Manuscript accepted on :04-Aug-2018
Published online on: 29-08-2018
Plagiarism Check: Yes
Reviewed by: Patricia Williams
Second Review by: Tasnuva Tunna
Final Approval by: Prof. em. Hans-Joachim Freisleben
E. Gayathri, K. Punnagai and D. Darling Chellathai
and D. Darling Chellathai
Department of Pharmacology, Sri Ramachandra Medical College and Research Institute, Chennai, India.
Corresponding Author E-mail: kpunnagai@yahoo.in
DOI : https://dx.doi.org/10.13005/bpj/1498
Abstract
Angiotensin Converting Enzyme Inhibitor (ACEI) and Angiotensin II type 1 receptor antagonist (ARBs) are the most efficient cardiovascular drugs and exhibited efficient cytostatic activity in vitro in many malignant and normal cells1.OBJECTIVE: This study aims to assess the anticancer activity of these two drugs in a dose dependant manner using A549 cell line through MTT assay and Cell cycle analysis.. MATERIALS AND METHODS: Ramipril and Olmesartan were added to A549 at various concentrations ranging from 10⁻⁶ to 10mM.The dot plot of the cytotoxicity results were used to extrapolate the IC50 values. The dot plot of flow cytometry results were used to extrapolate the DNA percentage in phases of cell cycle. The plates were read at 570 nm by using a PERCLIN ELMER (multimode reader). Measurements for concentration required for 50% inhibition was noted. RESULTS: Ramipril and Olmesartan were added to A549 at various concentrations ranging from 10⁻⁶ to 10mM.The dot plot of the cytotoxicity results were used to extrapolate the IC50 values. The dot plot of flow cytometry results were used to extrapolate the DNA percentage in phases of cell cycle.
Keywords
Anticancer activity; Cell Cycle Analysis; Lung cancer; MTT assay; Olmesartan and Ramipril
Download this article as:| Copy the following to cite this article: Gayathri E, Punnagai K, Chellathai D. D. Evaluation of Anticancer Activity of Olmesartan and Ramipril on A549 Cell Line. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Gayathri E, Punnagai K, Chellathai D. D. Evaluation of Anticancer Activity of Olmesartan and Ramipril on A549 Cell Line. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=21882 |
Introduction
Angiotensin converting enzyme inhibitor (ACEI) and Angiotensin II type1 receptor antagonist (ARB) are the most efficient hypertensive drugs. They are the first line drugs in cardiac failure and preferred drugs in hypertension with diabetes mellitus.2 It was established in various studies that ACEI and ARBs inhibits Angiogenesis, proliferation of cancer cells, migration of cells, and Tissue development.3,6 The drugs in current use are Ramipril and Olmesartan both are available orally with less adverse effects.
In the Renin Angiotensin system cascade the multifunctional potent endogenous chemical is angiotensin II which is an efficient vasoconstrictor7 (suganuma et al). Angiotensin II plays an important role in enhancing tumor angiogenesis and growth.8 Studies have shown that angiotensin receptor antagonist Candesartan inhibits tumor angiogenesis by preventing the lung metastasis of renal murine cells.8
Various studies have shown that anticancer agents produce deleterious side effects by damaging normal cells.9,11 So therefore use of ACE Inhibitors and ARBs can be efficient adjuvants in anticancer therapy with a propensity for less toxicity. A few studies report that ACEI and ARBs induce cancer12 and some studies have reported they exhibit anticancer property3. The present study aims to demonstrate the anticancer activity of the two drugs Ramipril and Olmesartan through MTT assay and cell cycle analysis on human A549 lung cancer cell line. The prevalence of new lung cancer accounts for 13% 13 in the world and In India it accounts for 6.9%.14
The study drug Ramipril is a selective oral Inhibitor of ACE which is currently used to treat blood pressure and cardiovascular disease.15 Latest studies shown that Ramipril inhibits the proliferation of human mesangial cell and regulates cell growth and division.16 This drug cause adverse reaction like cough, dizziness, nausea, vomiting and light headedness. Olmesartan is a selective oral inhibitor of Angiotensin 1 receptor which is also currently used to treat blood pressure and cardiovascular disease.17 It also has shown strong apoptotic activity against HeLa human cell lines.18 This drug causes adverse
reactions like dizziness, weakness and light headedness. Owing to the association of ACEI and ARBs with lung cancer this study was carried to evaluate the anticancer potential of Ramipril and Olmesartan through MTT assay and cell cycle analysis on human A549 lung cancer cell line.
Objective
This study aims to assess the anticancer potency of these two drugs through MTT assay and flow cytometry analysis on human A549 lung cancer cell lines.
