Manuscript accepted on :17-Oct-2018
Published online on: 06-11-2018
Plagiarism Check: Yes
Reviewed by: Amarendranath Choudhury
Mohankrishna Ghanta1 , Elango Panchanathan1
, Elango Panchanathan1 , Bhaskar V. K. S. Lakkakula2
, Bhaskar V. K. S. Lakkakula2 , Anbumani Narayanaswamy3
, Anbumani Narayanaswamy3 , Yogeshkumar Murkunde4 and Shonam Tamrakar4
, Yogeshkumar Murkunde4 and Shonam Tamrakar4
1Department of Pharmacology, Sri Ramachandra Medical College, Sri Ramachandra Institute of Higher Education and Research - DU, Chennai-600116, Tamil Nadu, India.
2Department of Molecular Genetics, Research Division, Sickle Cell Institute Chhattisgarh, Raipur- 492001, Chhattisgarh, India.
3Department of Microbiology, Sri Ramachandra Medical College, Sri Ramachandra Institute of Higher Education and Research - DU, Chennai-600116, Tamil Nadu, India.
4CEFTE, Sri Ramachandra Institute of Higher Education and Research - DU, Chennai-600116, Tamil Nadu, India.
Corresponding Author E-mail: drpelango@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1576
Abstract
Recent researches have suggested 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ), a soluble guanylate cyclase inhibitor may attenuate motor impairments in Parkinson's disease (PD). The antiparkinsonian activity of ODQ were studied on motor abnormalities induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to provide a better understanding of this drug group. The objective of the present study is to evaluate the effect of ODQ on behavioral parameters such as Beam walk test, Adhesive removal test and to assess the biochemical changes due to ODQ against MPTP induced PD mice model. Eighteen C57BL/6J male mice were included in the study, divided into three groups of 6 each. Group A mice were treated with vehicle (Normal saline). Group B mice were subjected to MPTP sub acute protocol. Group C mice were treated with MPTP as according to sub acute protocol and administered with ODQ subcutaneous injection after final MPTP dose. Behavioral tests like Beam walk test, Adhesive removal test, along with Biochemical correlation were done using standard methods. Narrow beam walk and adhesive removal behavior were significantly reversed, and Superoxide dismutase (SOD) levels were enhanced in ODQ treated group compared to MPTP intoxicated mice group. Soluble guanylate cyclase inhibitor ODQ, could be a potential treatment for maintaining the balance of antioxidant and oxidant biochemical environment during oxidative stress which may be helpful for treating PD, targeting one or more factors of its multiple etiological factors.
Keywords
Behaviour; 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; Parkinson's Disease; Superoxide Dismutase; MPTP
Download this article as:| Copy the following to cite this article: Ghanta M, Panchanathan E, Lakkakula B. V. K. S, Narayanaswamy A, Murkunde Y, Tamrakar S. 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one Attenuates Oxidative Trauma and Recuperate Beam Walk and Adhesive Removal Behavior in MPTP Parkinsonian Mice Model. Biomed Pharmacol J 2018;11(4). |
| Copy the following to cite this URL: Ghanta M, Panchanathan E, Lakkakula B. V. K. S, Narayanaswamy A, Murkunde Y, Tamrakar S. 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one Attenuates Oxidative Trauma and Recuperate Beam Walk and Adhesive Removal Behavior in MPTP Parkinsonian Mice Model. Biomed Pharmacol J 2018;11(4). Available from: http://biomedpharmajournal.org/?p=23927 |
Introduction
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) is a known selective inhibitor of soluble guanylate cyclase enzyme. Soluble guanylate cyclase (sGC) enzyme is a heterodimer consisting of two sub units namely alpha and beta. Beta subunit consists of heme site which acts as a receptor for nitric oxide. Nitric oxide stimulates sGC and causes synthesis of cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP).1 cGMP causes glutamate toxicity upon over regulation of sGC.2 ODQ inhibits sGC by binding to heme site and affects sGC- cGMP signalling pathway.3 Excitoxicity was a known factor for Parkinson’s disease (PD) due to high glutamergic inputs in Basal ganglia pathway. Also, it was considered for aggravating the disease condition and striatal neuronal cell death.4 Over regulation of glutamate receptors causes intracellular calcium ion accumulation resulting in formation of reactive oxygen species. Activation of glutamate receptors causes opening of calcium channel that are coupled to glutamate receptors, results calcium influx and accumulation. This leads to neurocyte excitation that may initiate apoptosis and neurodegeneration.5
Glutamate toxicity is also related to superoxide dismutase levels (SOD). SOD catalyses the free radicals. Compromise in SOD levels increased glutamate toxicity.6 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) increases SOD activity due to increased free radical generation which finally gets compromised.7 The imbalance in glutamergic transmission results in akinesia.8 This akinesia is a movement disorder causing difficulty in mobility. This is measured by behavioral tests. In this study we performed narrow beam walk test and Adhesive removal test. These tests were among the behavioral tests considered sensitive for identifying classical motor manifestations of PD in MPTP mice and also the functional recovery of the animal and treatment efficacy. The present study was performed to evaluate the effects of ODQ against MPTP induced oxidative stress and behavioral impairments in C57BL/6J mouse model.
