Fahrun Nur Rosyid1, Edi Dharmana2, Ari Suwondo3, K. Heri Nugroho H. S4 and Sugiarto5
1Department of Medical Surgical Nursing, School of Nursing, Universitas Muhammadiyah Surakarta, Surakarta, Indonesia.
2Department of Parasitology, Faculty of Medicine, Diponegoro University, Semarang, Indonesia.
3Department of Occupational Safety and Health, Faculty of Public Health, Diponegoro University, Semarang, Indonesia.
4Department of Internal Medicine, Faculty of Medicine, Diponegoro University, Semarang, Indonesia.
5Dr. Moewardi General Hospital of Surakarta, Indonesia.
Corresponding Author E-mail: fnr100@ums.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1505
Abstract
Diabetic foot ulcer (DFU) is among the many complications of diabetes and it takes a very long period of time to heal. It can lead to the amputation of the lower limb, thereby resulting to death or in most cases, a bad quality of life. The aim and objective of this study is to assess the effect of bitter melon leaves extracts on serum TNF-α levels and improvement of diabetic foot ulcers. The study technique used here is the randomized, double-blinded, placebo-controlled trial. Thirty patients suffering from DFU participated in the trial and according to PEDIS scores were divided into two groups, of which 15 patients were in the treatment group and administered with bitter melon leaves extract at a dose of 6 g/day and the remaining 15 patients were in the control group and were given placebo. This intervention was done for 4 weeks and the examination of serum TNF-α levels was carried out at baseline and at the end of treatment. The readings of the healing process for diabetic foot ulcers with PEDIS scores were also taken at baseline, weeks 2, 3 and 4. Data were analyzed using the paired t-test and the independent t test. After 4 weeks of treatment, there was a decrease in baseline serum TNF-α levels in the treatment and control groups (29.5 ± 8.6 pg/ml, P = 0.0001 and 202.5 ± 610.2 pg/ml, P = 0.001). There was no effect on serum TNF-α levels (P = 0.28). There was a decrease in PEDIS degrees from baseline, week 2, 3 and 4 in the treatment and control groups (2.7±0.5; 2.7±0.5; 2.7±0.6; 1.9±0.6 and 2.6±0.5; 2.6±0.5; 2.5±0.6; 2.2±0.8). However there was no effect on diabetic foot ulcer improvement both groups in week 2 (P = 0.46), week 3 (P = 0.57) and week 4 (P = 0.29). Bitter melon leaves extracts is proven to have no effect on the serum TNF-α levels and improvement of diabetic foot ulcers.
Keywords
Bitter Melon; Diabetes Mellitus; Diabetic Foot Ulcer; Momordica Charantia L; Serum TNF-α levels
Download this article as:| Copy the following to cite this article: Rosyid F. N, Dharmana E, Suwondo A, Nugroho K. H. H. S, Sugiarto S. The Effect of Bitter Melon (Momordica Charantia L.) Leaves Extract on TNF-α Serum Levels and Diabetic Foot Ulcers Improvement : Randomized Controlled Trial. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Rosyid F. N, Dharmana E, Suwondo A, Nugroho K. H. H. S, Sugiarto S. The Effect of Bitter Melon (Momordica Charantia L.) Leaves Extract on TNF-α Serum Levels and Diabetic Foot Ulcers Improvement : Randomized Controlled Trial. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22148 |
Introduction
The rate at which diabetes mellitus (DM) occur is increasing worldwide, both in developing and developed countries.1-2 Among the complications, diabetic foot ulcer (DFU) is common in diabetic patients and this has increased in the last few years.3-6 Studies have shown that more than 15% of patients with DM will experience DFU complications during their lifetime.7 Accurate data on the prevalence of DFU is difficult to obtain. However, its prevalence in many countries of the world is estimated at 4% to 27%.8-11 In addition, healing DFU takes a longer time and can result in cutting the lower limb, thereby leading to bad quality of life and death.12-14
