Vandana Dhiman1 , Anshita Aggarwal2
, Anshita Aggarwal2 , Sanjay Kumar Bhadada2
, Sanjay Kumar Bhadada2 , Naresh Sachdeva2
, Naresh Sachdeva2 , Nirmal Raj Gopinathan3
, Nirmal Raj Gopinathan3 and D. K. Dhawan1
and D. K. Dhawan1
1Department of Biophysics, Panjab University, Chandigarh, India,
2Departments of Endocrinology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
3Departments of Orthopedics, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Corresponding Author E-mail: bhadadask@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/1417
Abstract
Bisphosphonates (BPs) are widely used for treatment of osteogenesis imperfecta (OI). However, prolonged use may be associated with suppression of bone turnover, the exact molecular mechanism of which is poorly understood. The objective of this study was to evaluate the effect of zoledronic acid (ZOL) on precursor osteoclasts by studying caspase 3 activity. A total of 15 children participated in the study (n = 10 OI patients, n= 5 controls). Out of the 10 OI children, 5 had received a cumulative dose of <30 mg and 5 received > 30 mg of ZOL. Isolated mononuclear cells were studied for caspase 3 activity from all study participants. The mean age of study participants was 7 ±1.5 years. Six of them had OI type IV, two had type III and one had types I & II each. Radiographs showed “zebra stripe sign” and dense metaphyses; suggestive of acquired osteosclerosis. Bone turnover markers (PINP and CTx) were suppressed in all OI patients compared to controls. Caspase-3 activity was significantly increased in precursor osteoclasts cells at higher doses of BPs (>30 mg). Overzealous use of ZOL in OI suppresses bone turnover markers (P1NP, CTx) causes osteosclerosis and increased expression of caspase 3 activity in precursor osteoclasts which results in adynamic bone.
Keywords
Apoptosis; Bisphosphonates; Bone Mass; Fracture; Osteogenesis Imperfecta; Precursor Osteoclast Cells; Zebra Lines
Download this article as:| Copy the following to cite this article: Dhiman V, Aggarwal A, Bhadada S. K, Sachdeva N, Gopinathan N. R, Dhawan D. K. The Impact of Bisphosphonates on the Osteoclast Cells of Osteogenesis Imperfecta Patients. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Dhiman V, Aggarwal A, Bhadada S. K, Sachdeva N, Gopinathan N. R, Dhawan D. K. The Impact of Bisphosphonates on the Osteoclast Cells of Osteogenesis Imperfecta Patients. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20299 |
Introduction
Osteogenesis imperfecta (OI) is a fairly rare disorder (one in 15-20000 births).1, 2 The phenotypic spectrum ranges in severity from mild skeletal phenotypes with few to no fractures, to prenatal lethality. on molecular basis, OI is classified into 12 types.3 OI is characterized by fragility fractures, blue sclera, short stature, and hearing loss.4 Less commonly observed findings in OI are decreased pulmonary function, cardiac valvular regurgitation, chest wall deformities (pectus carinatum, barrel chest) and scoliosis.4
Although about 85-90% of cases are caused by structural or quantitative mutations in the collagen gene, recent studies show that this disorder is majorly due to defective collagen production.5 Among different types of OI, type II & III are relatively uncommon but are extremely aggressive and lethal.4 BPs are mainstay of therapy for OI. They are analogs of pyrophosphate, which inhibit the bone resorption by blocking the key enzyme farnesyl-pyrophosphate.6 In response to inhibition of prenylation of intracellular proteins by BPs, there is ultimately an increase in osteoclast apoptosis.7
BPs can also reduce number of osteoclasts inducing apoptosis in macrophages and osteoclast cells.8 In addition, BPs can directly inhibit the bone resorbing activity of osteoclasts.9 The mechanism by which BPs act directly on osteoclasts and osteoclast precursors has been reported to be partly due to inhibition of the mevalonate pathway.6 There is also a likely indirect effect via osteoblasts which have cellular link with osteoclast cells due to modulation of osteoblast secretion of soluble paracrine factors that influence osteoclast activity.10, 11
BPs, administered to children with OI, have been shown to increase bone volume by counteracting the high turnover cellular status of bone in classic OI.12-14 The new bone still contains defective collagen. The hypothesis behind the treatment is that an increased bone mass (even of impaired quality) leads to moderate reduction in the fracture risk.15 Zoledronic acid (ZOL) enhances osteoblastic activity and differentiation,16 which further increases the bone mass in OI patients.3 It is likely that BPs decrease the fracture rate but increase bone brittleness and also conceding the fact that BPs directly do not affect the defective collagen however they inhibit the bone resorption by osteoclasts.17, 18
The Present study was planned to compare the frequency of caspase 3 expressing cells (suggestive of apoptosis) in precursor osteoclast cells of OI children who received ZOL (high dose vs. low dose) with age-matched controls.
