Mohammad Abdulla1, Eyad Mallah1, Wael Abu Dayyih1, Walid Abu Rayyan1, Feras Darwish El-Hajji2, Mona Bustami1, Kenza Mansour1, Israa Al-Ani3, Nisreen Seder4 and Tawfiq Arafat1
1Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan.
2Faculty of Pharmacy and Medical Sciences, Applied Science University, Amman, Jordan.
3Faculty of Pharmacy and Medical Silences, Al-Ahliyya Amman University, Jordan.
4School of Biomedical Science, Faculty of Health Sciences, University Sultan Zainal Abidin, 21300 Kuala Nerus, Terengganu, Malaysia.
Corresponding Author E-mail: eyad782002@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1494
Abstract
Both sildenafil and RED BULL®(energy drink) are claimed to boost up energy.RED BULL®, one of the most commonly used energy drinks and its easily and widely available for daily use, the idea of this research work had arisen from observations about the concomitant use of sildenafil with RED BULL® by men seeking for better sexual performance. To study the effect of RED BULL® on the pharmacokinetic profile of sildenafil by using HPLC.The pharmacokinetic parameters (Cmax, Tmax and AUC) were determined in 10 rats following oral administration of 0.57 mg/ml sildenafil with and without RED BULL® in crossover design, to achieve this purpose,simple, rapid and accurate method for validation and determination of sildenafil in rat plasma in the presence of RED BULL® has been developed. This was performed using High-Performance Liquid Chromatography- Ultra Violet (HPLC-UV). The pharmacokinetic data showed that sildenafil plasma level was lower when combined with RED BULL® According to the results obtained, maximum concentration (Cmax) for sildenafil alone was (162.05 ng/ml) after 0.5 hours of administration. The Cmax decreased to (44.68 ng/ml) after 0.5 hoursof administration RED BULL® concomitantly with sildenafil which showed a significant effect on sildenafil plasma level (P<0.001). The area under the curve (AUC) decreased significantly from (370.53 ng/ml*hr) for sildenafil alone to (87.74 ng/ml*hr) when combined with RED BULL®. RED BULL® can alter sildenafil pharmacokinetic if they were taken together.
Keywords
Drug Interaction; Energy Drinks; Pharmacokinetic; Sildenafil; Validation
Download this article as:| Copy the following to cite this article: Abdulla M, Mallah E, Dayyih W. A, Rayyan W. A, El-Hajji F. D, Bustami M, Mansour K, Al-Ani I, Seder N, Arafat T. Influence of Energy Drinks on Pharmacokinetic Parameters of Sildenafil in Rats. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Abdulla M, Mallah E, Dayyih W. A, Rayyan W. A, El-Hajji F. D, Bustami M, Mansour K, Al-Ani I, Seder N, Arafat T. Influence of Energy Drinks on Pharmacokinetic Parameters of Sildenafil in Rats. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=21911 |
Introduction
Sildenafil citrate is an oral drug used as a therapy for erectile dysfunction.1 It is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1 Hpyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate.2
Sildenafil citrate structure is shown in figure 1.
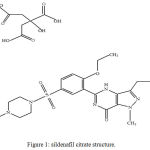 |
Figure 1: sildenafil citrate structure.
