Omar M. E. Abdel-Salam1 , Amany A. Sleem2, Marawan Abd El-Baset Mohamed Sayed2, Yasser A. Khadrawy3 and Fatma A. Morsy4
, Amany A. Sleem2, Marawan Abd El-Baset Mohamed Sayed2, Yasser A. Khadrawy3 and Fatma A. Morsy4
1Department of Toxicology and Narcotics.
2Department of Pharmacology.
3Department of Medical Physiology.
4Department of Pathology, National Research Centre, Cairo, Egypt.
Corresponding Author E-mail: omasalam@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1480
Abstract
The effect of Cannabis sativa extract on chemical kindling induced in rats by the repeated intraperitoneal (ip) injections of pentylenetetrazole (PTZ) was studied. Rats were treated with PTZ (35 mg/kg) once every 48 hours for 12 times alone or with ip Cannabis sativa (20 mg/kg expressed as Δ9-THC content) 30 min prior to PTZ injection. Seizures were recorded for 20 minutes. Control rats received ip saline. Cannabis treatment caused significant elevation of mean seizure score as compared to PTZ only group after the 5th, 6th and 7th PTZ repeated injections during seizure development. In particular, cannabis caused significant elevation in the frequency of myoclonic jerks, rearing (stage 3), turn over onto one side position and back position (stage 4), and generalized tonic-clonic seizures (stage 5) compared with the PTZ only group. PTZ caused significant elevations in brain lipid peroxidation (malondialdehyde), and nitric oxide along with deceased reduced glutathione level. In addition, brain acetylcholinesterase (AChE) activity significantly decreased compared to control value after PTZ treatment. Cannabis given to PTZ treated rats caused significant increase in brain malondialdehyde and AChE activity compared to PTZ only group. Reduced glutathione level was restored by cannabis. Histopathological studies indicated the presence of spongiform changes, degenerated and necrotic neurons, inflammatory cells, and gliosis in cerebral cortex and degeneration of some Purkinje cells in the cerebellum in both PTZ- and cannabis-PTZ-treated groups. It is concluded that in an epilepsy model induced by repeated PTZ administration, cannabis increased lipid peroxidation and mean seizure score.
Keywords
Cannabis Extract; Kindling; Neurodegeneration; Oxidative Stress; Pentylenetetrazole
Download this article as:| Copy the following to cite this article: Abdel-Salam O. M. E, Sleem A. A, Sayed M. A. E. M, Khadrawy Y. A, Morsy F. A. Cannabis Sativa Increases Seizure Severity and Brain Lipid Peroxidation in Pentylenetetrazole-Induced Kindling in Rats. Biomed Pharmacol J 2018;11(3). |
| Copy the following to cite this URL: Abdel-Salam O. M. E, Sleem A. A, Sayed M. A. E. M, Khadrawy Y. A, Morsy F. A. Cannabis Sativa Increases Seizure Severity and Brain Lipid Peroxidation in Pentylenetetrazole-Induced Kindling in Rats. Biomed Pharmacol J 2018;11(3). Available from: http://biomedpharmajournal.org/?p=22576 |
Introduction
Cannabis sativa is well known for its recreational uses.1 It is the most widely used illicit drug, being abused by about 183 million people World-wide in 2014.2 The psychotropic effects of cannabis are due to its main cannabinoid compound that is delta-9-tetrahydrocannabinol (D9-THC).3 Over 120 cannabinoids, a C21 terpenophenolic compounds have been identified in the plant4 and amongst them cannabidiol, Δ9-tetrahydrocannabivarin, and cannabidivarin are the most studied.5 These have different pharmacological actions from D9-THC and might even antagonize some of its actions.6-9 Cannabinoids act on at least two types of G protein coupled receptors; CB1 receptor predominantly expressed in the central nervous system and CB2 receptor predominantly expressed on immune cells in the periphery.10
Only recently, cannabis and cannabinoid-based medicines have come to attention as a remedy for different medical conditions. The oromucosal spray Sativex, composed of whole plant extract containing both D9-THC and cannabidiol (CBD) [THC:CBD=1:1] is being used for the treatment of spasticity, neuropathic pain and bladder dysfunction in multiple sclerosis.11 Dronabinol and nabilone are two oral formulations of a synthetic THC approved for treatment of nausea and vomiting due to chemotherapy and that refractory to conventional antiemetic therapy as well as weight loss associated with HIV infection and cancer.12,13 Medicinal cannabis is also being used for a variety of medical conditions including chronic pain, fibromyalgia, depression, arthritis, neuropathy14-16 and inflammatory bowel disease.18 The use of cannabis is also frequent among epileptic patients and in one study 20.3% of patients reported using cannabis after the diagnosis of epilepsy being made.19
