Adi Parwata1, Putra Manuaba2 and Sutirta Yasa3
1,2Department of Chemistry, Faculty Mathematics and Natural Sciences.
3Fakulty of Medical, Udayana University, Bali, Indonesia.
Coresponding Author E-mail: m.andita@yahoo.co.id
DOI : https://dx.doi.org/10.13005/bpj/1517
Abstract
Flavonoids can provide antioxidant effects by preventing the formation of ROS, directly capture ROS, protect lipophilic antioxidants and stimulate the increase of enzymatic antioxidants. Flavonoids are phenolic compounds that are widely found in medicinal plants, one of which is Gyrinops versteegii leaves. In this research to determine the potential of flavonoids in water extract Gyrinop versteegii as one source of natural antioxidants was investigated. This research begins with maceration of Gyrinops versteegii leaves with some solvents such as ethyl acetate, ethanol, methanol and water. Each of the extracts obtained measured the total content of Phenol. The extract, which had the highest total phenol content, measured the total flavonoid content and antioxidant capacity. The active extract as antioxidant was further isolated and identified its flavonoid content. Flavonoids obtained measured antioxidant capacity in vitro. Total phenol (mg GAE/100 g) of ethyl acetate extract = 443, ethanol extract =1.510, methanol extract = 6.069 and water extract = 14.979, total flavonoid contens = 2298, 977 mg QE/100 gram, containing phenol, flavonoid, tannin, alkaloid and steroid compounds. Antioxidant capacity with IC50 = 3,45 ppm (5 min.) and 3,05 (60 min.). Identification of isolates with UV-Vis spectroscopy showed 2 absorption bands namely band I at 352 nm and band II at 256 nm. Addition of AlCl3 / HCl shear reagent showed band I undergoing a 2 nm batochromic shift. These results indicate that the resulting flavonoid is suspected to be a flavonoid group of flavonol substituted -OH group at C-5 or 5-hydroxy-flavonol. and its antioxidant capacity or IC50 = 17,14 ppm. These results indicate that the isolated flavonoid has very strong antioxidant activity and is potentially developed as a natural antioxidant.
Keywords
Capacity Antioxidant; Gyrinops Versteegii; Total Phenol and Flavonoid
Download this article as:| Copy the following to cite this article: Parwata A, Manuaba P, Yasa S. The Potency of Flavonoid Compounds in water Extract Gyrinops versteegii Leaves as Natural Antioxidants Sources. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Parwata A, Manuaba P, Yasa S. The Potency of Flavonoid Compounds in water Extract Gyrinops versteegii Leaves as Natural Antioxidants Sources. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22659 |
Introduction
The utilization of medicinal plants or herbs (back to nature) in tackling health problems is increasing. This movement is motivated by environmental changes, lifestyle, and the development of disease patterns. The lack of negative effects arising in the use of medicinal plants and economically attract people to go back to use drugs derived from natural materials (back to nature). It is supported by thousands of herbs used in traditional Chinese Medicine (TCM) which is currently develoved in Hong Kong, Canada, The United States, Malaysia, Thailand, India, Singapore and Australia. Ayurvedic medicine is based on the system of Indonesia and India to used hundreds or more herbs for traditional medicine. Movement back to nature is followed by the research and development of medicinal plants in terms of bioactivity and effectiveness as drugs with experimental animals and patients. This proved more and more stocks are ready fitofarmaka developed into modern medicine. One developed study are a natural antioxidant and immunomodulatory. Antioxidant compounds contained in medicinal plants are one of the active compounds that can prevent a reaction – free radical oxidation reactions later. The compounds are antioxidants include phenolic compounds and flavonoids. These compounds can capture or provide the hydrogen atoms in free radicals that lipid peroxidation reactions and reactions of DNA damage can be prevented. Basically, the body is already producing or endogenous antioxidant such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) but has not been able to deal with the excess of free radicals in the body resulting in an imbalance of antioxidant production and the amount of free radicals (Akhlaghi, et al., 2009). This imbalance if not addressed can lead to oxidative stress oksidatif. Stres unresolved is what can lead to degenerative diseases such as diabetes mellitus and kanker. this imbalance can be overcome by exogenous antioxidant consumption (Adi Parwata,2016)Exogenous Antioxidants can be either synthetic and natural. Synthetic antioxidants that have been circulating in the community is Vitamin C, tocopherols , β-carotene, propyl gallate, butyl hydroksi anisole (BHA) and butyl hidoksi toluene which is used as an