Diabetic foot ulcers are characterized by increased fibroblast apoptosis, decreased fibroblast cell proliferation and prolonged inflammatory reactions. This is shown by the large amount of neutrophil granulocytes seen in the wound. Granulocyte neutrophils secrete proinflammatory cytokines, especially TNF-α and interleukin-1 β (IL-1β). Both of these cytokines can help in building up matrix metalloprotease (MMP)which destroys protein matrix and growth factors thereby slowing down the healing of wounds.15 In type 2 DM patients, TNF-α increases both locally and systemically.16 Furthermore, many references report an increase in TNF-α levels in diabetic ulcer tissue, both in animals and patients.17 Wound healing requires infection control, improvement of inflammation, regeneration of connective tissue matrix, angiogenesis or vasculogenesis, wound constriction and re-epithelialization.18
Cleaning out dead or infected skin or tissue from DFU is very expensive and some of the drugs taken by the patients have various side effects. Aside this, DFU patients have to normalize their blood glucose levels first so as not to slow down the process of wound healing. Some other therapy like adjuvant therapy can go alongside this to improve the effectiveness of this treatment. Moreover adjuvant therapy is also used for the promotion of health promotion and diseases treatment.19 This adjuvant therapy comes from natural products which have been widely used as traditional medicine to help in wound healing. One traditional medicine that can be used for wound healing is the Bitter Melon (Momordica charantia). It can be used to treat diabetes, hypertension, inflammation, fever, bacterial and viral infections20-21 as well as wounds.22-23 The aim of this study is to prove the effect of melon leaves extracts on serum TNF-α levels and diabetic foot ulcer improvement.
Materials and Methods
The Making of the Bitter Melon Leaves Extracts
Bitter melon is obtained from Boyolali, in Central Java of Indonesia. The selected bitter melon (Momordica charantia) leaves are the good ones, flat, fresh and green. They are dried in the sun and smoothed using mortar and pestle and weighed 100 g with an analytical scale. The bitter melon leaves are wrapped in filter paper and put into a Soxhlet extractor tube. Then ethanol solvent was added from the top until it spilled into the flask up to half. The flask is then heated at medium boiling temperature until all extracts are considered to have been extracted. Also, an evaporator is placed on a permanent pole so that it can make a slope of 30-40º with the table. The extraction coming out are put into the extraction flask. The evaporator is placed in such a way that some part of the extraction flask is submerged in aquades in a water bath. This water bath is connected to a power source and the temperature is raised to 70ºC. Therefore the evaporation results are left in the extraction flask in the form of a thick liquid while it is at 100% concentration. The bitter melon extract capsules and the placebo were made at the Clinical Pharmacology Laboratory, Faculty of Pharmacy, Muhammadiyah University of Surakarta, Indonesia and each capsule is made up of 500 mg bitter melon extract powder and placebo. The placebo was made from grade 102 microcrystalline cellulose. The charantin, saponins and flavonoids contained in bitter melon leaves extract were analyzed by HPLC method at the same faculty.
Clinical Trial
This study design used is the randomized controlled trial carried out at Dr. Moewardi General Hospital Surakarta, Indonesia. And its approval was gotten from the Health Research Ethics Committee, Dr. Moewardi General Hospital/School of Medicine Sebelas Maret University of Surakarta, Indonesia.
Patients
Patients eligible for this study were people having type 2 diabetes with diabetic foot ulcers degrees ulcers by pedis II and III. These are people between the ages of 30-65 years, having hemoglobin> 10 g/dl, BMI 18.5-22.9, levels of albumin> 3 g/dl, brachial ankle index value (ABI)> 0.6-1.3, and suffering from DM for a long time: 0-15 years. All the patients involved in the study agreed to sign the informed consent.
Excluded patients were people who had been planned for minor amputations (below or above knee), those having chronic hypoxia and sepsis, with ages <30 years or> 65 years. Other factors that made some excluded from this study include: stress, obesity, alcohol consumption, smoking, patients with co-morbidities (cardiovascular disease, lung and immunology), steroid therapy and chemotherapy, those having allergies with the use of bitter melon leaf extract and those that willingly withdraw. The flow chart that describes patients who are recruited and acted upon are shown in figure 1.