The brain tissue is vulnerable to oxidative damage. The high rate of oxygen consumption and the presence of redox active metals such as Fe++ and Cu++ account for an increased generation of reactive oxygen/nitrogen metabolites. Meanwhile, the brain is rich in polyunsaturated fatty acids, the target of these reactive species. On the other hand, there are limited antioxidant mechanisms compared with other organs.20,21 It is not surprising therefore that oxidative stress is a major contributing pathogenetic factor in neurodegenerative disorders such as Parkinson’s disease,22 Alzheimer’s disease,23Huntington’ disease24 and Autism.25 Oxidative stress has also been implicated in the development of epileptic seizures and/or neuronal damage in epilepsy.26-30 Moreover, in experimental models of epilepsy, the administration of antioxidants eg., caffeine or ascorbic acid were shown to ameliorate lipid peroxidation in the brain tissue and the development of epileptic seizures.31-34
In this study, the effect of Cannabis sativa extract was examined on brain oxidative stress and epileptic seizures induced by the repeated administration of pentylenetetrazole (PTZ) in rats. kindling caused by PTZ, a GABA(A) receptor antagonist is a clinically relevant model of human epilepsy.35
Materials and Methods
Animals
The study was conducted on male Sprague-Dawley rats weighing 180-200 g. Rats were group-housed under temperature- and light-controlled conditions and allowed standard laboratory rodent chow and water ad libitum. The experiments were done at 9 O’clock to avoid changes in circadian rhythm. The study was done in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985) and the institutional ethics committee. Seven to eight rats were used per group.
Drugs and Chemicals
Pentylenetetrazole (PTZ) was purchased from Sigma (St. Louis, USA). Cannabis sativa resin (hashish) was kindly provided by the Ministry of Justice of Egypt. Extract of Cannabis sativa. was obtained by chloroform treatment, and contained 20% of D9-THC as determined by GC mass. The method of extraction was that described by Turner and Mahlberg36 with modification.37
Chemical Kindling
The kindling procedure was induced by repeated ip injection of (35 mg/kg) of pentylenetetrazole (PTZ) (Sigma, St. Louis, USA) every 48 hours. After each PTZ treatment, rats were placed separately under glass funnels, and the appearance of clonic and tonic seizures were recorded during individual observations for 20 minutes. The resultant seizures were classified according to the following scale34:
Stage 0: no response
Stage 1: ear and facial twitching
Stage 2: convulsive waves through the body
Stage 3: myoclonic jerks, rearing
Stage 4: turn over onto one side position
Stage 5: turn over onto back position, generalized tonic-clonic seizures
Study Design
Rats were randomly divided into three groups (7-8 rats each). Rats were treated with 0.9% saline (group 1), PTZ at 35 mg/kg, ip, once every 48 hours for 12 times alone (group 2: control postive) with saline or with ip Cannabis sativa at 20 mg/kg (expressed as Δ9-THC content) 30 min prior to PTZ injection (group 3). Seizures were recorded for 20 minutes. Two hour after the last PTZ injection, rats were quickly euthanized by decapitation; their brains removed on ice cold glass plate, stored at -80C until the biochemical assays. One half of each brain was kept in 10% formol saline for histopathological processing.
Biochemical Assays
Determination of Lipid Peroxidation
Malondialdehyde (MDA), a product of lipid peroxidation was determined in tissue homogenates according to the method of Nair and Turne.38 In this assay thiobarbituric acid reactive substances (TBA) react with thiobarbituric acid to form TBA-MDA adduct which can be measured colorimetrically at 532 nm.
Determination of Reduced Glutathione
Reduced glutathione (GSH) was determined in tissue homogenates using the procedure of Ellman et al.39 The assay is based on the reduction of Ellman´s reagent (DTNB; 5, 5’-dithiobis (2-nitrobenzoic acid)) by the free sulfhydryl group on GSH to form yellow colored 5-thio-2-nitrobenzoic acid which can be determined using spectrophotometer at 412 nm.
Determination of Nitric Oxide
Nitric oxide was determined using colorimetric assay where nitrate is converted to nitrite via nitrate reductase. Griess reagent then act to convert nitrite to a deep purple azo compound that can be determined using spectrophotometer.40
Determination of Acetylcholinesterase Activity
The procedure used was a modification of the method of Ellmanet al.41 as described by Gorunet al.42 The principle of the method is the measurement of the thiocholine produced as acetylthiocholine is hydrolyzed. The color was read immediately at 412 nm.