|
The mechanism of erection involves therelease of nitric oxide (NO) in the Corpus cavernosum during sexual stimulation. The NO activates the enzyme guanylatecyclase, which increases the levels of cyclicguanosine monophosphate (cGMP).3
The production of cGMP leads to relaxation of smooth muscles in the corpus cavernosum and increases blood flow into the penis.4
Sildenafil citrate has no direct relaxant effect, but it increases the levels of cGMP by inhibiting phosphodiesterase type 5 (PDE5), which is responsible for degradation of cGMP.5 Sildenafil is primarily metabolized by the CYP3A4 enzyme, which is the principal enzyme responsible for the oxidative metabolism of themajority ofdrugs.6 The interaction between sildenafil and other drugs that are also metabolized by CYP3A4 should be taken into consideration when drugs are prescribed to avoid undesired pharmacological effects.6-8
RED BULL® is the best-selling energy drink in theUnited States of America and worldwide. It contains different ingredients such as sugar, caffeine, taurine, glucuronolactone and B vitamins.9 Caffeine is probably the most common ingredient in RED BULL®, as it acts as a stimulant.10 Taurine is a homocysteine derived amino acid, but not a constituent of proteins.11 It is present freely in the intracellular fluid and distributed widely in the sarcoplasm of the cardiac tissues, hepatocytes, central nervous system, and retina. Taurine mediates the homeostasis of different physiological functions12 including osmoregulation,antioxidation,detoxification, neuromodulation, and brain and retinal development.13 Moreover, a number of studies have documented some pharmacological functions of taurine against congestive heart failure, liver disease, hyperlipidemia.14
Several papers reported that Sildenafil pharmacokinetics are altered in response to food-drug (or drug-drug)interactions.5,15-17 It has been demonstrated the co-administration of sildenafil with potent CYP3A4 inhibitors such as azole antifungal agents, macrolide antibiotics, and protease inhibitorscauses a significant increase in plasma sildenafil levels.18,19 Another example of interaction is Sitaxentan (endothelin receptor antagonist), which when administered with sildenafil, demonstrated a weak, but a statistically significant interaction.20 The effect of concomitant administration of caffeine with certain drugs used for cardiovascular, CNS, gastrointestinal, infectious, respiratory and skin disorders has been extensively studied as well.21 whereas, the effect of other ingredients in Red Bullhas not been studied for the pharmaco kinetic effect on sildenafil. Caffeine has been reported to interact with many medications such as certain selective serotonin reuptake inhibitors (particularly fluvoxamine), antiarrhythmics (mexiletine), antipsychotics (clozapine), psoralens, idrocilamide and phenylpropanol amine, quinolones (enoxacin) and bronchodilators (furafylline and theophylline).10 Thus, pharmaco kinetic interactions at the CYP1A2 enzyme level may cause toxic effects during concomitant administration of caffeine and certain drugs used for cardiovascular, CNS, gastrointestinal, infectious, respiratory and skin disorders.9
Recently, drug-drug and drug-food interactions are being the center of our interest.22-26 The idea of this research work had arisen from observations of about the concomitant use of sildenafil with RED BULL® by men seeking for better aphrodisiac effect during sexual intercourse. Both Sildenafil and RED BULL® claimed to boost up energy.Since RED BULL® is one of the most commonly used energy drinks, it is easily and widely available for daily use. The current study was conducted to investigate the effect of RED BULL® ingestion on the pharmacokinetics of Sildenafil in rats plasmausing HPLC in a crossover study.
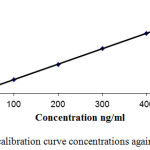 |
Figure 2: The plot of calibration curve concentrations against the ratios.
|
Materials and Methods
Reagents and Materials
Deionized Water, Nano pure (Fischer Scientific), Rats plasma (from animal house in ASU)
Methanol, advanced gradient grade (Fischer scientific),Acetonitrile (ACN) (Fisher), Triethylamine (TEDIA), Phosphoric acid (Medex), Sildenafil (from Dar al-Dawapharma),
Carbamazepine, EDTA, Distilled water.
Instrumentation
An HPLC (FinniganSurveyor) was used and composed ofChromQuestsoftware 4.2.34,
Solvent delivery systems pump (LC Pump Plus), Auto-sampler plus, UV-VIS plus Detector,
Sepax GP-C18, (150 x 4.6 mm), 5µm.
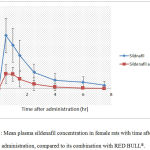 |
Figure 3: Mean plasma sildenafil concentration in female rats with time after drug administration, compared to its combination with RED BULL®.
|
Each data point represents the mean (n=10).
Preclinical Study and Protocol
Adult female Sprague-Dawley laboratory rats were supplied by the animal house of Applied Science University. The average weight of these rats was about 240g (200g -260), and they were in healthy condition. They were placed in anair-conditioned environment (20-25°C) and were exposed to 12 hours light/12 hours dark cycle daily.
All animals in the experiments were handled in compliance with FELASA guidelines (Federation of European Laboratory Animal Science Association) and the study protocol was approved by the research committee (February 2014) at the Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan.
10 rats were included in the drug interaction study in a crossoverstudy.The rats were divided into 2 groups (group A and group B), 5 rats each.In the first period of the experiment, group (A) had received sildenafil aqueous solution with a concentration of 0.57 mg/ml and a dose of 2.85/kg (200mg/70kg)alone as a single dose, while group (B) had received sildenafil aqueous solution as a single dose with RED BULL® (purchased from local market), respectively and spontaneously.