Study Procedure
The procedures of the study were explained to the participants and also gave them the informed consent form to read. At the first visit, patients were selected based on their eligibility. Those selected were then randomized into 2 groups, namely the treatment group and the control group. The treatment group were given pure leaves extract at a dose of 6 grams orally for 4 weeks while those in the control group were given the placebo at a dose of 6 grams orally also for 4 weeks. At the end of this study, medication adherence was determined by calculating the number of drugs taken and interviewing patients in the control group.
Outcome Measurement
The primary efficacy results were changes in serum TNFα levels and this was measured using the Enzyme-Linked Immuno Sorbant Assay (ELISA) method (R & D System, Minneapollis, USA). The measurement was done at baseline and week 4 in both groups. Secondary efficacy results are the process of healing diabetic foot ulcer which is assessed by the PEDIS sheet. Improvement of diabetic foot ulcers rated with a score pedis also done at baseline, and then weeks 2,3 and 4.
Statistical Analysis
The results of the study are presented as follows: number of patients (n), mean and SD. Data were analyzed using SPSS, if Р ≤ 0.05 then it is statistically significant. Non-parametric statistical methods will be used if the variables studied are not normally distributed. Analysis was done on the mean differences between the measurements of serum TNF-α levels and the wound healing process with the T test and ANOVA statistical tests.
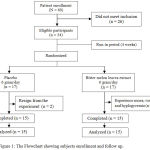 |
Figure 1: The Flowchart showing subjects enrollment and follow up.
|
Results
There were 30 diabetic foot ulcer patients who participated in this study. All patients were randomized and then given 6 g/day of bitter melon leaves extract in the treatment group (n = 15) and placebo (n = 15) in the control group. All baseline characteristics between the two groups are the same (Table 1). Examination of serum TNF-α levels at baseline and changes in mean from baseline and week 4 are shown in Table 2. Examination of diabetic ulcer healing processes assessed by PEDIS scores at baseline, weeks 2, 3 and 4 are shown in Table 3. All baseline parameters in the treatment group and control were the same and considering the age, gender, education, occupation, body weight, body mass index (BMI), HbA1c, duration of diabetes, antidiabetic medicine, osteomyelitis, antibiotics, duration of ulcer, PEDIS degree and ankle brachial index (ABI) of these two groups, there was no difference and no changes observed after the experiment. More so, after the 4 weeks of treatment, there was a decrease in baseline serum TNF-α levels in both groups (29.5±8.6 pg/ml, P = 0.0001 and 202.5±610.2 pg/ml, P = 0.001), but there was no effect on serum TNF-α levels (P = 0.282). There was a decrease in PEDIS degrees from baseline, weeks 2, 3 and 4 in the treatment and control groups (2.7±0.5; 2.7±0.5; 2.7±0.6; 1.9±0.6 and 2.6±0.5; 2.6±0.5; 2.5±0.6; 2.2±0.8), but there was no effect on diabetic foot ulcer improvement both in week 2 (P = 0.46), 3 (P = 0.57) and 4 (P = 0.29).
Discussion
Age and sex affect wound healing as a fundamental factor. Increasing age is a major risk factor for wound healing. Clinically and research in animals, both cellular and molecular, there is a relationship between increasing age and slowing wound healing.24-25 there is delayed wound healing at old age. This is related to changes in inflammatory responses, such as the delay in infiltration of T cells into the wound with changes in chemokine production and decreased production of macrophages.26 According to the observation of Swift et al (1999), there was a slowdown of reepithelization, collagen synthesis and angiogenesis in older mice when compared with the young ones.27