Materials and Methods
Animals
C57BL/6J male mice (25-30gm) were obtained for the study from Centre for Toxicology and Developmental Research (CEFT), Sri Ramachandra Institute of Higher Education and Research – Deemed University, Porur, Chennai, Tamil Nadu, India. Animals were housed in groups of 4 per cage. The temperature and humidity of the room was well controlled and maintained light-dark cycle on 12L:12D. They were provided with water and food ad libitum. The study was done according to national guidelines of proper care and use of animals in laboratory research (Indian National Science Academy, New Delhi, 2000). Ethical clearance was obtained from Institutional Animal Ethics Committee (IAEC/49th/SRU/502/2016), Sri Ramachandra Medical College and Research Institute- Deemed University, Porur, Chennai, India.
Chemicals
MPTP, ODQ were purchased from TCI Chemicals (India) Pvt. Ltd. Avery dotted Adhesives (Amazon Export Sales LLC, USA) were purchased. Mouse anti-Tyrosine hydroxylase (Sigma Aldrich, USA), goat anti-mouse IgG and Immuno CruzTM ABC Staining kit (Santa Cruz, USA). All other chemicals used for SOD assay were of analytical grade.
Experimental Procedures
Eighteen C57BL/6J mice included in the study were randomized into three groups. Group A (n=6) included mice treated with normal saline (0.9%). Group B (n=6) included mice treated with intraperitoneal injection (IP) of MPTP-HCL, Group C (n=6) included mice treated with IP injection of MPTP-HCL and subcutaneous injection of ODQ. MPTP-HCL was administered for five days of 7 doses. 20mg/kg dose was given twice daily, 12th hourly for two days followed by 20mg/kg dose once daily for subsequent three days.9 10mg/kg ODQ was administered subcutaneously on the last day one hour after the last dose of MPTP-HCL.
Behavioral Tests
Narrow Beam Walk Test
The beam walking test apparatus was featured with a wooden beam (L100 cm×W1 cm) which was mounted on a height of 1m above ground with help of two wooden towers. One end of the beam had a box with a hole so that the mice can enter from the beam into the box. In this test the mouse was trained to cross the beam as described elsewhere.10 To measure the motor coordination and balance, foot slips and traversal time to cross the beam were recorded as described previously.11
Adhesive Removal Test
In this test, the mice in the home cage were shifted to another cage. The test mouse remained in the home cage. With the help of forceps, the adhesive dot was applied to snout of the mice. Time of contact, time to remove the adhesive dot was recorded. The latency in touching the adhesive dots was used for evaluating sensory impairment. Maximum time for the test was 60s. If the mouse did not remove the adhesive dot with in 60s, then it was removed manually. Three trials were performed for each mouse.12
SOD Assay
Estimation of SOD activity in brain homogenate was done by spectrophotometric method.13 Inhibition reaction of NADH- Phenazine methosulphate – nitroblue tetrazolium formazan was measured at 560nm optical density. The enzyme activity was expressed as milliunits/mg protein. One single unit of enzyme activity implies the concentration of enzyme required to inhibit the chromogen production by 50% in one min at an optical density of 560nm.