Washing out period of2 weeks was implemented, after which the test items were switched between the 2 groups, Group (A) received sildenafil solution and RED BULL® while group (B) received sildenafil solutionalone.
The dose used is corresponding to 200 mg for human of 70 kg weight, 200 mg is the double dose of sildenafil regular dose. This dose was chosen to clarify any possible interaction, as it foundto be usedin theprevious clinical trial.27
The least weight of rats was 200g and the concentration was calculated based on this weight. Rats weighing200 mg took 1 ml of the solution, and then other rats took more than 1ml according to their weights.
The ratswhich were aimed to take RED BULL® in each part of the experiment were supplied with 15 ml, 16 hours before sildenafil solution administration. They receivedanother 5ml of RED BULL® orally after the administration of sildenafil solution.
A syringe with a specific needle was used for the oral administration of both sildenafil and RED BULL®solutions.For rats which were aimed to take sildenafil solution alone, drinking water was administrated insufficient amount all the time.
Blood samples were taken from the rats’ optical vein at the following time points: (zero, 0.5, 1, 1.5, 2.5, 4, 6, 7.5) hours.
Blood samples were drawn into EDTA-containingmicrotubes. The microtubeswere immediately centrifuged for 10 minutes at 5,000 rpm and separated plasma was transferred into labeled Eppendorf tubes then stored at -20°C until analysis.
Preparation of Solutions
Preparation of Sildenafil Solution for Oraluse
0.081 g of sildenafil citrate (equals to 0.057 g sildenafil) was dissolved in 100 ml of distilled water, vortex to have complete dissolution to get a concentration of 0.57 mg/ml.
Preparation of Stock Solution of Sildenafil
An equivalent weight of 10mg of sildenafil working standard was dissolved in 10 ml of methanol to getaconcentration of 1000 µg/ml stock solution of sildenafil.
Preparation of Stock Solution of Carbamazepine Internal Standard
An equivalent weight of 10 mg of carbamazepine working standard was dissolved in 100 ml of Acetonitrile to get a concentration of 100 µg/ml stock solution of carbamazepine.
Preparation of Working Solution of Carbamazepine I.S
20 µl were taken from carbamazepine stock solution (100µg/ml) and diluted to 100 ml using ACN which was considered to be anI.S working solution that contains 20 ng/ml of carbamazepine.
Method of Extraction
Thedescribed procedures were applied for subject samples, calibrator, and quality control samples.
In order to perform the sample extraction, disposable Eppendorf tubes were labeled and placedon a rack, then 100.0 µl aliquots of each test sample (blank, zero, standards, quality control low, quality control medium, quality control highand rat samples) were drawn into the tubes by pipette and mixed with 150.0 µl of internal standard (20 ng/ml carbamazepine in ACN), then vortex of each sample vigorously for 1.0 min, and finally Centrifugation at 14000 rpm for 15 minutes.
Chromatographic conditions and detection conditions were as follow in table I:
Table 1: Chromatographic conditions and detection conditions.
| Column Oven Temp | Auto-sampler
Temp |
Auto-sampler Injection Volume | Pump Flow Rate |
HPLC Conditions |
|
| 30˚C | 10˚C | 25 µl | 1.0 ml/min | ||
| Mixture of (57.5 % ACN, 42.5 Water), 675 µl Triethylamine /1L of mixture, pH=7.0 adjust pH with phosphoric acid | Mobile phase | Chromatography | |||
| Sepax GP-C18, (150 x 4.6 mm, 5µm) | Column type | ||||
| Sildenafil | Carbamazepine(I.S) | Expected Retention times
(minutes) |
|||
| 4.2 min | 2.5 min | ||||
| 230 nm | Wavelength | Detection Conditions | |||
Analytical Method Validation
Accuracy and Precision
The intra-day precision and accuracy were evaluated by analyzing six replicates of the QC samples (low, mid, high) and lower limit of quantification (LLOQ) samples on a single day. The inter-day precision and accuracy were determined by analyzing three runs of QC samples and LLOQ samples on three different days. The accuracy (%) was calculated by dividing a measured mean concentration over the nominal analyte concentration. Precision was presented as CV%. The acceptable limits of accuracy and precision should be below 15% except at the LLOQ, for which accuracy and precision should be below 20%.28
Linearity
Linearity was determined by a series of six injections to a seven calibration concentration levels for theanalyte. Peak areas of the calibration standards were plotted in the Y-axis against the nominal standard concentration, and the linearity of the plotted curve was evaluated through the value of the correlation coefficient (R2) which should be more than 0.98.28
Stability
Stability of the analyte in the rat serum was evaluated using both low and high QC samples, which were analyzed immediately after preparation and after the applied storage conditions.Evaluation included:autosampler stability, freeze-thaw stability “after 3 cycles”, and Short-term stability at room temperature “24 h”.