In general, there are differences in wound healing between the young and old. There is a correlation between age and late wound healing. This is indicated by an increase in platelet aggregation, inflammatory mediator secretion, late infiltration of macrophages and lymphocytes, impaired macrophage function, decreased secretion of growth factors, delayed re-epithelialization, decreased angiogenesis, collagen deposition, collagen and wound strength.24 Sex hormones have a role in healing wounds as seen in age. According to Gilliver et al. (2007), women and men of old age will experience lateness in the healing of acute wounds. This is due to a decrease in estrogen (estrone and 17β-estradiol), androgens (testosterone and 5α-dihydrotestosterone, DHT) and steroids precursor dehydroepiandrosterone (DHEA).28 The results showed that there were differences between older men and young in regulating gene expression estrogen in wounds.29 Estrogen affects wound healing by regulating various genes associated with regeneration, matrix production, protease inhibitors, epidermal function and genes associated with inflammation.29
According to this study, the mean BMI of subjects in both groups did not show obesity. Obesity affects wound healing. This is shown in experiments involving animals in which obesity is accompanied by impaired collagen structure and function, collagen deposition disorders, and wound healing disorders. This is part of changes in fat tissue structure.30 HbA1c value> 7% suggests that most patients have uncontrolled DM and have microvascular complications and neuropathy (ADA, 2011).31 Old DM can cause chronic hyperglycemia and an increase in microvascular permeability occur during the initial and advanced phases of the disease. Changes in capillary structure and function can cause molecular exchange through the endothelial membrane to the interstitium.32 Hyperglycemia can also lead to oxidative stress because the production of ROS is more than the antioxidant capacity.33 The formation of advanced glycation end products (AGEs) due to hyperglycemia can cause diabetic ulcer healing in mice.34 In diabetic wounds, there will be a disruption of cellular function, including a decrease in T cells and dysfunction of fibroblasts and epidermal cells, so that healing of the wound becomes affected.35-36 Long suffering from diabetes has a direct relationship with microvascular complications and neuropathy.31
In the peripheral arteries, hyperglycemia causes endothelial and vascular muscle dysfunction, and a decrease in vasodilator production by the endothelium causing constriction. Hyperglycemia in diabetic patients increases thromboxane A2, which is a vasoconstrictor and platelet aggregation agonist. Therefore there is an increased risk of plasma hypercoagulability. Hypertension and dyslipidemia also contribute to this peripheral artery disease. These things, if accumulated, will cause occlusive artery disease which then causes lower extremity ischemia and increases the risk of ulcer. Formed ulcers will be easily infected, develop into gangrene and end with the amputation of the lower leg.37-38 Old diabetic foot ulcer, in this study, is defined as a chronic ulcer that fails to follow the normal order of wound healing.39
Table 1: Baseline characteristics of patients.
| Variable | Treatment group (n = 15) | Control group (n = 15) | p-valuea |
| age (year) | 56±8.34 | 52.1±7.6 | 0.19 |
| sex | 0.46 | ||
| man (%) | 6 (40) | 4 (26.7) | |
| woman (%)
Postmenopausal (%) / absent(%) |
9 (60)
5 (33.3) / 4 (26.7) |
11 (73.3)
6 (39.9) / 5 (33.4) |
|
| Education | 0.64 | ||
| SD (%) | 1 (6.7) | 1 (6.7) | |
| SLTP (%) | 4 (26.7) | 2 (13.3) | |
| SLTA (%) | 8 (53.3) | 10 (66.7) | |
| PT (%) | 2 (13.3) | 2 (13.3) | |
| Occupation | 0.21 | ||
| House wife (%) | 8 (53.3) | 4 (26.6) | |
| Entrepreneur (%) | 6 (40) | 9 (60) | |
| Civil servant (%) | 0 | 1 (6.7) | |
| Pensionary (%) | 1 (6.7) | 1 (6.7) | |
| weight (kg) | 57.9±7.9 | 55,00±8.5 | 0.35 |
| Body Mass Index (kg/m2) | 21.5±1.3 | 21.83±1.3 | 0.54 |
| HbA1c (%) | 10.2±2.1 | 10.75±2.8 | 0.40 |
| Period of DM suffering (year) | 9.5±6.9 | 7.47±5.6 | 0.38 |
| Antidiabetic medicine
Yes (%) / No (%) |
15 (100) / 0 | 15 (100) / 0 | 1.00 |
| Osteomyelitis
Yes (%) / No (%) |
0 / 15 (100) | 0 / 15 (100) | 1.00 |
| Antibiotics
Yes (%) / No (%) |
15 (100) / 0 | 15 (100) / 0 | 1.00 |
| Period of ulcus suffering (Week) | 22.1±34.8 | 25.40±33 | 0.79 |
| PEDIS degree | 0.57 | ||
| 1. PEDIS 2 (%) | 4 (26.7) | 6 (40) | |
| 2. PEDIS 3 (%) | 11 (73.3) | 9 (60) | |
| Ankle Brachial Index | 0.22 | ||
| 1. ≤ 0,90 (%) | 2 (13.3) | 5 (33.3) | |
| 2. > 0,90 (%) | 13 (86.7) | 10 (66.7) |
Data is shown by n (%) or mean ± SD. P > 0.05 indicated that there is no significant difference between the groups.The p-values were obtained through independent test or Man-Witney test.