Material and Methods
Study Design
The Present study is a case series of 10 OI patients. The study was conducted from January 2014 to July 2014 at the Postgraduate Institute of Medical Education and Research, Chandigarh, India. Whole blood sample was taken from OI patients after obtaining an informed written consent from the parents of patients. The study was approved by institute ethics committee. Total 15 children participated in the study (n=10 OI patients, n=5 age matched, healthy controls).
Cumulative dose is defined as total dose of ZOL received by the patient. Out of the 10 OI children, 5 had received a low cumulative dose (<30mg) of ZOL while 5 had received a high cumulative dose (> 30 mg) over the last 3 years. The diagnosis of OI was suspected on basis of clinical features and radiological findings and was confirmed by mutational analysis.
Biochemical and Radiological Diagnosis
Total serum calcium, phosphate, alkaline phosphatase, were measured in the hospital laboratory by standard methods (Auto Analyzer Modular P 800; Roche Diagnostics). Plasma 25 hydroxyvitamin D, C terminal telopeptide (CTx) and procollagen type 1 amino terminal propeptide (P1NP) levels were also measured by Elecsys and cobase immunoassay analyzers (ECLIA). The measuring range for CTx as per the package insert was 10-6000 pg/mL with an analytical sensitivity of 10 pg/mL and an intra assay CV of 17.9%. The measuring range for P1NP as per the package insert was 5-1200 ng/mL with an analytical sensitivity of 5 ng/ml and an intra assay CV of 4.1%.
Extractions and Immunostaining of Osteoclast Cells
Sample of whole blood was layered over Ficoll-Hypaque density gradient and direct immunostaining for Peripheral blood mononuclear cells (PBMC) was performed. Mononuclear cells were stained with monoclonal antihuman RANK-PE (9A725, Thermofisher, scientific) and caspase-3 NucView 488 antibodies. Cells were incubated for 30 minutes at room temperature, followed by washing using phosphate buffer saline (PBS) with 2% fetal bovine serum (FBS). The cells were finally acquired using flow cytometer (BD FACS CANTO-II, Becton Dickinson, CA, USA) and data were analyzed using FACS Diva software. Single color tubes were used for compensation. Cells were first gated on the basis of forward and side scatter (P1), followed by gating of RANK positive cells (P2). caspase-3 activity was assessed in these RANK positive precursor osteoclast cells. The frequency of caspase-3 expressing precursor osteoclasts and median fluorescence intensity (MFI) of caspase-3 were compared among subject groups. The OI patient’s demography, biochemistry, radiology & ZOL dose are summarized in Table 1.
Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM). Data was checked for normality using KS test. Normally distributed data was compared using unpaired t test, while skewed data was compared using Mann Whitney test. All statistical analysis were performed using graph pad prism 5.0.
Result
Clinical findings of OI patients
The mean age of study participants (n=10) was 7 ±1.5 years. All (n=10) of them had short stature, blue sclera and 9 had dentinogenesis imperfecta. 6 of them had OI type IV, 2 had type III and one each had types I and II. Biochemical parameters namely, serum calcium were 9.3±0.24 mg/dl, phosphate 4.72±0.2 mg/dl, alkaline phosphatase 295.3±64.31 IU/L, 25-hydroxyvitamin D 31.66±4.32 ng/ml were in the normal range. In comparison to healthy controls, bone turnover markers were significantly suppressed in high and low dose ZOL treated group as given in Table 1.