Tyrosine Hydroxylase (TH) Immunohistochemical Staining
Immunohistochemical evaluation of TH positive neurons in mice brain was investigated. Five micrometer – thick paraffin sections through matched coronal levels were stained with mouse anti-TH (1:100) using standard immunoperoxidase techniques. TH immunoreactivity was detected with a biotinylated goat anti-mouse for TH secondary antibody at 1:100 dilutions and Immuno CruzTM ABC Staining kit. All slides were counterstained with Mayer’s hematoxylin and visualised in light microscopy. The expression of immunopositivity in sections was evaluated at a magnification of x 10 under light microscopy. The number of immunopositive cells were counted manually as described previously by Guo et al.(2016) with minor modification.14
Statistical Analysis
The means of all groups in the study was analysed by one-way ANOVA and for multiple comparisons Post hoc test was done using SPSS software version 16.0.
Results
The biochemical and behavioral tests mean values of all groups in study, their comparison using one-way ANOVA and Post hoc test are tabulated in Table 1.
Effect of ODQ on SOD Expression in Substantia Nigra of MPTP Treated Mice
In comparison to MPTP treated mice, ODQ treated MPTP mice showed enhancement in SOD levels (125.60±13.10). MPTP group mice exhibited decreased levels of SOD levels (74.80±12.38) in comparison to control mice group (132.62±14.84) [Figure 1].
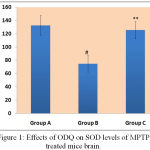 |
Figure 1: Effects of ODQ on SOD levels of MPTP treated mice brain.
|
SOD level were represented in Y-axis as unit/mg protein and the study groups were mentioned in X-axis. # P value compared between Group A and Group B is 0.0005 (< 0.05), ** P value compared between Group B and Group C is 0.0005(<0.05). P value < 0.05 is significant.
Effect of ODQ on Narrow Beam Walk Test Against MPTP Treated Mice
Beam walking test detects the dopaminergic neuronal loss in nigro-striatal pathway (central nervous system lesions).15 There was significant increase in beam walk traversal time of MPTP treated mice group (47.375±9.0) in comparison to control mice group (11.875±2.79). ODQ reversed the beam walking ability (25.625±7.52) which was statistically significant. Number slips while crossing the beam was noted which was noticed high in MPTP treated mice (4.875±2.29) compared to control group (0.75±0.46). ODQ showed significant improvement compared to MPTP treated group (2.875±1.35) [Figure 2].
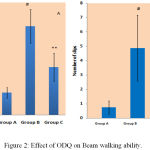 |
Figure 2: Effect of ODQ on Beam walking ability.
|
A- shows traversal time, # P value compared between Group A and Group B is 0.0005(< 0.05), ** P value compared between Group B and Group C is 0.0005(< 0.05). B- shows number of slips, # P value compared between Group A and Group B is 0.0005 (< 0.05), ** P value compared between Group B and Group C is 0.007 (< 0.05). P value < 0.05 is significant.
Effect of ODQ on Adhesive Removal Test Against MPTP Treated Mice
The time taken for removing the adhesive material by mice from its snout signifies the sensory and motor coordination abilities of the mice. MPTP treated mice showed poor ability to remove the adhesive dot, took more time and attempts compared to normal control mice and ODQ treated MPTP mice [Figure 3].
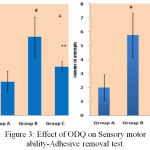 |
Figure 3: Effect of ODQ on Sensory motor ability-Adhesive removal test.
|
A- shows removal time in seconds on Y-axis, # P value compared with Group A is 0.0005(< 0.05), ** P value compared between Group B and Group C is 0.0005(< 0.05). B- shows number of attempts, # P value compared between Group A and Group B is 00.0005 (< 0.05), ** P value compared between Group B and Group C is 0.005 (< 0.05). P value < 0.05 is significant.
Effects of ODQ on TH- Positive Neurons in Substantia Nigra of MPTP Treated Mice
The TH-positive neurons in substantia nigra were shown as mean ± SE. The MPTP treated mice of group B (17.2±2.08) showed decrease in TH positive neurons when compared to group A, normal mice (45±3.24). Group C, ODQ treated mice showed higher expression of TH positive neurons (38.25±2.25) when compared to Group B [Figure 4].
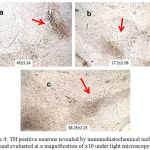 |
Figure 4: TH positive neurons revealed by immunohistochemical technique and evaluated at a magnification of x10 under light microscopy.
|
a, b, c corresponds to the study groups A, B, C respectively. The cells counted manually in ten random fields were expressed as Mean ± Standard Error of Mean.