Materials and Methods
Chemicals and Reagents
A549 cells were procured from National Centre for Cell Sciences, Pune. Ramipril, Olmesartan, 3- (4, 5-Dimethylthiazol-2-Yl), 2, 5-diphenyl tetrazolium (MTT), Dimethyl sulfoxide (DMSO) and propidium iodide were purchased from sigma aldrich. Fetal bovine serum (FBS), Phosphate-Buffered saline (PBS) was purchased from Gibco, Invitrogen Life Technologies. This study was done in Imrahl labs and VClin Bio lab at central research facility of Sri Ramachandra deemed to be university.
Mtt Assay
The cytotoxicity of the two drugs was performed according to the method of Mossmann19.Briefly, cells (1 × 105/well) were seeded in 24-well plates following which cells were incubated at 370C with 5% CO2 atmosphere. Upon reaching confluence the cells were washed in Phosphate Buffered Saline (PBS) and the medium was changed. Increasing concentrations of both the drugs obtained through serial dilution were added to the different wells and were marked respectively. One of the wells was treated only with the diluent which served as the control. The culture plates were incubated for 24 hrs at 370C with 5% CO2 atmosphere. After incubation, the treatment medium were collected from all the wells and washed with PBS (pH 7.4). 100µl/well (5mg/ml) of 0.5% 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl–tetrazolium bromide (MTT) was added to each well and the cell plate was incubated for 4 hours following which 1ml of DMSO was added in all the wells. This helps in dissolving the insoluble crystalline formazan product for effective absorbance measurement. The absorbance at 570 nm was measured with UV-visible Spectrophotometer using DMSO as the blank and the results were tabulated. Measurements for concentration required for 50% inhibition was noted.
Cell Cycle Analysis Flow Cytometry
Flow cytometry is a quantitative assay was done to measure the DNA content of cells. Cell cycle analysis was performed by method of Del Bino et al.20 This assay was done in VClin Bio lab. Cells were seeded into a 6 well plate 0.5×10⁶ cells/well. Upon reaching 80% confluence the cells were washed with PBS and drugs at different concentrations i.e. 5μM and 10μM and 15µM were added to the A549 cell lines. The treatment medium were collected after Trypsinisation then fixed with 70% ethanol. Then these cells were centrifuged at 1500 RPM then stored at 4ᵒc overnight and incubated for one hour. Then stained with propidium iodide in presence of 1% RNASE at 37ᵒc and analyzed the sample in a flow cytometer. A gate (R1) was drawn to show the total events. The dot plot of flow cytometry was drawn to extrapolate the DNA percentage in phases of cell cycle by setting a gate in the single population (G0/G1/S/G2/M). The emitted fluorescent light of the DNA dye (PI emits fluorescence in FL2 channel) generates an electronic signal that can be recorded as high (FL2H) for the intensity of the staining as well as measured as pulse-area (FL2A) and pulse-width (FL2W) of the samples. In the FL2-width vs FL2-area dot plot, a gate (G0/G1/S/G2/M) was set around the single population. Ten thousand events were acquired and the percentage of DNA content in each cell cycle phase were analyzed using Cell Quest pro software (Becton Dickinson,USA ).
Results
Mtt Assay
The concentrations ranged from 10⁻⁶ to 10mM, IC50 value of Ramipril was 0.012 mM respectively. The cell viability of Ramipril at different concentrations on lung cancer cell line were shown in Table- 1.The IC50 value of Olmesartan was 0.35mM.The cell viability of Olmesartan at different concentrations on lung cancer cell line were shown in fig 1.
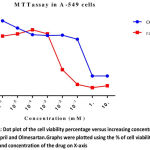 |
Figure 1
|

Flow Cytometry
We performed cell cycle analysis for determining the inhibitory effect of Ramipril and Olmesartan on lung cancer cell lines. Cells were treated with control, Ramipril 5µM, 10 µM, 15 µM and Olmesartan 300 µM. The percentage of cells in the G0/G1, S, G2/M phases was counted by flow cytometry shown in table-2. Ramipril when treated with different concentrations 5µM, 10 µM, 15 µM there was increase in the percentage of G2/M cells to 10%, 11%, 13% shown in fig.2.Similarly Olmesartan at 300 µM percentage of G2/M cells increased to 15% shown in fig.3 when compared with the control. Our results showed that Ramipril and Olmesartan arrested G2/M phase.