Statistical analysis
Results are expressed as mean ± SEM. The results of the biochemical assays analyzed using One Way ANOVA and Duncan’s multiple range test while the results of behavioral study were analyzed by Mann-Whitney using Graphpad Prism software, version 5 (inc., San Diego, USA). A probability value of less than 0.05 was considered statistically significant.
Results
Brain oxidative stress
Rats treated with PTZ exhibited significantly higher malondialdehyde (43.7% increase: 27.31 ± 1.2 vs. 19.0 ± 0.89; p<0.05) and nitric oxide values (65.8% increase: 34.5 ± 1.5 vs. 22.0 ± 1.3; p<0.05) compared to the saline control group. In addition, brain reduced glutathione significantly decreased by 23.4% (p<0.05) (4.9 ± 0.35 vs. 6.4 ± 0.21) with respect to control group after PTZ treatment.
Rats treated with PTZ + Cannabis sativa exhibited higher malondialdehyde concentrations in brain than those of PTZ only treated group (46.1% increase: 39.9 ± 1.45 vs. 27.31 ± 1.32; p<0.05). Nitric oxide was unchanged whereas brain reduced glutathione was increased by 25% (p<0.05) (6.13 ± 0.33 vs. 4.9 ± 0.35).
Brain acetylcholinesterase activity
In PTZ only treated rats, brain AChE activity significantly decreased by 47.5% (p<0.05) compared to the control value (1.68 ± 0.14 vs. 3.2 ± 0.18). Cannabis given to PTZ treated rats resulted in significant increase in AChE activity by %61.9% (p<0.05) compared to PTZ only group (2.72 ± 0.11 vs. 1.68 ± 01.4).
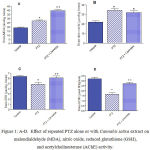 |
Figure 1: A-D. Effect of repeated PTZ alone or with Cannabis sativa extract on malondialdehyde (MDA), nitric oxide, reduced glutathione (GSH), and acetylcholinesterase (AChE) activity.
|
*: p< 0.05 vs. vehicle-treated group +: p< 0.05 vs. PTZ control group.
Kindling
Compared with the PTZ only group, treatment with Cannabis sativa caused significant elevation of the mean seizure score after the 5th, 6th and 7th PTZ repeated injection during seizure development (Fig. 2). Fig. 3 shows the average mean score over the study period. The mean seizure score was 2.52 ± 0.26 in the PTZ only group and 3.05 ± 0.24 in the PTZ + cannabis group (21% increase: p<0.05).
Fig. 4 shows that cannabis extract significantly elevated seizure frequency of myoclonic jerks, rearing (stage 3); turn over onto one side position; turn over onto back position (stage 4), and generalized tonic-clonic seizures (stage 5) by 62.5%, 125.1% and 166.7%, respectively, as compared to the positive epileptic group. Values are 3.25 ± 0.36 vs. 2.0 ± 0.38 for stage 3, 3.0 ± 0.37 vs. 1.3 ± 0.21 for stage 4 and 2.7 ± 0.33 vs. 1.0 ± 0.00 for stage 5, respectively.
Similarly, the sum of scores of myoclonic jerks, rearing (stage 3); turn over onto one side position; turn over onto back position (stage 4), and generalized tonic-clonic seizures (stage 5) displayed significant increments by 62.5%, 125.1% and 166.7% compared to the positive epileptic group, respectively (Fig. 5). Values are 9.8 ± 0.75 vs. 6.0 ± 1.13 for stage 3, 12 ± 1.46 vs. 5.3 ± 0.84 for stage 4 and 13.3 ± 1.67 vs. for stage 5, respectively.
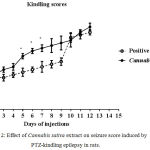 |
Figure 2: Effect of Cannabis sativa extract on seizure score induced by PTZ-kindling epilepsy in rats.
|
Each point in the line represents the mean ± S.E. of 7-8 experiments. Data were analyzed by Mann-Whitney. * P <0.05 vs. positive control.
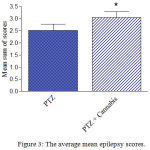 |
Figure 3: The average mean epilepsy scores.
|
Rats were injected with i.p. PTZ only (positive control) PTZ + orally given Cannabis sativa extract (30 minutes before PTZ). Data represents mean ± S.E. of 7-8 experiments. Data were analyzed by Mann-Whitney. * P <0.05 vs. positive control.
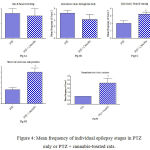 |
Figure 4: Mean frequency of individual epilepsy stages in PTZ only or PTZ + cannabis-treated rats.
|
Each bar represents mean ± S.E. of 7-8 experiments. Data were analyzed by Mann-Whitney. * P <0.05 vs. positive control.