Table 2: Effect of bitter melon leaves extract on serum TNF-α levels.
| Variable | Baseline | Δ in weeks 4 | p within groupa | p between groupb |
| TNF-α serum levels (pg/ml) | ||||
| Treatment group | 41.3±6.9 | -29.5± 8.6 | 0.0001 | 0.28 |
| Control groul | 235.7±671.5 | -202.47±610.2 | 0.001 |
Data is shownby mean ± SD.
The changes in thevalues of serum TNF-α levels showed that there is significant difference for time or treatment effects (Independent t Test) at p < 0.05.
ap < 0.05 significant difference of change values between treatment and control group at the same period of time (Paired t-test)
bp < 0.05 significant difference of change in values after 4 weeks (Independent t Test)
Table 3: Effect of bitter melon leaves extract on diabetic ulcer healing process.
| Variable | Time | Treatment group | Control group | pa |
| PEDIS degree | Baseline | 2.7±0.5 | 2.6±0.5 | 0.46 |
| weeks 2 | 2.7±0.5 | 2.6±0.5 | 0.46 | |
| weeks 3 | 2.7±0.6 | 2.5±0.6 | 0.57 | |
| weeks 4 | 1.9±0.6 | 2.2±0.8 | 0.29 |
Data is shown by mean ± SD.
The change values of PEDIS showed significantly difference for time or treatment effects (Independent t Test) at p < 0.05.
ap < 0.05 significant different of change values betwen treatment group and control group at the same periode of time (Independent t Test)
Diabetic foot ulcer comes with high levels of serum TNF-α,15,17,40-41 apoptosis of fibroblasts and decreased fibroblast cell proliferation17. This is followed by ulcer healing disorders. TNF-α is a sign of inflammation in tissue healing process. Previous studies have shown the relationship between TNF-α and wound healing. Decreased TNF-α levels, indicating control of inflammation and adequate healing progress.41 TNF-α stimulates MMP synthesis. And with high protease in the wound, it causes degradation of protein matrix and growth factors which are important factors in wound healing process. Therefore wound healing becomes disconnected and uncoordinated.15 Apart from this, TNF-α suppresses the tissue growth factor-β (TGF-β) thereby inducing myofibroblasts to proliferate important proteins in the reorganization of extracellular matrices such as α-smooth muscle actin (α-SMA), type 1A collagen and fibronectin. Therefore, it has implications on wound healing disorders.40 This research does not fit in with the study of Bao et al., (2013). He suggested that bitter melon can reduce the infiltration of macrophages into the adipose tissue. Because of this, it can reduce the expression of IL-6 and TNF-α in the adipose tissue. This indicates that bitter melon has the potential to be anti-inflammatory in obese mice42; Hsieh et al., (2013) reported that the use of bitter seed oil can reduce inflammation in adipose tissue43; Xu et al. (2014) stated that bitter melon can reduce serum C-reactive protein in obese mouse44; İlhan et al., (2015), reported that olive oil from bitter melon could improve wound healing and anti-inflammatory.45 But this particular study has not shown to affect the serum TNF-α levels.