 |
Figure 2
|
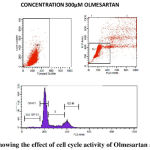 |
Figure 3
|
Discussion
Our study shows the potential antitumor activity of Ramipril and Olmesartan on human A549 lung cancer cell lines using MTT and flow cytometry analysis. From the graph half maximal inhibitory concentration (IC50) was calculated and it proves that Ramipril was more effective than Olmesartan. We found that Ramipril and Olmesartan exhibited half maximum inhibitory concentration (IC50) using MTT at 0.012& 0.35mM. Based on the MTT result we further preceded to flow cytometry analysis. In this assay Ramipril and Olmesartan induced G2/M phase arrest. Cell cycle progression in A549 when exposed to Ramipril at different concentrations i.e. 5, 10 and 15 µM, (figure 2) shifted the population of cells into the G2/M phase from G1 phase. In the control only 6% cells were in G2/M phase when Ramipril exposed to 5µM increased to 10% cells and at 10 and 15 µM increased to 11% and 13% in G2/M phase. There was a reduction in the G1 Phase when compared with the control. The Cell cycle progression in A549 cells, when exposed to Olmesartan at concentration (300µM) a similar increase in G2-M fraction was observed i.e. 15.22% when compared with the control i.e. 9.30% and induced G2/M phase arrest.G2/M cell cycle inhibits the cell entry into the mitotic stage. Ramipril and Olmesartan induced a dose dependent arrest of lung cancer cell lines at G2/M phase with reduced number of cells in the G0/G1 phase. From this study we could say that Ramipril and Olmesartan induced G2/M phase arrest. Further investigations are needed for the role of various proteins involved in the G2/M phase arrest.The antitumor effects were first described in a study by Lever et al3 in which breast cancer incidence decreased in patients receiving ACE inhibitors. Previously Attoub et al21 using MTT and cell cycle analysis showed Captopril inhibited the viability of LNM35 (human lung adenocarcinoma) and induced apoptosis in flow cytometry. Whereas Perindopril 13another ACE inhibitor, showed no direct cytotoxicity (invitro) against tumor cells but inhibited tumor growth in murine hepatocellular cancer cell lines by suppressing VEGF induced angiogenesis.
However kiyoaki et al showed that Telmisartan at 300 µM Angiotensin receptor antagonist inhibited cell viability and induced apoptotic cell death in prostate cells.22,25 Elham et al showed that Olmesartan inhibited cell viability (MTT) and also induced apoptotic cell death in flow cytometry on HeLa and MCF-7 cancer cell lines.18 However there are only limited studies for Ramipril and Olmesartan in lung cancer cell lines. This study demonstrates the antineoplastic activity of Ramipril and Olmesartan, invitro.
Previous studies demonstrated that Olmesartan block the RAS and NF-kappaB pathway18 whereas Ramipril inhibited platelet derived growth factor expression.15 NF-kappaB pathway and platelet derived growth factor is significant in the proliferation of cells. This could probably be the reason for the anticancer property in our study. Nowadays surgery, chemotherapy, radiation, hormones and immunotherapy are the main treatment approaches for the cancer. In the existing treatment methods resistance and toxicity is the serious problems encountered. This work is the first of its kind in demonstrating antineoplastic activity of Ramipril and Olmesartan, invitro. These drugs inhibit the angiogenesis, NF-kappaB pathway, stand as an anticancer drug. Ramipril and Olmesartan inhibited cell viability and also induced cell cycle arrest in A549 cell lines. In our study Ramipril was found to be more effective at lower concentrations than Olmesartan. This will pave way for generating newer drugs with the drugs existing in the market which are safe and effective.
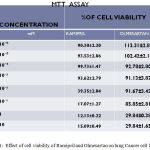 |
Table 1
|
Table 2: Shows the percentage of Ramipril and Olmesartan in different phases of cell cycle at various concentrations 5 µM, 10 µM and 15 µM and 300 µM.
| CONCENTRATION(µM) | GATED CELLS PERCENTAGE | |||
| SUB GO-G1 PHASE | G0-G1 PHASE | S PHASE | G2-M PHASE | |
| Control | 0.26 | 71.21 | 20.28 | 9.90 |
| Ramipril 5 µM | 0.28 | 68.21 | 21.68 | 10.52 |
| Ramipril 10 µM | 0.70 | 66.99 | 21.46 | 11.28 |
| Ramipril 15 µM | 6.51 | 60.91 | 19.97 | 13.24 |
| Olmesartan 300µM | 1.36 | 68.16 | 15.67 | 15.21 |
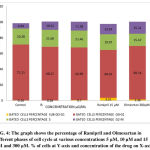 |
Figure 4
|
Limitations
Further confirmatory studies in this line are required to demonstrate the anticancer effect of Ramipril and Olmesartan on A549 lung cancer cell line.
Conclusion
The present study concludes that Ramipril is more efficient than Olmesartan in lung cancer (A549) cell line.
Acknowledgement
The authors are thankful to Department of Pharmacology, Imrahl Labs, VClin bio labs at central research facility of Sri Ramachandra deemed to be university
Conflict of Interest
None
References
- Molteni A, Ward W.F, Ts’ao C.H, Taylor J, Small Jr.W, Brizio-Molteni L et al. Cytostatic properties of some angiotensin I converting enzyme inhibitors and of angiotensin II type I receptor antagonists. Curr Pharm Des. 2003;9:751–761
CrossRef - See S and Stirling A.L. Candesartan cilexetil: an angiotensin II receptor blocker. Am J. Health Syst Pharm. 2000;57:739-746.