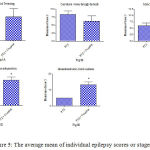 |
Figure 5: The average mean of individual epilepsy scores or stages.
|
Rats were injected with i.p. PTZ only (positive control) PTZ + orally given Cannabis sativa extract (30 minutes before PTZ). Data represents mean ± S.E. of 7-8 experiments. Data were analyzed by Mann-Whitney. *P<0.05 vs. positive control.
Histopathology
Sections from the brain of saline-treated rats shows normal histological structure of the cerebral cortex (Fig. 6A) and cerebellum (Fig. 6B). The cerebral cortex of rats treated with only PTZ exhibited spongiform changes, congestion of cerebral blood vessels, and hemorrhage in meninges above the surface. There were sings of degeneration and necrosis in some neurons and gliosis. Affected neurons become shrunken and eosinophilic. The nuclei became condensed and lost their crisp contours . Degeneration of Purkinje cells was observed (Fig. 6C & 5D). Rats treated with PTZ and along with cannabis. Rats treated with PTZ along with cannabis showed no improvement in pathological changes. Examination of the cerebral cortex revealed congestion of cereberal blood vessels and the presence of inflammatory cells. Affected neurons were shrunken and eosinophilic with gliosis (Figs 6E & 5F). In the cerebellum there was degeneration of some Purkinje cells after PTZ injections (Fig. 6G). Following both PTZ and cannabis, the cerebellum showed degeneration of Purkinje cells and also thinning of the granular layer (Fig. 6H).
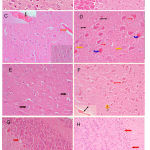 |
Figure 6: Hx & Ex stained sections. (A) Cerebral cortex: control rat shows normal histological structure. (B) Cerebellum: control rat shows normal histology. (C) Cerebral cortex: PTZ only showing congestion of cerebral blood vessel (red arrow), hemorrhage in meninges above the surface (black arrow).
|
The granular layer cells have large vesicular nuclei with well defined nucleolus, while pyramidal cells are smaller in size with gliosis (right of figure). (D) Cerebral cortex: PTZ only showing spongiform changes in cerebral cortex, eosinophilic neuron (blue arrow), necrotic neuron (yellow arrow) and gliosis (red arrow). (E) Cerebral cortex: PTZ along with cannabis 20 mg/kg showing granular layer cells having large vesicular nuclei with well defined nucleolus (black arrow), while pyramidal cells are smaller in size with gliosis (red arrow). (F) Cerebral cortex: PTZ along with cannabis 20 mg/kg showing no improvement in histological changes in the form of congestion of cerebral blood vessel (black arrow). Affected neurons become shrunken and eosinophilic (red arrow). There are inflammatory cells (yellow arrow), and hemorrhage in meninges above the surface (black arrow). (G) Cerebellum: only PTZ showing a degeneration of some Purkinje cells (red arrow) (Hx & Ex 400). (H) Cerebellum: PTZ along with cannabis 20 mg/kg showing degeneration of Purkinje cell (red arrow) and thinning of the granular layer (Hx & Ex 200).
Discussion
The results of the present study indicates that treatment with Cannabis sativa resin extract rich in D9-THC caused significant elevation of mean seizure scores in rats receiving PTZ, thereby, suggesting that cannabis increases the susceptibility for epileptic seizures. The administration of PTZ resulted in increased brain oxidative stress as indicated by the increase in the lipid peroxidation product malondialdehyde,21 thereby, suggesting the increased production of reactive oxygen metabolites. The presence of oxidative stress and the increase in brain malondialdehyde in brain of rats treated with PTZ or other epileptogenic agents eg., pilocarpine has been reported previously.30,43-45 Studies also indicated increased lipid peroxidation in plasma of children with epilepsy and nuclear magnetic resonance changes.26 Our results also show depletion of reduced glutathione by PTZ treatment. Glutathione, a tripeptide of l-glutamate, l-cyseine and l-glycine is the brain’s most important antioxidant and free radical scavenger. The cysteine thiol moiety accounts for the antioxidant action of reduced glutathione and is oxidized by free radicals in the cell to oxidized GSH disulfide (GSSG)46 and the ratio of its reduced (GSH)/oxidized (GSSG) form greatly determines the cellular redox state.46,47 The decrease in reduced glutathione in the brain of PTZ-treated rats thus occurs most probably as a result its consumption by the increased formation of reactive oxygen metabolites. Other studies found a significant decrease in GSH levels and in Cu,Zn-Superoxide dismutase and catalase activities in erythrocytes after PTZ injection in rats.27 Significantly decreased GSH/water ratio was also found in the parietooccipital region of epileptic patients independently from seizure activity.48
The present findings indicate markedly increased brain nitric oxide content following PTZ injections which is in accordance with other published data in kainic acid- or PTZ-induced seizures.49,50 The increase in nitric oxide of neuronal origin during seizure development has been implicated in the initiation of seizure-like events51 and in causing endoplasmic reticulum stress and peroxynitite (ONOO-)-mediated oxidative/nitrosative damage.45 Moreover, inhibition of neuronal nitric oxide synthase was shown to inhibit convulsive seizures caused by PTZ in rats.50 It is thought that this increase in nitrosative stress is involved in neurodegeneration seen in epilepsy.32,52
Our results shows in addition markedly depressed brain AChE activity in rats receiving PTZ. This inhibitory effect of PTZ on AChE activity has been reported in crude homogenates of rat brain53 and an increase in cholinergic activity is linked to seizure initiation by the agent.54 Acetylcholine release increases in the epileptic rat brain55 and seizures could be induced in rodents by cholinergic agents like pilocarpine30,56 and organophosphorus nerve agents.57 Abnormal AChE immunostaining and loss of AChE fibers have been demonstrated in some regions in specimens from temporal lobe epileptics.58 Moreover, neuronal nicotinic receptor mutations have been implicated in autosomal dominant nocturnal frontal lobe epilepsy.59 These data suggests a role for increased neuronal cholinergic activity in epileptogenesis.