Wound healing is not a simple linear process but an integration of dynamic interactive processes and overloads of many cells, extracellular matrices and dissolved mediators.46 In response to these injuries, the body has physiological functions of wound healing. This healing process consists of the initial phase, intermediate and advanced phases. Each phase has a different biological process and cell role. In the initial phase, hemostasis occurs where the broken blood vessels in the wound will be stopped by the occurrence of a vasoconstriction reaction. This is to restore blood flow and inflammation to remove damaged tissue and prevent bacterial infections. In the intermediate phase, mesenchymal cell proliferation, epithelialization and angiogenesis occur. Aside these, there is also a contraction of the wound and collagen synthesis in this phase. But in the final phase, there is wound formation/remodeling.47 The results in this study are not in accordance with the research of Grover and Yadav, (2004), where it was reported that bitter melon can help in wound healing20; Özbakıs et al., (2005) states that bitter melon has beneficial effects on wound by reducing the inflammation48; meanwhile Sharma et al., (2009) reported that bitter melon extract powder could significantly improve tissue regeneration, epithelial wound contraction in ulcer mouse models49; Teoh et al. (2009) reported that giving bitter melon extract can improve and accelerate the process of wound healing in diabetic animals50; Piskin et al. (2012) reported that giving bitter melon extract can increase and speed up the wound healing process in rabbits51; Hussan et al. (2014) reported that bitter melon ointment can accelerate ulcer healing in diabetic mouse and increase TGF-β52 expression52; Ahuja and Mittal (2014) reported that bitter melon leaves extract significantly improved wound healing in diabetic mouse23; Singh et al. (2017) reported that extracts of bitter melon topically can increase granulation tissue formation and angiogenesis. Therefore this can improve wound healing. The wound closure is influenced by the formation and maturation of collagen.53 In diabetic ulcers, this can occur in the healing of wounds. This is due to hyperglycemia, protein denaturation, oxidative stress.23 Therefore this study has shown to improve diabetic ulcers.
Conclusion
Bitter melon leaves extract has not shown to affect the serum TNF-α levels or improve diabetic foot ulcers according to these findings.
Acknowledgements
Sources of Support: This study was funded by the Directorate of Research and Community Service, the Directorate General of Reinforcement Research and Development, Ministry of Research, Technology and Higher Education, based on Decree No. 28/E/OPT/2017. We thank the Director of Dr. Moewardi General Hospital, Surakarta, Central Java, Indonesia and all participants in this study.
Conflicts of Interest
There is no conflict of interest.
Ethics Approval and Consent to Participate
Prospective participants are invited to participate in this study. All participants get the explanation about the purpose of this study which is to assess the effect of bitter melon leaves extracts on serum levels of TNF-α and diabetic ulcer healing process. Written informed consent was obtained from each participant before the study and participants are allowed to withdraw from this research at any time they wish. Also, the ethical clearness for this study was obtained from the Commission of Health Research Ethics Hospital Dr. Moewardi/Sebelas Maret University of Surakarta, Indonesia (Number: 902/XI/HREK/2016).
References
- Whiting D.R, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311-21.
CrossRef - IDF atlas (7th edition update). Brussels, Belgium. International Diabetes Federation. Available at http://www. diabetesatlas.org. 2015. [lastaccessed16.12.16]
- Rice J.B, Desai U, Cummings A.K, Birnbaum H.G, Skornicki M, Parsons N.B. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37:651–8.
CrossRef - Aalaa M, Malazy O.T, Sanjari M, Peimani M, Mohajeri – Tehrani M. Nurses’ role in diabetic foot prevention and care; a review. J Diabetes Metab Disord. 2012;11:24.
CrossRef - Alavi A, Sibbald R.G, Mayer D, Goodman L, Botros M, Armstrong D.G, Woo K, Boeni T, Ayello E.A, Kirsner R.S. Diabetic foot ulcers: Part II. Management. J Am Acad Dermatol. 2014;70(21):e1-2124.
- Cavanagh P.R, Lipsky B.A, Bradbury A.W, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725-1735.
CrossRef - Leone S, Pascale R, Vitale M, Esposito S. Epidemiology of diabetic foot. Infez Med. 2012;20:8–13.
- Shahi S.K, Kumar A, Kumar S, Singh S.K, Gupta S.K, Singh T.B. Prevalence of Diabetic Foot Ulcer and Associated Risk Factors in Diabetic Patients From North India. J DFC. 2012;3:83-91.
- Richard J.L, Schuldiner S. Epidemiology of diabetic foot problems. Rev Med Interne. 2008;29:222-30.
CrossRef - Nather A, Bee C.S, Huak C.Y, Chew J.L, Lin C.B, Neo S, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008;22:77–82.