The mean concentration should be within ±15% of the nominal concentration.28
Results and Discussion
Validation
The validation of sildenafil was conducted according to EMA guideline.28 Selected chromatograms for sildenafil validation (blank, zero, LLOQ) are shown in figures (4, 5 and 6).
 |
Figure 4: Sildenafil blank chromatogram.
|
 |
Figure 5: Sildenafil zero chromatogram.
|
 |
Figure 6: Sildenafil 20 ng\ml chromatogram.
|
Accuracy and Precision
Table IIrepresents inter-dayprecision and accuracy for quality control samples of sildenafil in three days of validation. All of the obtained accuracy and precision data were within the required range which is (85-115 % for all concentration except forLLOQ, which is 80-120 %) for the accuracy, and (20 % for LLOQ and 15 % for other concentrations) for the precision.
Table 2: Inter-day precision and accuracy data for sildenafil samples in the three days of validation.
| Conc./Days | 20 ng/ml | 60 ng/ml | 250 ng/ml | 425 ng/ml |
| Mean (n=6) | 20.398 | 60.535 | 257.891 | 426.620 |
| STD | 1.324 | 2.436 | 6.425 | 7.376 |
| CV% | 6.492 | 4.023 | 2.491 | 1.729 |
| Accuracy% | 101.99 | 100.89 | 103.16 | 100.38 |
Linearity
R2 which represents the strength of correlation coefficient for standard calibration curve was greater than 0.99 during validation course. Data of standard curve with regards to correlation, slope, R2, and intercept are shown in tables III.
Therefore, validation results passed the required criteria in term of linearity and accuracy
Table 3: Raw data of the calibration curve for sildenafil.
| Correlation | Slope | Factor | R2 | Intercept |
| 0.999963 | 0.002158 | 463.45431 | 0.999927 | -0.002794 |
Stability
TablesIV shows data for short-term stability indicated by two QC concentrations (low, high) for sildenafil after preparation procedure (auto-sampler stability), T=10°C and for room temperature or processing temperature. Regarding to the stability of plasma samples during freezing and thawing processes; two QC samples were stored and frozen in afreezerat the intended temperature-60°Cand thereafter thawed and processed at room temperature twice for 72 hours.All results were within the accepted range of 85%-l 15%.
Table 4: Short and long-term stability data for Sildenafil.
| Sildenafil QC low samples stability auto-sampler procedure at 10°C. | Sildenafil QC high samples stability auto-sampler procedure at 10°C. | ||||||||
| Time | Measured Conc. | Mean Measured | Accuracy % | Stability | Time | Measured Conc. | Mean Measured | Accuracy % | Stability |
| 0.00 Hour | 61.959 | 61.195 | 103.27 | 100.00 | 0.00 Hour | 427.436 | 426.263 | 100.57 | 100.00 |
| 60.624 | 101.04 | 423.162 | 99.57 | ||||||
| 61.002 | 101.67 | 428.190 | 100.75 | ||||||
| 24.00 Hours | 59.474 | 59.902 | 99.12 | 97.89 | 24.00 Hours | 426.317 | 423.081 | 100.31 | 99.25 |
| 60.462 | 100.77 | 424.062 | 99.78 | ||||||
| 59.771 | 99.62 | 418.864 | 98.56 | ||||||
| Sildenafil QC low samples stability at room temperature (bench stability). | Sildenafil QC high samples stability at room temperature (bench stability). | ||||||||
| Time | Measured Conc. | Mean Measured | Accuracy% | Stability | Time | Measured Conc. | Mean Measured | Accuracy% | Stability |
| 0.00 Hour | 61.959 | 61.195 | 103.27 | 100.00 | 0.00 Hour | 427.436 | 426.263 | 100.57 | 100.00 |
| 60.624 | 101.04 | 423.162 | 99.57 | ||||||
| 61.002 | 101.67 | 428.190 | 100.75 | ||||||
| 24.00 Hours | 59.693 | 58.031 | 99.49 | 94.83 | 24.00 Hours | 426.246 | 422.897 | 100.29 | 99.21 |
| 57.071 | 95.12 | 420.336 | 98.90 | ||||||
| 57.329 | 95.55 | 422.109 | 99.32 | ||||||