In this study, the effect of Cannabis sativa resin extract rich in D9-THC on the development of brain oxidative stress and seizures due to PTZ was examined. Cannabis was shown to increase seizure severity. Other studies showed increased kindling due to PTZ, picrotoxin or electroshock by D9-THC in mice.60 Recently, Malyshevskata et al.61 reported the development of electrographic seizures in mice by administering D9-THC (10 mg/kg) or the synthetic cannabinoid agonist JWH-018 with the effect being mediated by CB1 receptors. In humans, convulsions have been reported after the use of synthetic cannabinoids.62,63 We also found that treatment with cannabis resin was associated with an increase in brain malondialdehyde. Nitric oxide was unchanged but there was restoration of brain reduced glutathione by cannabis. This latter observation is intriguing for it suggests that the mere increased in reduced glutathione was not sufficient to inhibit lipid peroxidation in this model of brain damage. Moreover, our results show that the administration of cannabis resin increased brain AChE activity which is in accordance with previously published data.37 Finally, we observed that cannabis did not decrease neuronal damage caused by repeated PTZ injections.
Cannabis is a complex mixture of over 600 different compounds and beside terpenophenolic cammabinoids there are non-cannabinoid phenols, flavonoids eg, flavocannabiside and flavosativaside, flavonol glycosides such as kaempferol 3-O-sophoroside and quercetin 3-Osophoroside, and fatty acids.64,65 Concerning cannabinoids, these differ in their receptor pharmacology with D9-THC acting as a partial agonist of CB1 and CB2 receptors, while cannabidiol and D9-tetrahydrocannabivarin exhibits antagonistic effects.7,9,66 Moreover, cannabigerol exerts alpha2-adrenoceptor agonist and 5HT1A receptor antagonist activities.67 These facts makes the effect of the whole plant extract different from that of only D9-THC.68,69 In this study, how
ever, D9-THC was the major cannabinoid identified in the extract, making it the most likely constituent that accounted for the observed effects of cannabis in this model of epilepsy.
In summary, the present study demonstrates that the use of Cannabis sativa resin extract rich in D9-THC results in increased sensitivity to PTZ-induced seizures.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interests
The authors declare that there are no conflicts of interest.
References
- Huestis M. A. Cannabis (Marijuana)—effects on human behavior and performance. Forensic Sci Rev. 202;14:15.
- Market analysis of plant-based drugs-Opiates, cocaine, cannabis. United Nations Office on Drugs and Crime, World Drug Report. United Nations publication, Sales No. E.17.XI.9, Vienna, Austria. 2017:37-45.
- Mechoulam R., Gaoni Y. The absolute configuration of delta-1-tetra hydrocannabinol the major active constituent of hashish. Tetrahedron Lett. 1967;12:1109-11.
CrossRef - Morales P., Hurst D. P., Reggio P. H. Molecular Targets of the Phytocannabinoids-A Complex Picture Prog. Chem Org Nat Prod. 2017;103:103–131.
CrossRef - ElSohly M. A., Radwan M. M., Gul W., Chandra S., Galal A. Phytochemistry of Cannabis sativa L. In: Kinghorn A. D., Falk H., Gibbons S., Kobayashi J (eds). Phytocannabinoids Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Progress in the Chemistry of Organic Natural Products. Switzerland, Springer International Publishing. 2017;1-36.