CrossRef - Bakri F.G, Allan A.H, Khader Y.S, Younes N.A, Ajlouni K.M. Prevalence of Diabetic Foot Ulcer and its Associated Risk Factors among Diabetic Patients in Jordan. J Med J. 2012;46:118–25.
- Lipsky B.A, Weigelt J.A, Sun X, Johannes R.S, Derby K.G, Tabak Y.P. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011;34:1695–1700.
CrossRef - Monteirosoares M, Boyko E.J, Ribeiro J, Ribeiro I, Dinisribeiro M. Risk stratification systems for diabetic foot ulcers: a systematic review. Diabetologia. 2011;54:1190–1199.
CrossRef - Martinsmendes D, Monteirosoares M, Boyko E.J, Ribeiro M, Barata P, Lima J. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632–638.
CrossRef - Lobmann, R, Schultz G, and Lehnert H. Proteases and Diabetic Foot Syndrome: Mechanisms and Therapeutic Implications. Diabetes care. 2005;28(2):462-71.
CrossRef - Maltezoz E, Papazoglou D, Exiara T, Papazoglou L, Karathanasis E, Christakidis D and Ktenidou-Kartali S. Tumour Necrosis Factor-α Levels in Non-diabetic Offspring of Patients with Type 2 Diabetes Mellitus. JIMR. 2002;30:576-83.
CrossRef - Siqueira M.F, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J, Braasch C, and Graves D.T. Impaired wound healing in mouse models of diabetes is mediated by TNF-α dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia. 2010;53(2):378-88.
CrossRef - Velazquez O.C. Angiogenesis and vasculogenesis: Inducing the growth of new blood vessels and wound healing by stimulation of bone marrow–derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45:39-47.
CrossRef - National Center for Complementary and Alternative Medicine (NCCAM). Complementary, Alternative, or Integrative Health: What’s in a Name? S. Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA. Available online:http://nccam.nih.gov/health/whatiscam (accessed on 24 Juni 2016).
- Grover J.K and Yadav S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004;93:123–132.
CrossRef - Leslie T. Bitter Melon (Momordica charantia). In Herbal Secrets of the Rainforest; Leslie, T., Ed.; Sage Press: Austin Texas, T.X, USA. 2011;1–100.
- Basch E, Gabardi S and Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am J Health Syst Pharm. 2003;60(4):356-9.
- Ahuja D and Mittal A. Effect of Momordica charantia leaves extract on biophysical and biochemical parameters of wound in experimentally induced diabetes in rats. jddt. 2014;2(13):33-39.
- Gosain A, DiPietro L.A. Aging and wound healing. World J Surg. 2004; 28:321–326.
CrossRef - Keylock K.T, Vieira V.J, Wallig M.A, DiPietro L.A, Schrementi M, Woods J.A. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr CompPhysiol. 2008;294:179-184.
CrossRef - Swift M.E, Burns A.L, Gray K.L, DiPietro L.A. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001; 117:1027–1035.
CrossRef - Swift M.E, Kleinman H.K, DiPietro L.A. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479-1487.
- Gilliver S.C, Ashworth J.J, Ashcroft G.S. The hormonal regulation of cutaneous wound healing. Clin Dermatol. 2007;25:56-62.
CrossRef - Hardman M.J, Ashcroft G.S. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008;9:80.
CrossRef - Biondo-Simoe M.L.P, Zammar G.R, Fernandes R.S, Biondo-Simos R, Mello F.S.R, Noronha L. Obesity and abdominal wound healing in rats. Acta Cir. Bras. 2010;25(1).
- American Diabetes Association. Standard of Medical Care in Diabetes. Diabetes Care. 2011;34 (Supplement 1):S11-S61.
CrossRef - Bouskela E, Botttino D.A, Tavares J.C. Microvascular permeability in diabetes. In: Schmid-Schonbein G.W., Neil Granger D., editors. Molecular Basis for Microcirculatory Disorders. Paris : Springer-Verlag France. 2003;545-55.
CrossRef - Vincent A.M, Russell J.W, Low P, Feldman E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612-628.