Discussion
PD pathology includes mostly pronounced antioxidant role. The antioxidant enzyme role in dopaminergic neurons of basal ganglia is to compensate continuous generation of free radicals. MPTP toxicity alters status of antioxidant enzymes by damaging mitochondria.16 So MPTP toxicity may be measured by estimating the antioxidant enzyme levels. In our study efficacy of ODQ was evaluated by estimating the SOD enzyme and motor behavioral changes against MPTP treated mouse model of PD. Sub-acute protocol of MPTP used for induction of PD in mice of this study showed reduction in TH positive neurons. SOD levels were found to be reduced in MPTP model.17,18 In our study MPTP decreased SOD levels in mice brain. There are studies which reported decreased levels of SOD enzyme in Parkinson’s disease patients.19 Excitotoxicity due to free radicals may be inhibited by administering superoxide dismutase.20 Glutamate antagonist was shown to decrease post synaptic membrane excitation mediated by glutamate receptor coupled calcium channels of neurocytes. This was found to be beneficial in patients of comatose state.21,22 Another study stated that intense brain irritation by glutamate like compound may be alleviated by glutamate antagonists ensuring neuroprotection2. In our study, ODQ enhanced superoxide dismutase activity compared to MPTP treated mice. Hypoactivity in mice within half an hour of MPTP treatment and lasting up to 40 wks post treatment was reported in many studies.23,24 Onset of hypoactivity was same in our study. Behavioral assessment was done by beam walk test and adhesive removal test to evaluate motor impairments. Beam walking test was initially used for evaluating sensory motor abnormalities in stroke, Huntington’s disease and PD patients.25-27 This test also provides evaluation for fine motor initiation, coordination and postural balance of individual animal. MPTP toxicity was reported to increase the traversal time and increase the number of slips (in coordination) in beam walk test. Our study results showing mobility time was consistent with other studies.28 ODQ showed beneficial effects by increasing the mobility time and decreased the number of slips. Adhesive removal test confirms sensorimotor abilities of the animal.29 Increased adhesive removal time was reported in PD models and this is also seen in our study.12,30 ODQ reduced the adhesive removal time indicating the reversal of motor ability. Taking our study results in to consideration it may be stated that ODQ attenuate glutamate toxicity resulted by MPTP toxicity and improved SOD enzyme activity. This also may show the role of sGC – cGMP pathway in MPTP toxicity and in treatment of PD.
Conclusion
The results of our study show that MPTP up-regulated glutamate receptor and affected SOD activity in brain which might result in compromised antioxidant activity of mice. This may result in neurological insult that might cause neurodegenerative diseases. As MPTP toxicity is specific to corpus striatum and striatal dopaminergic neurons, it results in Parkinson’s disease. ODQ which is a specific soluble guanylate cyclase inhibitor attenuated MPTP toxicity and showed beneficial effects in this preclinical study.
Conflict of Interest
There is no conflict of interest.
Acknowledgements
The authors would like to thank the Founder Chancellor, Sri Ramachandra Institute of Higher Education and Research, for providing necessary funds and facilities. We also thank Mr. S. Ravi Kumar, and Mrs. Mangayarkarasi, CEFTE, Sri Ramachandra Institute of Higher Education and Research for their support during animal experiments.
Funding Source
Sri Ramachandra University Founder Chancellor Shri. N.P.V. Ramasamy Udayar Research Fellowship.
References
- Derbyshire E. R., Marletta M. A. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533-559.
CrossRef - Ghanta M., Panchanathan E., Lakkakula B., Narayanaswamy A. Retrospection on the Role of Soluble Guanylate Cyclase in Parkinson’s Disease. J Pharmacol Pharmacother. 2017;8:87-91.
- Feelisch M., Kotsonis P., Siebe J., Clement B., Schmidt H. H. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Molecular pharmacology. 1999;56:243-253.
CrossRef - Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neuro degeneration. Pflugers Arch. 2010;460:525-542.
CrossRef - Brown J. I., Baker A. J., Konasiewicz S. J., Moulton R. J. Clinical significance of CSF glutamate concentrations following severe traumatic brain injury in humans. J Neurotrauma. 1998;15:253-263.
CrossRef - Li Y., Copin J. C., Reola L. F., et al. Reduced mitochondrial manganese-superoxide dismutase activity exacerbates glutamate toxicity in cultured mouse cortical neurons. Brain Res. 1998;814:164-170.