- Pertwee R. G., Thomas A., Stevenson L. A., Ross R. A., Varvel S. A., Lichtman A. H., Martin B. R., Razdan R. K. The psychoactive plant cannabinoid, Delta9-tetrahydrocannabinol is antagonized by Delta8- and Delta9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150(5):586-94.
CrossRef - Thomas A., Stevenson L. A., Wease K. N., Price M. R., Baillie G., Ross R. A., Pertwee R. G. Evidence that the plant cannabinoid Delta9-tetrahydrocannabivar in is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146(7):917-926.
CrossRef - Cascio M. G., Gauson L. A., Stevenson L. A., Ross R. A., Pertwee R. G. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159(1):129-141. doi: 10.1111/j.1476-5381.2009.00515.x.
CrossRef - Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 2007;150(5):613-623.
CrossRef - Svíženská I., Dubový P., Šulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures —A short review. Pharmacol Biochem Behav. 2008;90(4):501-11. doi: 10.1016/j.pbb.2008.05.010.
CrossRef - Meuth S. G., Vila C., Dechant K. L. Effect of Sativex on spasticity-associated symptoms in patients with multiple sclerosis. Expert Rev Neurother. 2015;15(8):909-18. doi: 10.1586/14737175.2015.1067607.
CrossRef - Seamon M. J., Fass J. A., Maniscalco-Feichtl M., Abu-Shraie N. A. Medical marijuana and the developing role of the pharmacist. Am J Health Syst Pharm. 2007;64(10):1037-44.
CrossRef - Maida V., Daeninck P. J. A user’s guide to can nabinoid therapies in oncology. Curr Oncol. 2016;23(6):398-406. doi: 10.3747/co.23.3487.
CrossRef - Ware M. A., Adams H., Guy G. W. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59:291–295.
CrossRef - Aggarwal S. K., Carter G. T., Sullivan M. D., ZumBrunnen C., Morrill R., Mayer J. D. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J Opioid Manag. 2009;5:257-86.
CrossRef - Fiz J., Durán M., Capellà D., Carbonell J., Farré M. Cannabis use in patients with fibromyalgia: effect on symptoms relief and health-related quality of life. PLoS One. 2011;6:e18440.
CrossRef - Lal S.,Prasad N.,Ryan M.,Tangri S.,Silverberg M. S.,Gordon A., Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891–896.
CrossRef - Hamerle M., Ghaeni L., Kowski A., Weissinger F., Holtkamp M. Cannabis and other illicit drug use in epilepsy patients. Eur J Neurol. 2014;21(1):167-70. doi: 10.1111/ene.12081.
CrossRef - Floyd R. A. Antioxidants ox idative stress and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245.
CrossRef - Sies H., Jones D. P. Oxidative stress. In Encyclopaedia of stress. Ed Fink G., Diego S. Elsevier. 2007;45-48.
CrossRef - Drechsel D. A., Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med. 2008;44:1873–1886.
CrossRef - Jiang T., Sun Q., Chen S. Oxidative stress A major pathogenes is and potential therapeutic target of anti oxi dative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol. 2016;147:1-19. doi: 10.1016/j.pneurobio.2016.07.005.
CrossRef - Túnez I., Sánchez-López F., Agüera E., Fernández-Bolaños R., Sánchez F. M., Tasset-Cuevas I. Important role of oxidative stress biomarkers in Huntington’s disease. Med. Chem. 2011;54(15):5602–5606.
CrossRef - Abdel-Salam O. M. E., Youness E. R., Mohammed N. A., Elhamed A. W.A. Nuclear factor-kappa B and other oxidative stress biomarkers in serum of autistic children. Open J Mol Integ Physiol. 2015;5:18‒27.
CrossRef - Turkdogan D., Toplan S., Karakoc Y. Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. Child Neurol. 2002;17:673-676.
CrossRef - Akbas S. H., Yegin A., Ozben T. Effect of pentylenetetrazol-induced epileptic seizure on the antioxidant enzyme activities, glutathione and lipid peroxidation levels in rat erythrocytes and liver tissues. Clin Biochem. 2005;38(11):1009-14.
CrossRef - Patsoukis N., Zervoudakis G., Georgiou C. D., Angelatou F., Matsokis N. A., Panagopoulos N. T. Thiol redox state and lipid and protein oxidation in the mouse striatum after pen tylenetetrazol-induced epileptic seizure. Epilepsia. 2005;46(8):1205–1211.
CrossRef - Sobaniec W., Solowiej E., Kulak W., Bockowski L., Smigielska-Kuzia J., Artemowicz B. Evaluation of the influence of anti epileptic therapy on antioxidant enzyme activity and lipid peroxidation in erythrocytes of children with epilepsy. J Child Neurol. 2006;21:558–562. DOI 10.2310/7010.2006.00115.