- Lever A.F, Hole D.J, Gillis C.R, McCallum I.R, McInnes G.T, MacKinnon P.L, Meredith P.A, Murray L.S, Reid J.L and Robertson J.W. Do inhibitors of angiotensin‑I‑converting enzyme protect against risk of cancer? Lancet . 1998;352:179‑184.
CrossRef - Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16:299.
CrossRef - Fujita M, Hayashi I, Yamashina S, Itoman M and Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis and metastasis. Biochem Biophys Res Commun. 2002;294: 441‑447.
CrossRef - Folkman J, Watson K, Ingber D, Hanahan D. Inductionof angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339: 58–61.
CrossRef - Suganuma T, Ino K, Shibata K, et al. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion,angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005;11:2686–94.
CrossRef - Miyajima A, Kosaka T, Asano T, Asano T, Seta K, Kawai T and Hayakawa M. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002;62:4176-4179.
- Nussbaumer P, Bonnabry J.L,Veuthey F. Sandrine, Analysis of anticancer drugs: a review. Talanta . 2011;85:2265–2289.
CrossRef - Monsuez J.C, Charniot N, Vignat J.Y. Artigou, Cardiac side-effects of cancer chemotherapy. Inter. J. Cardiol. 2010;144:3–15.
CrossRef - Dropcho J. The neurologic side effects of chemotherapeutic agents, Continuum (Minneap Minn.). 2011;17:95–112.
CrossRef - Yoshiji H,Yoshii J, Ikenaka Y, et al. Suppression of the renin-angiotensin system attenuates vascular endothelial growth factor-mediated tumor development and angiogenesis in murine hepatocellular carcinoma cells. J. Oncol. 2002;20:1227–31.
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France. International Agency for Research on Cancer. 2013. [accessed on January 21, 2014]. GLOBOCAN. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available from: http://globocan.iarc.fr. 2012;1:0.
- Indian Council of Medical Research; 2013. [accessed on January 21, 2014]. National Cancer Registry Programme. Three Year Report of Population Based Cancer Registries. 2009-2011.
- Becker R.H.A, Schölkens B.A, Unger T, Linz W. Ramipril: Review of pharmacology. Am J Cardiol. 1987;59:3-11.
CrossRef - Grandaliano G, Ranieri E, Monno T, Gesualdo L, Schena F. Ramipril Inhibits in vitro Human Mesangial Cell Proliferation and Platelet-Derived Growth Factor Expression. Nephrol. 1999;7:229–235.
CrossRef - Redon J, Fabia M.J. Efficacy in angiotensin receptor blockade: a comparative review of data with olmesartan. J. Renin Angiotensin Aldosterone Syst. 2009;10:147–56.
CrossRef - Bakhtiari A, Hosseini M.T, Boroushaki S.H.M. Synergistic, cytotoxic and apoptotic activities of olmesartan with NF-kB inhibitor against HeLa human cell line. Toxicol. Mech. Methods. 2015;2:25:1–8.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol Methods. 1983;65:55-63.
CrossRef - Bino D.Z. Darzynkiewicz C,Degraef R, Mosselmans D, Fokan and Galand P. Comparison of methods based on annexin-V binding, DNA content or TUNEL for evaluating cell death in HL-60 and adherent MCF-7 cells. Cell Proliferation. 1999;32:25–37.
CrossRef - Attoub S, Gaben M.A, Suhail d, Al-Salab M.A.H, Sultana A, John A.b Gary M, Jan N.c, Mester d and Petroianua G. Captopril as a Potential Inhibitor of Lung Tumor Growth and Metastasis. New York Academy of Sciences. 2008;1138:65-72.
CrossRef - Funao K, Matsuyama M, Kawahito Y, Sano H, Chargui J, et al.Telmisartan is a potent target for prevention and treatment in human prostate cancer. Oncol Rep. 2008;20:295–300.
- Funao K, Matsuyama M, Kawahito Y, Sano H, Chargui J, et al. Telmisartan as a peroxisome proliferator-activated receptor-gamma ligand is a new target in the treatment of human renal cell carcinoma. Mol Med Rep. 2009;2:193–198.
- Uemura H, Ishiguro H, Nakaigawa N, Nagashima Y, Miyoshi Y, et al. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Mol Cancer Ther. 2003;2:1139–1147.
- Ishiguro H, Ishiguro Y, Kubota Y, Uemura H . Regulation of prostate cancer cell growth and PSA expression by angiotensin II receptor blocker with peroxisome proliferator-activated receptor gamma ligand like action. J.American association for cancer resea. 2007;67:924–932.