additive in some food and beverage packaging circulating in the community . Based on several studies of synthetic antioxidants consuming excess can cause toxicity effects and lower health (Wong S.P., et.al., 2006). Based on this reason many researchers are looking for alternative antioxidants derived from nature either of vegetables , fruits and traditional medicinal plants . In addition to vitamins contained in vegetables and fruits some phenolic compounds such as flavonoids that of the medicinal plants can be used as a natural antioxidant . One of the medicinal plants that could potentially are the leaves of plants Gyrinops versteegii and Aquilaria sp. (Tri (Noviyanti, 2011; Mulyaningsih, et.al., 2014)Gyrinop versteegii and Aquilaria sp is a genus of the plant family Thymelaeaceae . This plant is a producer of agarwood or resin which has a high -quality essential oils and very fragrant. Agarwood or resin can be formed at the plant when naturally infected by the fungus fusarium sp or injected directly with the inoculant of the fungus fusarium sp. (Tri (Noviyanti, 2011; Mulyaningsih, et.al., 2014)Crude extract of Aquilaria crassna can reduce ischemia induces cardiac cells die or injured by initiating a p38 mitogen and further activating phosphorylation of protein kinase (MAPK). This extract can cure diarrhea and skin infections caused by Staphylococcus aureus (Jermsri, et.al., 2012 and Kamonwannasit,, et.al., 2013). Japanese society has traditionally use agarwood as herbal medicines to treat of stress or tranquilizers , protect the operation of the organs vital such as heart , lungs and liver. maintaining the health of the stomach, help sore throats with its anaesthetic, and detoktifikasi body (Noviyanti, 2011; Tri Mulyaningsih, et.al., 2014). Traditionally in India, China,Tibet, Eropa, Timur Tengah, Malaysia dan Indonesia, agarwood used for sedative, antiseptic, expectorant, deodorants, anti- asthma, anesthesia, anti-fungal, antibacterial, anorexia, headache, gout, inflammatory, Mycobacterium tuberculosis drugs, especially humans, cirrhosis of the liver, abdominal pain, aphrodisiac, thyroid cancer, lung tumor, analgesic, cardiovascular disease, kidney disease, inhibit Salmonella typhi and Salmonella flexneri bacilli (Gunasekera,et.al,1981;Taylor,et.al, 2001; Tri Mulyaningsih, et.al., 2014).Gyrinops verstegii leaf water extract can decrease oxidative stress through decreased MDA levels and increased SOD enzyme activity and catalase in Wistar Rats given maximum physical activity (Adi Parwata, 2016). This extract can also inhibit DNA damage through decreased levels of 8-OHdG in Wistar rats given maximum physical activity.Chemical constituents of plants Gyrinops versteegii including noroxo-agarofuran, agarospirol, 3,4-dihidroxy-dihydrofuran, vaterolactone , indol dan isolonifolen, coniferyl alkohol, guaiacol, catecol dan pyrogalol, veratrol, ambrox, p-methoxy-benzylaceton, aquilochin, Jinkohol, jinkohol ermol, and kusunol (Noviyanti, 2011).Gyrinops verstegii is active as an antioxidant allegedly caused by the content of phenol and flavonoid compounds. Flavonoids can provide antioxidant effects by preventing the formation of ROS, directly capture ROS, protect lipophilic antioxidants and stimulate the increase of enzymatic antioxidants Flavonoids can directly capture superoxide free radicals so that the production of SOD can be improved. This condition can keep the balance of free radicals and enzymatic antioxidants so that the sustainability of oxidative stress can be inhibited (Akhlaghi, et al., 2009). Flavonoid 5-hydroxy-7-methoxy-flavanone has activity as an antioxidant and inhibits the growth of fibrosarcoma. This flavonoids also have activity as antiangiogenesis by decreasing VEGF and COX-2. These flavonoids can increase apoptosis by increasing p53 (Adi Parwata, 2016).Based on the use as a medicinal plant and its chemical content, Gyrinop versteegii plant potential to be developed as an alternative natural antioxidant. In this research, the researchers want to prove that the content of flavonoids as antioxidants so that the results of the extract can be developed into standardized herbal (OHT) remedies based on the active ingredient of flavonoids
Materials and Methods
Material
Fresh Gyrinops versteegii leaves, obtained from the village of Marga, Subdistrict Marga, Tabanan, Bali, Indonesia, male rats Wistar from BBVet , standard food of, ethanol GR(E Merck), ethyl acetate GR (E Merck), HCl GR (E Merck), NHCO3 GR(E Merck), NH4Cl GR (E Merck), methanol GR (E Merck), TCA, TBA, TEP, BHT (Sigma), PBS, xanthin oxidase, Na-EDTA, H2O2, BSA 0,5%, 2.5 mM NBT, MDA Assay Kit from NWK Northwest, CMC-Na and Whatmann Filter Paper No.4 (E Merck)
Equipment
UV-Vis spectrophotometer (Varian), analytic Digital Balance Scale (Ohaus), Brand Memmert oven, polypropilin tube (Colom 18), rotary vacuum evaporator Brand Buchii, Vortex, water bath.