- Freitas R. M. Investigation of oxidative stress involvement in hippo campus in epilepsy model induced by pilocarpine. Neurosci Lett. 2009;462(3):225-229. doi: 10.1016/j.neulet.2009.07.037.
CrossRef - Ayyildiz M., Coskun S., Yildirim M., Agar E. The effects of ascorbic acid on penicillin-induced epileptiform activity in rats. Epilepsia. 2007;48(7):1388–1395.
CrossRef - Mishra V., Shuai B., Kodali M., Shetty G. A., Hattiangady B., Rao X., Shetty A. K. Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenes is with suppression of oxidative stress and inflammation. Sci Rep. 2015;5:17807. doi: 10.1038/srep17807.
CrossRef - de Oliveira C. C., de Oliveira C. V., Grigoletto J., Ribeiro L. R., Funck V. R., Grauncke A. C., de Souza T. L., Souto N. S., Furian A. F., Menezes I. R., Oliveira M. S. Anticonvulsant activity of β-caryophyllene against pentylenetetrazol-induced seizures. Epilepsy Behav. 2016;56:26-31. doi: 10.1016/j.yebeh.2015.12.040.
CrossRef - Sefil F., Kahraman I., Dokuyucu R., Gokce H., Ozturk A., Tutuk O., Aydin M., Ozkan U., Pinar N. Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp Med. 2014;7(9):2471-7. Collection. 2014.
- Dhir A. Pent ylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci. 2012. Chapter 9:Unit 9. 37. doi: 10.1002/0471142301.ns0937s58.
CrossRef - Turner J. C., Mahlberg P. G. Separation of acid and neutral cannabinoids in Cannabis sativa L. using HPLC. In: Agurell S, Dewey WL, Willete RE, editors.Chemical pharmacol ther agents. USA: Academic Press. 1984;79-88.
- Abdel-Salam O. M. E., Youness E. R., Khadrawy Y. A., Sleem A. A. Ace tylcholinesterase, but yrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis tramadol or both. Asian Pac J Trop Med. 2016;9(11):1066-1071.
CrossRef - Nair V., Turner G. A. The thiobarbituric acid test for lipid peroxidation: structure of the adduct with malondialdehyde. Lipids 1984;19:804-805.
CrossRef - Ellman G. L. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70-77.
CrossRef - Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993;7(2):340-360.
CrossRef - Ellman G. L., Courtney K. D., Andreas V Jr., Feather-Stone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm. 1961;7:88-90.
CrossRef - Gorun V., Proinov I., Baltescu V., Balaban G., Barzu O. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparation. Anal Biochem. 1978;86:324-326.
CrossRef - Frantseva M.V., Velazquez P. J. L., Tsoraklidis G., Mendonca A. J., Adamchik Y., Mills L. R., Carlen P. L., Burnham M. W. Oxidative stress is involved in seizure-induced neurodegeration in the kindling model of epilepsy. Neuroscience. 2000;97(3):431-5.
CrossRef - Pence S., Erkutlu I., Kurtul N., Bosnak M., Alptekin M., Tan U. Antiepileptogenic effects of glutathione against increased brain ADA in PTZ-induced epilepsy. Int J Neurosci. 2009;119(5):616-29. doi: 10.1080/00207450802055440.
CrossRef - Zhu X., Dong J., Han B., Huang R., Zhang A., Xia Z., Chang H., Chao J and Yao H. Neuronal nitric oxide synthase contributes to PTZ kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front Cell Neurosci. 2017;11:377. doi: 10.3389/fncel.2017.00377. e Collection 2017.
CrossRef - Dringen R. Metabolism and functions of glut a thione in brain. Progress in Neurobiology. 2000;62:649-671.
CrossRef - Wu G., Fang Y. Z., Yang S., Lupton J. R., Turner N. D. Glut athione metabolism and its implications for health. J Nutr. 2004;134:489 – 492.
CrossRef - Mueller S. G., Trabesinger A. H., Boesiger P., Wieser H. G. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57(8):1422-7.
CrossRef - Mülsch A., Busse R., Mordvintcev P. I., Vanin A. F., Nielsen E. O., Scheel-Krüger J., Olesen S. P. Nitric oxide promotes seizure activity in kainate-treated rats. Neuroreport. 1994;5(17):2325-8.
CrossRef - Watanabe M., Miyai A., Danjo S., Nakamura Y., Itoh K. The threshold of pentylenetetrazole-induced convulsive seizures,but not that of non convulsive seizures is controlled by the nitric oxide levels in murine brains. Exp Neurol. 2013;247:645–652.