| Sildenafil QC low samples stability at freeze and thaw. | Sildenafil QC high samples stability at freeze and thaw. | ||||||||
| Time | Measured Conc. | Mean Measured | Accuracy% | Stability | Time | Measured Conc. | Mean Measured | Accuracy% | Stability |
| 0.00 Hour | 61.959 | 61.195 | 103.27 | 100.00 | 0.00 Hour | 427.436 | 426.263 | 100.57 | 100.00 |
| 60.624 | 101.04 | 423.162 | 99.57 | ||||||
| 61.002 | 101.67 | 428.190 | 100.75 | ||||||
| 72.00 Hour | 58.712 | 58.871 | 97.85 | 96.20 | 72.00 Hour | 423.093 | 424.899 | 99.55 | 99.68 |
| 58.713 | 97.86 | 426.925 | 100.45 | ||||||
| 59.187 | 98.65 | 424.678 | 99.92 | ||||||
Pharmacokinetic data of sildenafil in presence of RED BULL®
The plasma concentrations of sildenafil with or without RED BULL®weremeasured inthe population rats using a sample size of 10 rats for the drug alone and then repeated for the combination of the drug with RED BULL® in a crossoverstudy with washout period of 2 weeks.
7 discrete samples were obtained from rats following drug administration tothelast time interval of 7.5 hours.
This study was conducted on female rats since the sildenafil bioavailability is more comparable to humans (regardless the gender) and the plasma levels of sildenafil male rats are much lower when compared to its bioavailability in female rats (16) (17).
The plasma levels of sildenafil fed ratshas reached its maximum level at the first 0.5 hours(Cmax) (162.05 ng/ml) (Tmax)when administered alone as depicted in figures (3, 7).
 |
Figure 7: Sildenafil sample chromatogram after 0.5 hours of administration for one group.
|
Whereas, sildenafil plasma levelswereintimately decreased whenadministered with RED BULL® as Cmaxwas only 44.68 ng/mlatthe first 0.5 hours, as shown in figures(3, 8). The sharp decrease in sildenafil levels as a consequent of administration with RED BULL® reached a proportion of 72% decrease (P<0.001) compared with sildenafil fed rats alone.
Figure 3shows Mean plasma sildenafil concentration of female rats in a crossover study, sildenafil was administered alone and in combination with RED BULL®.Each data point represents the mean (n=10).
The pharmacokinetic interaction of Sildenafil and RED BULL® could happen at any stage of ADME: “Absorption, Distribution, Metabolism, and Excretion.”
However, the absorption of sildenafil from the intestine happens through a passive mechanism, no carriers are needed so we rule out this is the cause of the difference.29 The pH of RED BULL®solutions (with and without Sildenafil) is 3.28, which indicates theminimum effect on stomach pH.In addition, we noted that complete dissolution occurred for Sildenafil in RED BULL®.
 |
Figure 8: Sildenafil with RED BULL®sample chromatogram after 0.5 hours of administration for one group.
|
However, this cannotrule out the presence of interaction at the absorption level in the stomach as the presence of REDBULL willeffect of the gastric emptying time.26
By plotting the data on thesemilog scale as shown in figure (9), the slope (which represents elimination rate constant) of Sildenafil when administered with RED BULL beverage was steeper, this indicates a higher elimination rate of the drug when combined with RED BULL.
Figure 9: Mean plasma sildenafil concentration in female rats with time after drug administration, compared to its combination with RED BULL® on asemilog scale. Each data point represents the mean (n=10).
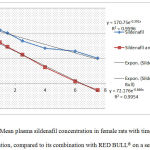 |
Figure 9: Mean plasma sildenafil concentration in female rats with time after drug administration, compared to its combination with RED BULL® on asemilog scale.
|
Each data point represents the mean (n=10).