Methods Extraction of Gyrinops Versteegii Leaves
Extraction of Gyrinops versteegii leaves followed prosedure Harborn, 1996, Biswas R. et.al. 2005 and Ashafa, 2010. The fresh of Gyrinops versteegii leaves that have been dried to a powder blended with a size of 40 mesh. The powder of Gyrinops versteegii leaves, macerated with ethyl acetate, ethanol, methanol and water for 24 hours, strain extract and liquid extract obtained is evaporated until thick. Condensed extract ethyl acetate, ethanol, methanol and water collected is weighed and stored at a temperature of -200C . This extract is used for test or further analysis.
Antioxidant Capacity Analysis
Antioxidant Capacity Analysis followed procedure Almey (2010). The analysis begins with making of a standard solution of gallic acid 0-100 mg/L. Weighed 0.1 grams each extract, then diluted with methanol to a volume of 5 mL flask and then in the vortex so that a homogeneous solution. This homogeneous solution is centrifuged at 3000 rpm for 15 minutes. Each solution has been pipetted 0.5 mL of this homogeneous, then add 3.5 ml of 0.1 mM DPPH in methanol at a test tube and then in the vortex. This solution was incubated at 250C for 30 minutes so DPPH reacts with the sample. Each solution was measured absorbance at λ max = 517 nm. Antioxidant capacity was calculated using linear regression equation Y = ax + b. Antioxidant capacity can be seen from the results % inhibite and IC50. IC50 value is the value which is the concentration of test samples that provides damping DPPH oxidation by 50 %. IC50 value can be calculated from the linear regression equation (y =ax+b). Some of the extract concentration was measured percent of inhibition and included in the calibration curve. Extract concentration (ppm) as absis (x), while % inhibition as coordinates (y). The calculation result y = 50 included in the equation in order to obtain the value of x as the IC50 value of each sample. Levels of antioxidants can be seen from the IC50 IC50 < 50 ppm is said to be very powerful antioxidant, said to be strong IC50 50-100 ppm, said moderate is IC50 100-150 ppm and IC50 was > 151 is said to be weak as antioxidants.The most active extracts as antioxidants analyzed its chemical content by some of the color reagent and the other analysis.
Total Phenol Analysis
Total Phenol Analysis followed the procedure (Wolfe et al., 2003 and Almey, 2010). Extract of ethyl acetate, ethanol, methanol and water dissolved in 5 mL volumetric flask. Pipette 0.4 mL, put in a test tube, add 0.4 mL reagent Folin – Clocalteu, divortex until homogeneous, allow 5-10 minutes, add 4.2 mL of Na2CO3, then let stand for 90 minutes at room temperature. further absorbance is measured at maximum wavelength at 760 nm. Create a standard curve of gallic acid in 85% methanol with a concentration of 10-100 mg/L. Levels of Total Phenol is calculated by linear regression formula Y=ax+b. Data calculation results are expressed in units Gallic acid equivalent (GAE /100 gram samples. Extracts of the most active and has the highest antioxidant capacity is used as a treatment in Wistar rats to measure the levels of MDA.
Total Flavonoid Contens Analysis
Total Flavonoid contens Analysis followed the procedure Chang and Wen (2002) , Ashafa (2010) and Ordon-ez et al. (2006). Extract of water dissolved in 5 mL eyhanol in 10 mL volumetric flask, vortexed until homogeneous. Pipette 2,0 mL, put in a test tube, add 2,0 mL AlCl3 2%, vortexed until homogeneous then incubation in room temperature for 25 minutes. Furthermore absorbance at λ max = 415 nm. Total Flavonoid contens are integrated in the quersetin standard calibration curve (mg QE / gram).
Isolation and identification of flavonoid from water extract of Gyrinops verstegii leaves
Isolation and identifikasi of flavonoid from water extract of Gyrinops verstegii leaves followed prosedure Biswas R. et.al.(2005). The standardized Gaharu leaf powder is macerated with hot water. The obtained liquid extract is concentrated with an evapourator rotary. The concentrated extract obtained further in the press dryer until obtained by viscous extract. Subsequently the viscous extract obtained is separated by column chromatography with eluent which is a mixture of n-hexane: ethyl acetate: ethanol = 8: 2: 1. The fraction of the separation results was then tested for its flavonoid content. A positive fraction containing flavonoids is purified by thin layer chromatography with some eluent of different polarity.
The obtained purification result is recrystallized with methanol to obtain needle-shaped crystals. The obtained crystals were further identified with 10% NaOH to determine the flavonoid species and identified by UV-Vis spectroscopy and shear reagents such as NaOH, AlCl3 and AlCl3 / HCl. These crystals are also identified by IR spectroscopy to know the functional groups. and analyzed again antioxidant activity with DPPH method.