Table 1a: Clinical characteristics of OI patients with high dose (case 1-5) and low dose (case 6-10)
| Characteristic | Case1 | Case2 | Case3 Case4 Case5 | Case 6 | Case7 Case 8 Case 9 Case 10 | |||||
| Age (Years) | 8 | 8 | 10 7 6 | 8 | 5 9 1 9 | |||||
| Gender | M | M | M F M | M | M M M M | |||||
| Short stature | Yes | Yes | Yes Yes Yes | Yes | Yes Yes Yes Yes | |||||
| Weight (kg) | 9.3 | 10.2 | 9.4 15.0 10.0 | 9.3 | 20 12.6 15.0 12 | |||||
| Blue Sclera | + | + | + | + | + | + | + | + | + | + |
| Dentinogenesis imperfecta | + | + | + | + | + | + | + | + | – | + |
| Number of fractures | 6 | 7 | 5 | 9 | 5 | 7 | 6 | 5 | 2 | 5 |
| Scoliosis | Yes | Yes | Yes | Yes | No | No | Yes | No | No | No |
| Leg deformities | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total received dose | 36 | 68 | 64 | 64 | 40 | <30 | <30 | <30 | <30 | <30 |
| of ZOL (mg) Type of OI | IV | III | IV | IV | VII | IV | IV | IV | I | III |
|
S.ca (8.7-10.2 mg/dl) |
8.4 | 10 | 10.2 | 10.2 | 9.7 | 8.0 | 8.76 | 8.8 | 9.60 | 9.5 |
| S.Po4 (2.7-4.5mg/dl) | 5.4 | 4.2 | 5 | 4.6 | 5.2 | 4.6 | 4.92 | 4.92 | 3.22 | 5.1 |
| ALP (40-129 IU/L) | 217 | 216 | 135 | 167 | 276 | 771.4 | 318.6 | 518.0 | 106 | 212 |
| 25(OH)vit D | 20.22 | 30.07 | 27.33 | 24.62 | 26.25 | 27.03 | 37.01 | 31.06 | 68.43 | 24.62 |
| (11.1-42.9 ng/ml)
CTX (299± 137 pg/ml) |
0.236 | 0.316 | 0.199 | 0.258 | 0.314 | 0.937 | 0.379 | 1.04 | 0.276 | 0.486 |
| P1NP (15.13- 58.5 ng/ml) 129.8 143.5 59.37 116.2 132 460.0 114.4 439 219.2 128 | ||||||||||
Radiological Findings of the OI Patients
Radiographs of the patients on high dose of ZOL showed the presence of “zebra stripe sign”: indicative of cyclical ZOL therapy & also presence of dense metaphyses; suggestive of acquired osteosclerosis (Figure 1). There were various deformities in the bone as well as thickened cortices.
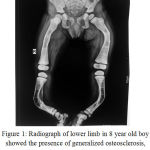 |
Figure 1: Radiograph of lower limb in 8 year old boy showed the presence of generalized osteosclerosis ,Zebra strip sign and dense metaphyses.
|
Induction of Apoptosis in Osteoclasts
We observed that the OI subjects who received high dose of ZOL had significantly increased frequency of caspase-3 expressing precursor osteoclast cells (median; 99.73%) as compared to those who received low dose (median; 53.92%) (p <0.0159), as well as control subjects (median; 35.96%) (p=0.005) (Figure 2a). As expected, the expression of caspase-3 in precursor osteoclasts was also higher in these subjects as compared to the low dose group (p <0.0079) and controls (p <0.005) value control vs high (Figure 2b). Thus, we observed significant difference in caspase-3 expression between the low dose group and high dose group of OI patients.
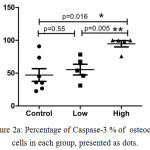 |
Figure 2a: Percentage of Caspase-3 % of osteoclast cells in each group, presented as dots.
|
The results are expressed as the mean ±standard error of the mean (n=5 for each group.*P<0.016 (control vs high dose), P<0.55 (control vs low dose ) and **P<0.005 (Low dose vs high dose)
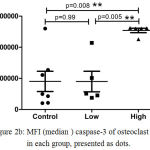 |
Figure 2b: MFI (median) caspase-3 of osteoclast cells in each group, presented as dots.
|
The results are expressed as the mean ±standard error of the mean (n=5 for each group.*P<0.008 (control vs high dose), P<0.99 (control vs low dose ) and **P<0.005 (Low dose vs high dose)
 |
Figure 2c: Representative flow cytometric plots showing the gating strategy for the analysis of RANK+Caspase+ cells.