The lower AUC, Cmaxis accompanied by anincrease in the clearance of the drug and shortening of the half-life. These changes elimination parameters indicate an enhancement in elimination processes of the drug which mainly eliminated by metabolism in theliver.
By reviewing the literatures, it has been demonstrated that taurine has an activating effect on the expression level of CYP 3A4 in the presence of CYP3A4 inducer. Taurine enhances the induction of CYP3A4 mRNA in a concentration-dependent manner.30 Taurine is a potent inducer of CYP3A4 mRNA via pregnane X receptor (PXR), an orphan member of the steroid/retinoid/thyroid hormone receptor superfamily of ligand-activated transcription factors.31,32 It was recently reported that PXR serves as a functional bile acids receptor, and lithocholic acid, which is a kind of bile acid, induces CYP3A mRNA.33-35 Attention should be paid to drug interactions when taurine-containing medicinal agents, foods, and drinks are administered together with both sildenafil and drugs metabolized mainly by CYP 3A4.35
Moreover,in a similar manner of drug interaction, high levels of taurine have lowered the plasma concentrations of acetaminophen and rifampicine by increasing the enzyme activity of CYP3A4.36,37 Moreover, we tried to find a single clinical report about overdoseabuse ofsildenafil and REDBULL but up to the date there is no case report about the adverse effect of Sildenafil intake with energy drink. Our findings couldexplain the reason of why there are no reports on sildenafil interaction with such drinks but even though we don’t know anything about the sildenafil clinical effect since it has reached to a sub therapeutic dose.
In addition, we propose that the change in sildenafil plasma levels in female rates is an enzyme induction mechanism due to asignificant change in elimination parameters. WhereasKelhas been changed significantly from 0.45 hr-1 for sildenafil alone to 0.56 hr-1when given in combination with RED BUL beverage. The terminal elimination t1/2 was shortened significantly from 1.51 hr to 1.15 hr in combination, and the clearance increased also highly significantly from to 135 L/hr/kg to 535.6 L/hr/kg in combination.
Also, the MRT-t was shortened. All these changes suggest an enhancement in elimination processes by metabolism of sildenafil by hepatic enzymes. However, the interaction still could have done at any stage of ADME.
Moreover, caffeine has a great impact on the cardiovascular system by causing increased heart rate and blood pressure which results in increased cardiac output.38 So theblood flow to theliver will increase, and the metabolism of sildenafil in presence of caffeine may increase because of this increase in blood flow.
This interaction between sildenafil and RED BULL®could be of clinical significance, because the decrease of sildenafil concentrations to subtherapeutic levels may make the effect of sildenafil clinically insignificant. Table V shows Pharmacokinetic data of sildenafil.
Table 5: Pharmacokinetic data of sildenafil.
| Sildenafil | Sildenafil and RED BULL | |
| T-max(hr) | 0.5 | 0.5 |
| C-max(ng/ml)* | 162.05 | 44.68 |
| AUC(ng/ml*hr)* | 370.53 | 87.74 |
| AUMC-t (ng.hr2/ml) | 810.90 | 155.73* |
| MRT-t (hr) | 2.18 | 1.66* |
| Kel (hr-1) | 0.45 | 0.56* |
| T1/2 (hr) | 1.51 | 1.15* |
| Systemic Cl (L/hr/kg) | 135 | 535.6* |
All parameters are within a CI of 5%.
The difference between both Cmax and AUC of sildenafil alone and sildenafil withRED BULL®was statistically significant (P<0.001).
Conclusion
There is a pharmaco kinetic interaction between sildenafil and RED BULL® when administrated concomitantly. Such interaction effectssignificantly (P<0.001) the Cmax and AUC of sildenafil. The interaction between sildenafil and RED BULL® could happen in all stages with high probability to be executed in metabolism stage.
This in vivo trialon rats suggests further investigations,such as assessing the possible interaction between sildenafil and RED BULL® in humans, checking the pharma cod ynamic effect of this combination, testing sildenafil levels in plasma in presence of certain concentrations of caffeine and explaining the exact mechanism of this interaction and the responsible compounds.
Acknowledgment
This research was funded by the deanship of scientific research at University of Petra. The technical support from Faculty of Pharmacy and Medical Sciences at University of Petra and the animal house department of Applied Science University was highly appreciated.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Berzas J, Rodriguez J, Castaneda G, Villaseñor M. Voltam metric behavior of sildenafil citrate (viagra) using square wave and adsorptive stripping square wave techniques: Determination in pharmaceutical products. Analytica chimica acta. 2000;417:143-8.