CrossRef - Kovács R., Rabanus A., Otáhal J., Patzak A., Kardos J., Albus K., Heinemann U., Kann O. Endogenous nitric oxide is a key promoting factor for initiation of seizure-like events in hippo campal and entorhinal cortex slices. J Neurosci. 2009;29(26) 8565-8577. DOI: https://doi.org/10.1523/JNEUROSCI.5698-08.2009.
CrossRef - Rowley S., Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62;121–131. 10.1016/j.freeradbiomed.2013.02.002.
CrossRef - Mahon P. J., Brink J. J. Inhibition of acetylcholinesterase in vitro by pentylenetetrazol. J Neurochemist. 1970;17(7):949–953. DOI: 10.1111/j.1471-4159.1970.tb02248.x
CrossRef - Meilleur S., Aznavour N., Descarries L., Carmant L., Mamer O. A., Psarropoulou C. Pen tylenetetrazol-induced seizures in immature rats provoke long-term changes in adult hippo campal cholinergic excitability. Epilepsia. 2003;44(4):507-17.
CrossRef - Coutinho-Netto J., Boyar M., Bradford H. F., Birdsall N. J., Hulme E. C. cetylcholine release and muscarinic receptors in cortical synaptosomes from epileptic rats. Exp Neurol. 1981;74(3):837-46.
CrossRef - Turski L., Ikonomidou C., Turski W. A., Bortolotto Z. A., Cavalheiro E. A. Review cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine a novel experimental model of intractable epilepsy. Synapse. 1989;3(2):154-71.
CrossRef - Acon-Chen C., Koenig J. A., Smith G. R., Truitt A. R., Thomas T. P., Shih T. M. Evaluation of acetylcholine seizure activity and neuro pathology following high-dose nerve agent exposure and delayed neuroprotective treatment drugs in freely moving rats. Toxicol Mech Methods. 2016;26(5):378-88. doi: 10.1080/15376516.2016.1197992.
CrossRef - Green R. C., Blume H. W., Kupferschmid S. B., Mesulam M. M. Alterations of hippo campal ace tylcholinesterase in human temporal lobe epilepsy. Ann Neurol. 1989;26(3):347–351.
CrossRef - Raggenbass M., Bertrand D. Nicotinic receptors in circuit excitability and epilepsy. J Neurobiol. 2002;53:580–589.
CrossRef - Karler R., Calder L. D., Sangdee P., Turkanis S. A. Interaction between delta-9-tetrahydrocannabinol and kindling by electrical and chemical stimuli in mice. Neuro pharmacology. 1984;23(11):1315-20.
CrossRef - Malyshevskaya O., Aritake K., Kaushik M. K., Uchiyama N., Cherasse Y., Kikura-Hanajiri R., Urade Y. Natural (∆9-THC) and synthetic (JWH-018) can nabinoids induce seizures by acting through the can nabinoid CB1 receptor. Sci Rep. 2017;7(1):10516. doi: 10.1038/s41598-017-10447-2.
CrossRef - Schep L. J., Slaughter R. J., Hudson S., Place R., Watts M. Delayed seizure-like activity following analytically confirmed use of previously unreported synthetic cannabinoid analogues. Hum Exp Toxicol. 2015 ;34(5):557-60. doi: 10.1177/0960327114550886.
CrossRef - Schneir A. B., Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol. 2012;8(1):62-4. doi: 10.1007/s13181-011-0182-2.
CrossRef - ElSohly M. A., Slade D. D. Chemical constituents of marijuana: The complex mixture of natural can nabinoids. Life Sci. 2005;78(5):539-48.
CrossRef - Brenneisen R. Chemistry and analysis of phytocannabinoids and other cannabis constituents. In ElSohly M. A (ed). Forensic science and medicine: marijuana and the can nabinoids. Totowa NJ: Humana Press Inc. 2006;17-49.
- Pertwee R. G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids delta 9-tetrahydrocannabinol cannabidiol and delta 9-tetrahydrocannabivar in. Br J Pharmacol. 2008;153(2):199-215.
CrossRef - Cascio M. G., Gauson L. A., Stevenson L. A., Ross R. A., Pertwee R. G. Evidence that the plant can nabinoid can nabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159:129-141.
CrossRef - Russo E. B., McPartland J. M. Cannabis is more than simply D 9-tetra hydrocannabinol. Psychopharmacology. 2003;165:431-432.
CrossRef - Hayakawa K., Mishima K., Hazekawa M., Sano K., Irie K., Orito K., Egawa T., Kitamura Y., Uchida N., Nishimura R., Egashira N., Iwasaki K., Fujiwara M. Can nabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157-164.
CrossRef