Result and Discussion
Result
Analysis water content of Gyrinops verstex leaves powder
Measurement of water content of Gyrinops verstegii leaves powder was used oven method. The measurement of the water content was performed three replications as revealed in Table 1,
Table 1: Water content of Gyrinops verstegii leaves powder.
| Sample
|
Start weight (gram) | End weight
(gram) |
Water contents
(% w/w) |
| 1 | 1,16993 | 1,06943 | 8,5905 |
| 2 | 1,46305 | 1,33747 | 8,5834 |
| 3 | 1,19854 | 1,09582 | 8,5870 |
| % Average ± SD | 8,5870±0,004 | ||
The result of water content of simplicia powder of Gyrinops versteegii leaves = 8, 98 % w/w. This result is in accordance with the standard of simplicia ingredients of Indonesian herbal medicine. These results indicate that the water content is <10% w/w. This result is in accordance with the standard of simplicia ingredients of Indonesian herbal medicine which states that the water content of herbal medicine simplicia powder Indonesia must be <10% w / w.
Analysis Total Phenol Contents
Extract of water, methanol, ethanol and ethyl acetate from the leaves of Gyrinop versteegii obtained through the extraction process. Extraction is a process of withdrawal of the desired component of an ingredient. This study used a method of maceration. Which is the simplest extraction method that is by simply soaking the leaves of Gyrinop versteegii with solvent water, methanol, ethanol and ethyl acetate in a simple container with a time of 24 hours , then filtered. The filtrate were then evaporated with a rotary evapourator to obtain a thick extract. Extraction results obtained brownish viscous extract. The total phenol content of each extract is extract water, methanol, ethanol and ethyl acetate as revealed in table 2.
Table 2: The Total phenol content of each extract.
| No. | Extract | Total Phenol Contents |
| (mg GAE /100 gr) | ||
| 1 | Water | 14.979 |
| 2 | Methanol | 6.069 |
| 3 | Ethanol | 1.51 |
| 4 | Ethyl acetate | 443 |
The table above shows that the gaharu leaf water extract has the highest total phenol content.The water extract then measured the total flavonoid content and its antioxidant activity.
Analysis Antioxidant Capacity
Antioxidant capacity of measurement results of Gyrinops versteegii leaves water extracts are revealed in the table 3.
Table 3: IC50 of extract in 5 and 60 minutes treatment.
| Solvent | Conc.
(ppm) |
Abs.
5 min. |
%
Inhibit |
IC50 (ppm)
5 min. |
%
Inhibit |
Abs.
60 min. |
IC50 ppm
60min |
| Water | 0,00
0,82 1,64 3,27 4,91 6,54 |
0,966
0,812 0,653 0,432 0,267 0,188 |
0,00
15,94 32,35 55,28 72,36 80,59 |
3,45 | 0,00
20,22 40,34 65,41 79,37 81,34 |
0,987
0,791 0,592 0,343 0,205 0,185 |
3,05 |
Note : Abs. = Absorbance ; min = minutes
Based on the above results, it can be proved water extract of Gyrinops versteegii leaves had the highest total phenol contents and antioxidant capacity with IC50 < 50 ppm. This means that the water extract of Gyrinops versteegii leaves potential to be developed into a source of natural antioxidants , the next need to be tested its total flavonoid contens and Chemical Ingredients of water extract of Gyrinops versteegii leaves.
Determination of Total Flavonoids Contens of Gyrinops verstegii
The measurement of Total flavonoid contens from Gyrinops verstegii leaves water extract preceded by making standard curve of quercentine with some concentration as revealed in table 4.
Tabel 4: Absorbance of quercentine Standard
| No. | Concentration of
standard quercetine (ppm) |
Absorbance |
| 1. | 0 | 0 |
| 2. | 2 | 0,065 |
| 3. | 5 | 0,175 |
| 4. | 8 | 0,265 |
| 5. | 10 | 0,333 |
| 6. | 15 | 0,565 |
This result is used to make the calibration curve like the following:
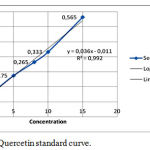 |
Figure 1: Quercetin standard curve.
|
Measurement of total flavonoid content performed three replications so that the total flavonoids = 2298, 977 mg QE/100 gram as revealed in table 5:
Table 5: Total Flavonoids contents.
| Sample | Weight | Totaln Flavonoid Contents |
| 1 | 1,246 | 2297,149 |
| 2 | 1,248 | 2300,804 |
| 3 | 1,247 | 2298,977 |
| Average ±SD | 2298,977 ± 1,8275 | |
Determination of Chemical Ingredients Water extract of Gyrinops versteegii leaves
The result of measurement of chemical content showed that the water extract of Gyrinops versteegii leaves contains terpenoid and flavonoid compounds , as revealed in Table 6,
Table 6: The Chemical Ingredients of extract.
| No. | Reagent | Compounds |
| 1 | Willstater | (+) Flavonoid |
| 2 | NaOH 10% | (+) Flavonoid |
| 3 | Meyer | (-) Alkaloid |
| 4 | Leiberman-Burchard | (+) Steroid |
| 5 | Water | (-) Saponin |
| 6 | FeCl3 | (+) Phenolic |
Note : (+) = containing compounds to be analyzed, (-) = not contains compounds being analyzed Based on the above results and discussions, in particular the high content of flavonoid compounds and the very strong antioxidant activity of water extracts, the researchers wanted to isolate and identify the type of flavonoids contained. Furthermore, the isolated flavonoids were analyzed for their antioxidant activity.