CrossRef - Umrani D.N, Goyal R.K. Pharmacology of sildenafil citrate. Indian journal of physiology and pharmacology. 1999;43:160-4.
- Moncada S, Higgs A. The l-arginine-nitric oxide pathway. New England Journal of Medicine. 1993;329:2002-12.
CrossRef - Ralph D, McNicholas T. Uk management guidelines for erectile dysfunction. BMJ: British Medical Journal. 2000;321:499.
CrossRef - Cheitlin M.D, Hutter A.M, Brindis R.G, Ganz P, Kaul S, Russell R.O, Zusman R.M, Forrester J.S, Douglas P.S, Faxon D.P. Use of sildenafil (viagra) in patients with cardiovascular disease. Circulation. 1999;99:168-77.
CrossRef - Mallah E.M, Rayyan W.S, Dayyih W.A, Elhajji F.D, Mansour K.A, Al-Majali I.S, Arafat T.A. Dose-dependent synergistic effect of pomegranate juice on the bioavailability of sildenafil in rats by using hplc method. LATIN AMERICAN JOURNAL OF PHARMACY. 2016;35:1277-84.
- Krenzelok E.P, Krenzelok E. Sildenafil: Clinical toxicology profile. Journal of Toxicology: Clinical Toxicology. 2000;38:645-51.
CrossRef - Mallah E, Saleh S, Rayyan W.A, Dayyih W.A, Elhajji F.D, Mima M, Awad R, Arafat T. The influence of eruca sativa (arugula) on pharmaco kinetics of sildenafil in rats. Neuro endocrinology Letters. 2017;38:295-300.
- Michael J.G, Jorge R.M.M, José R.G, Carlos M.R, Dharma R.P. Energy drinks and health: A brief review of their effects and consequences. Ciencias de la Conducta. 2012;27:23-34.
- Desbrow B, Leveritt M. Well-trained endurance athletes’ knowledge, insight, and experience of caffeine use. International journal of sport nutrition and exercise metabolism. 2007;17:328-39.
CrossRef - Curran C.P, Marczinski C.A. Taurine, caffeine, and energy drinks: Reviewing the risks to the adolescent brain. Birth Defects Research. 2017;109:1640-8.
CrossRef - Chesney R. Taurine: Its biological role and clinical implications. Advances in pediatrics. 1985;32:1-42.
- NAKAMuRA T, OGAsAwARA M, KoyAMA I, NEMoTo M, YosHiDA T. The protective effect of taurine on the biomembrane against damage produced by oxygen radicals. Biological and Pharmaceutical Bulletin. 1993;16:970-2.
CrossRef - Mochizuki H, Oda H, Yokogoshi H. Dietary taurine potentiates polychlorinated biphenyl-induced hypercholesterolemia in rats. The Journal of nutritional biochemistry. 2001;12:109-15.
CrossRef - O’rourke M, Xiong-Jing J. Sildenafil/nitrate interaction. Circulation. 2000;101:e90-e.
- McLeod A, McKenna C, Northridge D. Myocardial infarction following the combined recreational use of viagra® and cannabis. Clinical cardiology. 2002;25:133-4.
CrossRef - Bushra R, Aslam N, Khan A.Y. Food-drug interactions. Oman Med J. 2011;26:77-83.
CrossRef - Corbin J.D, Francis S.H, Webb D.J. Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction. Urology. 2002;60:4-11.
CrossRef - Jetter A, Kinzig‐Schippers M, Walchner‐Bonjean M, Hering U, Bulitta J, Schreiner P, Sörgel F,Fuhr U. Effects of grapefruit juice on the pharmaco kinetics of sildenafil. Clinical Pharmacology & Therapeutics. 2002;71:21-9.
CrossRef - Stavros F, Kramer W.G, Wilkins M.R. The effects of sitaxentan on sildenafil pharmaco kinetics and pharmacodynamics in healthy subjects. British journal of clinical pharmacology. 2010;69:23-6.
CrossRef - Carrillo J.A, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clinical .pharma cokinetics. 2000;39:127-53.
