Attia I., Ghada I. M., Noha H. R., Heba M. E. l., Niveen M. E. l. Can Glypican3 be Diagnostic for Early Hepatocellular Carcinoma among Egyptian Patients? Asian Pac J Cance Prev. 2014;14(12):7345-7349.
- Youssef M., El-Sharkawy S., Abbas N., et al. Clinical utility of Glypican-3 in hepatocellular carcinoma. Int J Integr Biol. 2010;10:41-7.
- Yan D., He Q., Chen Y., et al. Detection of α-fetoprotein and glypican-3 mRNAs in the peripheral blood of hepatocellular carcinoma patients by using multiple FQ-RT-PCR. J Clin Lab Anal. 2011;25:113-7.
CrossRef - Gomaa A., Hendy O., Aboraia G., et al. The diagnostic Value of Peripheral blood Glypican-3 in patients with hepatocellular carcinoma. World J Med Sci. 2012;7:105-12.
- Yongle W. u., Liu H., Weng H., Zhang X., Li P., Chun-Lei F.,et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. International Journal of Oncology. 2015;66(3):1275-1285.
- Sung Y. K., Hwang S. Y., Farooq M., Kim J. C and Kim M. K. Growth promotion of HepG2 hepatoma cells by antisense-mediated knockdown of glypican-3 is independent of insulin-like growth factor 2 signaling. Exp Mol Med. 2003;35:257-262.
CrossRef - Sun C. K., Chua M. S., He J and So S. K. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-beta2. Neoplasia. 2011;13:735-747.
CrossRef