CrossRef - Huijberts M.S, Schaper N.C, Schalkwijk C.G. Advanced glycation end products and diabetic foot disease. Diabetes Metab Res Rev. 2008; 24(1):S19-S24.
CrossRef - Loots M.A, Lamme E.N, Zeegelaar J, Mekkes J.R, Bos J.D, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111:850-857.
CrossRef - Sibbald R.G, Woo K.Y. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab Res Rev. 2008;24(1):25-30.
CrossRef - Clayton W Jr. A Review of The Pathophysiology, Classification, and Treatment of Foot Ulcers in Diabetic Patients. ProQuest Health and Medical Complete. 2009.
- Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition. 2010;26:862–6.
CrossRef - Liu Z.J, Velazquez O.C. Hyperoxia, Endothelial Progenitor Cell Mobilization, and Diabetic Wound Healing. Redox Signal. 2008;10: 1869-82.
CrossRef - Goldberg M.T, Han Y.P, Yan C, Shaw M.C, dan Garner M.L. TNF-α Suppresses α-Smooth Muscle Actin Expression in Human Dermal Fibroblasts: An Implication for Abnormal Wound Healing. J Invest Dermatol. 2007;127(11):2645–55.
CrossRef - Leung P.C, Wong M.W.N, dan Wong W.C. Limb salvage in extensive diabetic foot ulceration : an extended study using a herbal supplement. Hong Kong Med J. 2008;14:29-33.
- Bao B, Chen Y.G, Zhang L, Xu N.Y.L, Wang X. Momordica charantia (Bitter Melon) Reduces Obesity-Associated Macrophage and Mast Cell Infiltration as well as Inflammatory Cytokine Expression in Adipose Tissues. PLoS ONE. 2013;8(12):e84075.
CrossRef - Hsieh C.H, et al. Altered white adipose tissue protein profile in C57BL/6J mice displaying delipidative, inflammatory, and browning characteristics after bitter melon seed oil treatment. PloS One. 2013;8(9):e72917.
CrossRef - Xu J, et al. Bitter gourd inhibits the development of obesityassociated fatty liver in C57BL/6 mice fed a high-fat diet. J Nutrit. 2014;144(4):475-83.
CrossRef - İlhan M, Bolat I.E, Süntar İ, Köklü H.K, Çankal D.A.U, Keleş H.A. Topical application of olive oil macerate of Momordica charantia L. promotes healing of excisional and incisional wounds in rat buccal mucosa. Arch Oral Biol. 2015;60(12):1708-13.
CrossRef - Guo S, DiPietro L.A. Factors Affecting Wound Healing. J Dent Res. 2005;89(3):219-29.
CrossRef - Pusponegoro A.D, Dalam L, Sjamsuhidajat R, Jong D.W. penyunting. Buku Ajar Ilmu Bedah. Edisi ke-2. EGC. 2010;66-88.
- Özbakis D.G, Gürsan N. Effects of Momordica charantia L. (Cucurbitaceae) on indomethacin-induced ulcer model in rats. Turk J Gastroenterol. 2005;16:85-88.
- Sharma S. Sharma M.C, Kohli D.V, Chaturvedi S.C. Formulation, evalution, wound healing studies of benzene-95% absolute ethanol extract of leaves. Journal of Optoelectronicsand Biomedical Materials. 2009;1(4):375.
- Teoh S.L, Latiff A.A, Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol. 2009; 34(7):815-822.
CrossRef - Piskin A, Altunkaynak B.Z, Tumentemur G, Kaplan S, dan Yazici O.B. The beneficial effects of Momordica charantia (bitter gourd) on wound healing of rabbit skin. J Dermatolog Treat. 2012;1-8. Early Online.
- Hussan F, Teoh S.L, Muhamad N, Mazlan M. Latiff Momordica charantia ointment accelerates diabetic wound healing and enhances transforming growth factor-β expression. J Wound Care. 2014;23(8): 400, 402, 404–7.
CrossRef - Singh R, Ignacio G.G, Krishnamurthy G, Singh A.K. Bitter Melon Extract Promotes Granulation Tissue Growth and An giogenes is in the Diabetic Wound. Adv Skin Wound Care. 2017;30(1):16-26.
CrossRef