Isolated and Identified of Flavonoid on Water extract of Gyrinops versteegii leaves
Prior to the identification of spectroscopic isolates in purity test and flavonoid type. The flavonoid isolate obtained is a light yellow needle crystal. The purity test with thin layer chromatography (TLC) with multiple eluents of different polarity is obtained by a single spot as revealed in table 7,
Table 7: Purity Test of flavonoid isolate by TLC.
| No. | Mobile phase | Ratio | Result |
| 1 | n-BuOH: CH3COOH : H2O | 4:1:5 | 1 stain |
| 2 | CHCl3 : EtOH | 3:1 | 1 stain |
| 3 | n-BuOH: CH3COOH : H2O | 3:1 | 1 stain |
| 4 | n-C6H6 :CH3COOC2H5 | 1:1 | 1 stain |
| 5. | CH3COOH: H2O : HCl | 30:10:1 | 1 stain |
| 6. | n-C6H6 :CH3COOC2H5 | 3:1 | 1 stain |
The results above show that the flavonoid suspected isolates are considered pure by thin layer chromatography. Furthermore, this isolate was analyzed flavonoid group and its flavonoid structure by spectroscopy Analysis of chemical constituents showed that isolates indicated positive flavonoids of flavonol or dihidroflavonol, as revealed in Table 8:
Table 8: Chemical Ingredients of isolates.
| No. | Reagent | Discoloration | Indicated |
| 1. | Willstater | Colorless to pink | Flavonoid |
| 2. | Bate Smith Metcalfe | Colorless to red | Flavonoid |
| 2. | NaOH 10% | Colorless to brown | Flavonoid flavonol |
| 3. | FeCl3 | Colorless to pink | Flavonoid |
Note : All the Reagent result flavonoid
Identification with a UV-Vis Spectrophotometer
Identification with UV-Vis spectrophotometer showed the l max = 256 nm (band II) and 352 nm (band I). Subsequent analysis when isolates in methanol was coupled with AlCl3 + HCl, the absorption band II has a bathochromic shift of about 2 nm as shown by the following figure 2.
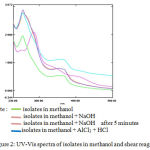 |
Figure 2: UV-Vis spectra of isolates in methanol and shear reagents.
|
Identification with a Infra Red spectrophotometer
Identification by IR spectroscopy showed some peaks as shown by the data in the following table 9:
Table 9: Identification isolates by IR spectroscopy.
| No. | Wavenumber(cm-1) | Intensity | Indicated |
| 1 | 3453,58 | Sharp | -OH |
| 2 | 2925,09 | Sharp | -CH aliphatic |
| 3 | 1729,22 | Sharp | >C=O |
| 4 | 1642,42 | Sharp | >C=C< aromatic |
| 5 | 1249,88 | Sharp | -C-O |
The results of antioxidant activity measurements showed that the isolates had strong antioxidant activity with 17,14 ppm. These results are shown in the following Table 10:
Table 10: Measurement of antioxidant capacity of isolates.
| Sample | Concentration | Absorbance | % inhibition | Linear equations | IC50 (ppm) | |
| (ppm) | Blanco | test sample | ||||
| Isolate | 2 | 0.17 | 0.16 | 5,89 | y = 2,883x + 0,588 | 17,14 |
| 4 | 0.139 | 13,24 | R² = 0,994 | |||
| 6 | 0.138 | 18,84 | ||||
| 8 | 0.134 | 23,2 | ||||
| 10 | 0.121 | 28,86 | ||||
IC50 results are obtained from the graph between concentration (mg / L) versus% oxidation resistance of free radicals by isolates as shown in the following figure 3.