|
Firstly cells were gated on the basis of FSC and SSC followed by gating of RANK+ cells (P2) which is mainly expressed by osteoclasts cells. Further these osteoclasts cells were analyzed for Caspases activity (P3)
Discussion
We have reported that high dose of ZOL acid caused suppression of bone turnover markers and osteosclerosis of growing skeleton. We also demonstrated radiological changes, namely, presence of dense metaphyses in the patients on high dose which occurred after therapy. We also demonstrated significantly increased expression of caspase-3 activity in osteoclasts isolated from PMBCs of ZOL (>30 mg) treated OI children compared to healthy controls. Among the 10 OI patients, 6 had OI type IV, 2 had type III and one each had types I and II. Out of these, types I and IV are relatively mild, with the patient remaining ambulatory, while types II and III have a more severe phenotype. All patients had fragility fractures, and blue sclera and 9 had dentinogenesis imperfecta. None of the patients had hearing loss. In a study from North India, of 20 OI patients, dentinogenesis imperfecta and blue sclera were seen in 50% of the patients. Similar to our study, all patients had fractures while none of them had hearing loss.19
Recent studies have shown that malocclusion depends upon the severity of the OI type.20 In our study, we found that all patients had dentinogenesis imperfecta. The recommended dose of ZOL for children is 0.05 mg/kg body weight to be repeated every six months21 but as we observed that doses as high as 16 mg per year had been received by our patients. The fact that bone turnover was slowed down was evident by the fact that the bone turnover markers (CTX, P1NP) were suppressed in the OI patients on prolonged ZOL therapy compared to the controls. Similar suppression of bone turnover in OI patients on pamidronate therapy was also shown in a previous study.22 The fact that BPs accumulate in the bone and residual levels are measurable even after years of therapy further raises concern for their safety, particularly in growing skeleton. Hence, drug holidays have been advocated for patients receiving BPs therapy for osteoporosis.23 In present study we have demonstrated that precursor osteoclast apoptosis was increased as shown by high caspases3 positive precursor osteoclast cells and high percentage of apopto precursor osteoclasts in OI patients on ZOL therapy as compared to the control group. In a study by Hughes et al, three BPs (risedronate, pamidronate, and clodronate) were shown to cause a 4- to 24-fold increase in the proportion of apoptotic osteoclasts in vitro.24 Of the three compounds, risedronate, the most potent inhibitor of bone resorption in vivo, was the strongest inducer of osteoclast apoptosis in vitro.24 In another study by Rogers et al, also demonstrated that BPs induce osteoclast apoptosis, in part by inhibiting the activity of enzymes in the mevalonate pathway and promoting caspase cleavage of mammalian sterile 20-like (Mst) kinase 1.25 Since in normal bone remodeling, bone resorption and formation are coupled to each other, this osteoclast apoptosis is expected to ultimately decrease bone formation as well.
This kind of “acquired osteopetrosis” has been previously reported by Whyte et al in a child of OI treated with pamidronate.26 In our study participants the dose of ZOL was more than five times the dose usually prescribed in pediatric population. Though the metaphyseal sclerotic “banding” seen on radiology is an expected finding in patients of OI on cyclic BPs therapy, but it should resolve after completion of therapy. However, it persisted in our patients even after cessation of therapy.
Limitations of study include small sample size and lack of base line data of bone markers for comparison. Nonetheless our study gives a new direction to work upon and warrants detailed studies for looking at mechanistic details.
BPs are known to increase bone mineral density in OI patients,27 however high dose can cause osteopetrosis.26 We have studied caspase3 activity in precursor osteoclasts. The study can be reproduced in a large number of patients, with analysis of entire apoptosis pathway.
Conclusion
Overzealous use of ZOL in OI children suppresses bone turnover markers (CTx, P1NP) and also causes osteosclerosis. High dose ZOL inhibits osteoclastic activity (increased expression of caspase 3 activity) which in turn predisposes to atypical fractures and delayed fracture healing. This is an exploratory study that highlights that the dosing of BPs needs to be standardised with the help of focused clinical trials so that its adverse effects on bones of OI patients can be prevented.
Acknowledgement
The study was supported by the Indian Council of Medical Research Senior Research Fellow award to Ms. V. Dhiman (ICMR SRF Fund # 45/10/2014-HUM-BMS). Mahinder Wadhwa and Ravi Garg for helping in interpretation of flow cytometer result.
Reference
- Stoll C, Dott B, Roth M.P, Alembik Y. Birth prevalence rates of skeletal dysplasias. Clinical genetics. 1989;35(2):88-92.
CrossRef - Forlino A, Marini J.C. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657-71.
CrossRef - Marom R, Lee Y.C, Grafe I, Lee B. Pharmacological and biological therapeutic strategies for osteogenesis imperfecta. American journal of medical genetics Part C, Seminars in medical genetics. 2016;172(4):367-83.