CrossRef - Shaikhli T.A, Dayyih W.A, Mallah E, Hamad M, Qinna N, Arafat T. Determination of atorvastatin pharmacokinetic parameters by lc/ms-ms with traditional liquorice beverage. Advances in Analytical Chemistry. 2015;5:17-24.
- Mallah E, Al Ani N, Dayyih A.W, Awad R. Simultaneous determination of sildenafil and glimepiride in rat plasma by using lc-ms method and their applications in pharmacokinetic interactions. J Clin Pharm. 2014;1:1007-20.
- Tbeekh H.T.A, Dayyih W.A.A, Mallah E.M, Qinna N.A, Awad R.M, Arafat T.A. Pomegranate juice affects on pharmacokinetic parameters of metronidazole by using hplc-ms. World J Pharm Pharm Sci. 2014;3:150-4.
- Tamimi L, Abudayyih W, Mallah E, Arafat T. Pioglita zone hcllevels and its pharma cokinetic application in presence of sucralose in animals serum by hplc method. Pharm .Anal. Acta. 2014;5:2.
- Awad R, Mallah E, Al Khawaja B, Dayyih W.A, El-Hajji F, Matalka K.Z, Arafat T. Pomegranate and licorice juices modulate metformin pharmacokinetics in rats. Neuro endocrinology letters. 2016;37.
- Nichols D.J, Muirhead G.J, Harness J.A. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: Absolute bioavailability, food effects and dose proportionality. British journal of clinical pharmacology. 2002;53:5-12.
CrossRef - BMWP D.A.B, CONSULTATION E, PARTY A.B.B.W. Committee for human medicinal products (chmp). 2007.
- Osman M.A, Maghraby E.G.M, Hedaya M.A. Intestinal absorption and presystemic disposition of sildenafil citrate in the rabbit: Evidence for site‐dependent absorptive clearance. Biopharmaceutics & drug disposition. 2006;27:93-102.
CrossRef - Labudova O, Yeghiazarjan C, Höger H, Lubec G. Taurine modulates expression of transporters in rat brain and heart. Amino Acids. 1999;17:301-13.
CrossRef - Lehmann J.M, McKee D.D, Watson M.A, Willson T.M, Moore J.T, Kliewer S.A. The human orphan nuclear receptor pxr is activated by compounds that regulate cyp3a4 gene expression and cause drug interactions. Journal of Clinical Investigation. 1998;102:1016.
CrossRef - Pascussi J.M, Drocourt L, Fabre J.M, Maurel P, Vilarem M.J. Dexamethasone induces pregnane x receptor and retinoid x receptor-α expression in human hepatocytes: Synergistic increase of cyp3a4 induction by pregnane x receptor activators. Molecular Pharmacology. 2000;58:361-72.
CrossRef - Staudinger J.L, Goodwin B, Jones S.A, Hawkins-Brown D, MacKenzie K.I, LaTour A, Liu Y, Klaassen C.D, Brown K.K, Reinhard J. The nuclear receptor pxr is a lithocholic acid sensor that protects against liver toxicity. Proceedings of the National Academy of Sciences. 2001;98:3369-74.
CrossRef - Xie W, Radominska-Pandya A, Shi Y, Simon C.M, Nelson M.C, Ong E.S, Waxman D.J, Evans R.M. An essential role for nuclear receptors sxr/pxr in detoxification of cholestatic bile acids. Proceedings of the National Academy of Sciences. 2001;98:3375-80.
CrossRef - Matsuda H, Kinoshita K, Sumida A, Takahashi K, Fukuen S, Fukuda T, Takahashi K, Yamamoto I, Azuma J. Taurine modulates induction of cytochrome p450 3a4 mrna by rifampicin in the hepg2 cell line. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2002;1593:93-8.
CrossRef - Ueshima Y, Tsutsumi M, Takase S, Matsuda Y, Kawahara H. Acetaminophen metabolism in patients with different cytochrome p‐4502e1 genotypes. Alcoholism: Clinical and Experimental Research. 1996;20.
CrossRef - Laine J, Auriola S, Pasanen M, Juvonen R. Acetaminophen bioactivation by human cytochrome p450 enzymes and animal micro somes. Xenobiotica. 2009;39:11-21.
CrossRef - Temple J.L, Dewey A.M, Briatico L.N. Effects of acute caffeine administration on adolescents. Experimental and clinical psychopharmacology. 2010;18:510.
CrossRef