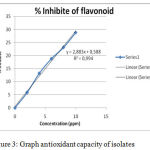 |
Figure 3: Graph antioxidant capacity of isolates.
|
Discussion
Acquisition of water content less than 10%, in accordance with the standards set by Materia Medika Indonesia. Materia Medika Indonesia is the standard of a medicinal plant powder or simplicia of medicinal material derived from plants. This suitability identifies the simplicia Gyrinops versteegii can be used for research in the development of Traditional Medicinal Plants. Research in the development of Traditional Medicines from Herbs to Standardized Herbal Medicine and Phytopharmaca can be done in the form of standardization of simplicia, standardization of extract, bioactivity test, isolation, identification and determination of its active chemical content, bioactivity test of isolates and formulations. In this research, standardization of extract, bioactivity test, isolation and identification of flavonoids content of extract and biocativity test of flavonoid isolate were produced so that Gyrinops verstegii extract can be used as natural antioxidant based on flavonoid content (Koleva, 2002).The Results of in vitro studies prove water extract of Gyrinops versteegii leaves have the highest antioxidant capacity compared with other solvents because it has the smallest IC50. ie 3.45 mg/L(5 minutes) and 3.05 mg/L(60 min). The IC50 means that the concentrations of 3.45 and 3.05 mg/L, the water extract of Gyrinops versteegii leaves already can inhibit 50% of the oxidation reaction of free radicals (Table 2). The potent antioxidant capacity is thought to be caused by a high content of total phenol and flavonoid contents. Phenolic compounds as flavonoids in the water extract of leaves Gyrinops versteegii able to inhibit and neutralize free radicals (DPPH). Based on these results that the water extract of Gyrinops versteegii leaves can be regarded as a natural antioxidant and developed further analizing in vivo antioxidant activity (Yu et.al, 2002).The results of antioxidant bioactivity measurements in vivo showed that Gyrinops verstexi leaves water extract at doses of 100 and 200 mg / kg BB could significantly (p <0.05) decrease MDA levels in wistar rats that given maximum physical activity (Adi Parwata, 2016). Bioactivity in lowering levels of MDA caused by the quantity of phenolic compounds (as flavonoids) at doses is able to reduce or neutralize ROS reaction products of metabolism of the body with fatty acids having a double bond (PUFA) . This would result in lipid peroxidation product called MDA (Middleton et.al., 2000 ; F.Hosseinimehr et.al. , 2006; Mathew et.al., 2006)Oral intake of Gyrinops versteegii leaf extract at doses of 50, 100 and 200 mg / kg BW can significantly (p<0,05) increase SOD enzyme activity and catalase in Wistar rats given maximum activity (Adi Parwata, 2016). This is due to the quantity of the content of this class of compounds phenol / flavonoid which is more and work synergistically, can directly capture or neutralize the free radical superoxide, peroxynitrite so flavonod stimulate the formation of enzymatic antioxidants SOD and endotil dysfunction can be reduced. Phenol compounds such as flavonoids can also function as an inducer that activates Nrf2 in the cytoplasm. Nrf2 in the cytoplasm will dissociate and translocate to the nucleus. In the nucleus Nrf2 associates to the promoter of a gene called Antioxidant Response Element (ARE), thus triggering the expression of the antioxidant coding gene to produce SOD (Middleton Jr. et al., 2000; Akhlaghi M.and Brian B.,2009).Phytochemical screening showed that the water extract of Gyrinops versteegii leaves contains phenolic compounds, steroid, terpenoids and flavonoids (Diouf, et.al., 2009). The compounds are believed to be active here as a natural antioxidant compounds. Natural phenol compounds (such as flavonoids, chlorophyll, terpenoids) usually contains many -OH groups,>C=O and >C=C< groups. These group is able to donate electrons or one atom of hydrogen for ROS to ROS becomes stable and form new free radicals that are less reactive. This resulted in a reaction barrier between ROS lipid peroxidation reaction with unsaturated fatty acids long-chain (PUFAs) can be suppressed and the results peroxidation MDA will decrease (Akhlaghi M.and Brian B.,2009). The results of this study supported some research content of compounds such as flavonoids, tannins and anthocyanins contained in chocolate (cocoa fiber) can reduce the production of MDA compound when consumed for three weeks by the Wistar rats with hypercholesterolaemia conditions (Lecumberri, et al.,2007). Flavonoid in Adiantum capillus-veneris L. has a high antioxidant capacity and may reduce levels of MDA (Jiang, et al.,2011). Flavonoids in lotus leaf (Nelumbo Gaertn nuficera) can reduce oxidative stress by lowering levels of MDA (Xu, H.C. and Wang, M.Y.2014).Identification with UV-Vis spectrophotometer showed the l max = 256 nm (band II) and shoulder = 352 nm (band I). This was in agreement to the literature in which flavonoids flavonol which is substituted OH in C-5 show two absorption bands i.e. between 250-280 nm (band II) and 350-385 nm that caused by the cinnamoyl and benzoyl groups of flavanones as shown by the following figure 4, (Silverstein et al , 1981; Adi Parwata, 2016).
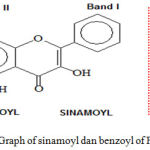 |
Figure 4: Graph of sinamoyl dan benzoyl of Flavonoids.
|
The occurrence of the batochromic shift in band I identifies the flavonoid flavonol group substituted by -OH groups in C-5 can be precented by the reaction of flavonol in methanol with AlCl3 as shown by the following figure 5 (Silverstein et.al.,1981 ; Markam, 1988).