CrossRef - Regner A., Schunemann D. P., Grivicich I., et al. Effects of toxic doses of glutamate on Cu-Zn and Mn/superoxide dismutases activities in human glioma cell lines. J Neurooncol. 2005;71:9-17.
CrossRef - Lange K. W., Kornhuber J., Riederer P. Dopamine glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev. 1997;21:393-400.
CrossRef - Tremblay M. E., Saint-Pierre M., Bourhis E., Levesque D., Rouillard C., Cicchetti F. Neuroprotective effects of cystamine in aged parkinsonian mice. Neurobiol Aging. 2006;27:862-870.
CrossRef - Ferguson M. C., Nayyar T., Deutch A. Y., Ansah T. A. 5-HT2A receptor antagonists improve motor impairments in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2010;59:31-36.
CrossRef - Rajasankar S., Manivasagam T., Surendran S. Ashwagandha leaf extract a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neuroscience letters. 2009;454:11-15.
CrossRef - Lee S., Oh S. T., Jeong H. J., et al. MPTP-induced vulnerability of dopamine neurons in A53T alpha-synuclein overe xpressed mice with the potential involvement of DJ-1 downregulation. The Korean journal of physiology & pharmacology official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2017;21:625-632.
CrossRef - Kakkar P., Das B., Viswanathan P. N. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130-132.
- Guo Z., Xu S., Du N., Liu J., Huang Y., Han M. Neuroprotective effects of stemazole in the MPTP-induced acute model of Parkinson’s disease: Involvement of the dopamine system. Neuroscience letters. 2016;616:152-159.
CrossRef - Luong T. N., Carlisle H. J., Southwell A., Patterson P. H. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011.
- Perier C., Vila M. Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:009332.
CrossRef - Kim J. B., Kopalli S. R., Koppula S. Cuminum cyminum Linn (Apiaceae) extract attenuates MPTP-induced oxidative stress and behavioral impairments in mouse model of Parkinson’s disease. Tropical Journal of Pharmaceutical Research. 2016;15:765-772.
CrossRef - Zhang F., Lu J., Zhang J. G., Xie J. X. Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neurons in an MPTP-induced Parkinson’s disease model in C57BL/6J mice. Neural regeneration research. 2015;10:308-313.
CrossRef - Ihara Y., Chuda M., Kuroda S., Hayabara T. Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson’s disease relationship to clinical data. Journal of the neurological sciences. 1999;170:90-95.
CrossRef - Afonso V., Champy R., Mitrovic D., Collin P., Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324-329.
CrossRef - Davalos A., Castillo J., Serena J., Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28:708-710.
CrossRef - Saniova B., Drobny M., Kneslova L., Minarik M. The outcome of patients with severe head injuries treated with amantadine sulphate. J Neural Transm (Vienna). 2004;111:511-514.
CrossRef - Ferger B., Spratt C., Earl C. D., Teismann P., Oertel W. H., Kuschinsky K. Effects of nicotine on hydroxyl free radical formation in vitro and on MPTP-induced neurotoxicity in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:351-359.
CrossRef - Fredriksson A., Eriksson P., Archer T. MPTP-induced deficits in motor activity: neuroprotective effects of the spintrapping agent alpha-phenyl-tert-butyl-nitrone (PBN). J Neural Transm (Vienna). 1997;104:579-592.
CrossRef - Carter R. J., Lione L. A., Humby T., et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248-3257.
CrossRef - Hwang D. Y., Fleming S. M., Ardayfio P., et al. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice behavioral characterization of a novel genetic model of Parkinson’s disease. J Neurosci. 2005;25:2132-2137.
CrossRef - Drucker-Colin R., Garcia-Hernandez F. A new motor test sensitive to aging and dopaminergic function. J Neurosci Methods. 1991;39:153-161.
CrossRef - Hong J., Sha S., Zhou L., Wang C., Yin J., Chen L. Sigma-1 receptor deficiency reduces MPTP-induced parkinsonism and death of dopaminergic neurons. Cell Death Dis. 2015;6:e1832.
- Glajch K. E., Fleming S. M., Surmeier D. J., Osten P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson’s disease. Behav Brain Res. 2012;230:309-316.
CrossRef - Fleming S. M., Ekhator O. R., Ghisays V. Assessment of sensorimotor function in mouse models of Parkinson’s disease. J Vis Exp. 2013.