CrossRef - Forlino A, Cabral W.A, Barnes A.M, Marini J.C. New perspectives on osteogenesis imperfecta. Nature reviews Endocrinology. 2011;7(9):540-57.
CrossRef - Marini J.C, Blissett A.R. New genes in bone development: what’s new in osteogenesis imperfecta. The Journal of clinical endocrinology and metabolism. 2013;98(8):3095-103.
CrossRef - Huang X, Huang S, Guo F, Xu F, Cheng P, Ye Y, et al. Dose-dependent inhibitory effects of zoledronic acid on osteoblast viability and function in vitro. Molecular medicine reports. 2016;13(1):613-22.
CrossRef - Boyce B.F. Advances in the regulation of osteoclasts and osteoclast functions. Journal of dental research. 2013;92(10):860-7.
CrossRef - Arnett T.R, Orriss I.R. Metabolic properties of the osteoclast. Bone. 2017.
- Murakami H, Takahashi N, Tanaka S, Nakamura I, Udagawa N, Nakajo S, et al. Tiludronate inhibits protein tyrosine phosphatase activity in osteoclasts. Bone. 1997;20(5):399-404.
CossRef - Martin T.J, Udagawa N. Hormonal regulation of osteoclast function. Trends in endocrinology and metabolism: TEM. 1998;9(1):6-12.
CrossRef - Martin T.J, Ng K.W. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. Journal of cellular biochemistry. 1994;56(3):357-66.
CrossRef - Pizones J, Plotkin H, Parra-Garcia J.I, Alvarez P, Gutierrez P, Bueno A, et al. Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. Journal of pediatric orthopedics. 2005;25(3):332-5.
CrossRef - Antoniazzi F, Zamboni G, Lauriola S, Donadi L, Adami S, Tato L. Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. The Journal of pediatrics. 2006;149(2):174-9.
CrossRef - Biggin A, Munns C.F. Long-Term Bisphosphonate Therapy in Osteogenesis Imperfecta. Current osteoporosis reports. 2017;15(5): 412-8.
CrossRef - Dwan K, Phillipi C.A, Steiner R.D, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. The Cochrane database of systematic reviews.CD005088. 2016;10.
CrossRef - Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Grundker C., et al. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochemical and biophysical research communications. 2002;291(3):680-6.
CrossRef - Misof B.M, Roschger P, Baldini T, Raggio C.L, Zraick V, Root L., et al. Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone. 2005;36(1):150-8.
CrossRef - Van Beek E.R, Lowik C.W, Papapoulos S.E. Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: the role of protein geranylgeranylation in the action of nitrogen-containing bisphosphonates on osteoclast precursors. Bone. 2002;30(1):64-70.
CrossRef - Bhadada S.K, Santosh R, Bhansali A, Upreti V, Dutta P. Osteogenesis imperfecta. The Jianournal of the Association of Physics of India. 2009;57:33-6.
- Jabbour Z, Al-Khateeb A, Eimar H, Retrouvey J.M, Rizkallah J, Glorieux F.H., et al. Genotype and malocclusion in patients with osteogenesis imperfecta. Orthodontics & craniofacial research. 2018.
- Sato A, Ouellet J, Muneta T, Glorieux F,H, Rauch F. Scoliosis in osteogenesis imperfecta caused by COL1A1/COL1A2 mutations – genotype-phenotype correlations and effect of bisphosphonate treatment. Bone. 2016;86:53-7.
CrossRef - Rauch F, Travers R, Plotkin H, Glorieux F.H. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. The Journal of clinical investigation. 2002;110(9):1293-9.
CrossRef - Adams A.L, Adams J.L, Raebel M.A, Tang B.T, Kuntz J.L, Vijayadeva V., et al. Bisphosphonate Drug Holiday and Fracture Risk: A Population-Based Cohort Study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2018.
- Hughes D.E, Wright K.R, Uy H.L, Sasaki A, Yoneda T, Roodman G.D, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. h Journal of bone and mineral researc:. the official journal of the American Society for Bone and Mineral Research. 1995;10(10):1478-87.
- Rogers M.J, Crockett J.C, Coxon .FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49(1):34-41.
CrossRef - Whyte M.P, Wenkert D, Clements K.L, McAlister W.H, Mumm S. Bisphosphonate-induced osteopetrosis. The New England. journal of medicine. 2003;349(5):457-63.
CrossRef - Glorieux F.H, Bishop N.J, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. The New England journal of medicine. 1998;339(14):947-52.
CrossRef