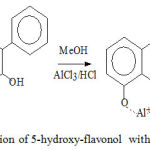 |
Figure 5: Reaction of 5-hydroxy-flavonol with AlCl3/HCl.
|
The results of identification with Infrared Spectrophotometer (FTIR) show the functional groups -OH,> C = O,> C = C <aromatis and > C-O. The results of identification with Infrared Spectrophotometer (FTIR) show the functional groups -OH,> C = O,> C = C <aromatis and> C-O. This is according to the structure flavonoid flavonol substituted -OH group at C-5 (Silverstein et.al.,1981; Markam, 1988).
Result of antioxidant capacity analysis from flavonoid isolate obtained IC50 = 17,14 ppm. these results suggest that isolate flavonoids have very strong antioxidant bioactivity because IC50 <50 ppm. This proves that the flavonoid contained in Gyrinops versteegii leaf water extract has the potential to be used as an alternative natural antioxidant. As antioxidant flavonoid isolated can prevent the occurrence of sustainable ROS reaction. Flavonoids can provide antioxidant effects by preventing the formation of ROS, directly capture ROS, protect lipophilic antioxidants and stimulate the increase of enzymatic antioxidants so as to prevent oxidative stress that can cause several degenerative diseases such as heart disease, diabetes millitus and cancer (Zeng et.al., 2001; Yu et.al., 2002; Akhlaghi M.and Brian B.,2009)
Flavonoids can bind to hydroxy radicals (*OH) so that DNA damage can be prevented. Prevention of DNA damage automatically prevents the occurrence of cancer due to DNA damage (Chabowska, et al., 2009 ; Gupta, et al., 2010). Flavonoids can directly capture superoxide and peroxinitrite. Through superoxide capture, flavonoids can increase the bioavailability of NO and inhibit the formation of peroxinitrite or flavonoids can directly capture peroxinitrit that damage the endothelium vacorelaxation and disrupt the endothelium, thus ultimately leading to better blood circulation in the coronary arteries and can automatically prevent heart disease (Middleton et.al., 2000 ; F.Hosseinimehr et.al. , 2006; Mathew et.al., 2006 and Maisuthisakul, 2007).
Conclusion
The flavonoid isolate from water extract of Gyrinops versteegii leaves is 5-hydroxy-flavonol and have bioactivity as natural antioxidant.
Acknowledgement
I would like to appreciated with colleagues who faithfully support laboratory research until completion of research. We did not forget to thank profusely to Rector, Chairman and staff of the Institute for Research and Community Services University Udayana, Denpasar, Bali and staff on the funds provided to finance the research.
References
- Parwata A., Sukardiman., Mulya H. S and Widhiartini A. Inhibition of Fibrosarcoma Growth by 5-Hydroxy-7-Ethoxy-Flavanons from Kaempferia pandurata Roxb. Biomedical & Pharmacology Journal. 2016;9(3):941-48.
CrossRef - Parwata A., Manuaba P., Yasa S., Wita. Gaharu Leaf Water Extract Reduce MDA and 8-OHdG Levels and Increase Activities SOD and Catalase in Wistar Rats Provided Maximum Physical Activity. Bali Medical Journal. 2016;5(3):79-83.
CrossRef - Akhlaghi M and Brian B. Mechanisms of flavonoid protection against myocardial ischemia–reperfusion injury. Journal of Molecular and Cellular Cardiology. 2009;46:309–17.
CrossRef - Almey A., Khan A. J., Zahir S., Suleiman M., Aisyah Rahim K. Total phenolic content and primary antioxidan activity of methanolic and ethanolic extract of aromatic plants’ leaves. International Food Research Journal. 2010;17:1077-88.
- Ashafa A. O. T., Grierson D. S and Afolayan A. J. In Vitro Antioxidant Activity of Extracts From the Leaves of Felicia Muricata Thunb. an Underutilized Medicinal Plant in the Eastern Cape Province, South Africa Afr J Tradit Complement Altern Med. 2010;7(4):296–302.
- Biswas R., Dasgupta A., Mitra A., Roy S. K., Dutta P. K., Achari B., Dastidar S. G., dan Chatterjee T. K. Isolation, purification and characterization of four pure compounds from the root extract of Pluchea indica Less and the potentiality of the root extract and the pure compounds for anti micro-bial activity. European Bulletin of Drug Research. 2005;13:63-70.
- Chabowska S. A.,Beck A., Poreba R., Andrzejak R. J., Juchniewicz A. Evaluation of DNA Damage in People Occupationally Exposed to Arsenik and Some Heavy Metals. Polish J. of Environ. Study. 2009;18 (6):1131-39.
- Diouf P. N., Stevanovic T., Cloutier A. Study on chemical composition, antioxidant and anti- inflammatory activities of hot water extract from Picea mariana bark and its proanthocyanidin- rich fractions. Food Chem. 2009;113:897–902.
CrossRef - Gupta R. K., Kesari A. N., Kesari S., Santosh K. S and Watal G. Studies on glycemic and lipidemic effect of Murraya koenigii in experimental animal. Journal of Ethno pharmacology. 2010;12:305-11.
- Gunasekera S. P. Kinghorn A. D., Cordell G. A. Plant anticancer Agents XXX. Constituents of Aquilari malaccens is. J.Natural Products. 1981;44(5). 569-572.
CrossRef - Harborne J. B. Metode Fitokimi a Penuntun Cara Modern Menganalisa Tumbuhan. (Kosasih Padmawinata). ITB. Bandung. 1996.
- Jermsri A. A.,Jiraviriyakul A. A., Unajak S., Kumphune S. Effect of Aquilaria crassna crude extract simulated ischemia induced cardiac cell death, International Journal of Pharma and Bio Science. 2012;3 (3):604-13.
- Jiang M. Z., Yan H., Wen Y., Li X. M. In vitro and in vivo studies of antioxidant activities of flavonoids from Adiantum capillus-veneris L. African Journal of Pharmacology. 2011;5(18):2079-85. China.
- Kamonwannasit S., Kumkrai P., Nantapong N., Kupittayanant S., Chudapongse N. Antioxidant and antibacterial activities of the extract of Aquilaria crassna leaves, Planta Medica. J. Med. Plant and Natural Product Research. 2013;12(2013):1-20.
- Koleva I. I., Beek V .T. A., Linssen J. P. H., de Groot A., Evstatieva L. N. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17.
CrossRef - Lecumberri E.,Goya L.,Mateos R., Alfa M., Ramos R., Izquierdo-Pulido M and Bravo L. A Diet Rich in Dietary Fiber from Cocoa mprovees Lipid Profil and Reduces MDA in Hyper cholesterolemic Rats. Nutrition. 2007;23:332-41.
CrossRef - Markam K. R., Cara Identi fikasi Flavonoid, terjemahan oleh Kosasih Padmawinata. Penerbit ITB Bandung. 1988;1-59.
- Maisuthisakul P., Suttajit M., Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–18.
CrossRef - Mathew S., Abraham E. T. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2006;44:198–206.
CrossRef - Middleton Jr. E., Kandaswami C and Theoharides T. C. The Effect of Plant Flavonoids on Mammalian Cells : Implication for Inflammation Heart Disease and Cancer. Pharmacological Review. 2000;52:673-751.
- Noviyanti E., Santoso B., Wiyono M. T. Study of eaglewood (gaharu) resulting from inoculation of Fusarium sp. on Aquilaria micro carpa, in Proceeding of Gaharu Workshop Development Gaharu Production Technology a Forest Community Based Empowerment, R&D Centre for Forest Conservation and Rehabilitation FORDA, Ministry of Forestry. Bogor. 2011;53-76.
- Silverstein R. M., Bassler G. C and Morril T. C. Spectrometric Identification of Organic Compounds, 4th Edition, Jhon Wiley and Spns Inc. New York. 95;305:181-189.
- Ordon-ez A. A. L., Gomez J. D., Vattuone M. A., Isla M. I. Antioxidant activity of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006;97:452–458.
CrossRef - Hosseinimehr F. S. J., Shahabimajd N. Antioxidant activity phenol and flavonoid contents of some selected Iranian medicinal plants. Afric J Biotech. 2006;11:1142–45.
- Mulyaningsih T., Marsono D. S and Yamada I. Selection of Superior Breeding In fraspecies Gaharu of Gyrinops versteegii (Gilg) Domke. Journal of Agricultural Science and Technology B. 2014;4:485-92.
- Taylor L., County M. Database Entry for Aquilaria agallocha Rain Tree Nutrition n Inc. Texas. 2001.
- Wong S. P.,Leong L. P and Koh J. H. W. Antioxidant activities of Aqueous extracts of selected plant. Food Chem. 2006;99:775-83.
CrossRef - Wolfe K., Wu X., Liu R. H. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614.
CrossRef - Xu H. C and Wang M. Y. Effect of flavonoid from Lotus (Nelumbo nuficera Gaertn) leaf on biochemical parameters related to oxidative stress induced by exhaustive swimming exercise of mice. Biomedical Research. 201;25(1):1-5. China.
- Yu L., Haley S., Perret J., Harris M., Wilson J., Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624.
CrossRef - Zheng W., Wang S. Y. Antioxidant activity and phenolic compounds in selected herbs. J.Agric Food Chem. 2001;49:5165-5170.
CrossRef







