Wassef Girgiss Nicola1, Mina Wassef Girgiss1, Aly Mohamed Ezz El-Arab2, Dawoud Fakhry Habib3, Mohamed Elsayed Elnemr4, Nadia Mohamed Ahmed3 and Eman Refaat Youness3
1Internal Medicine, Endocrinology and metabolism-Department of Internal Medicine, Medical Division, National Research Centre (NRC), Cairo, Egypt.
2Nutrition-Department of Nutrition and Food Science, National Research Centre (NRC), Cairo, Egypt.
3Biochemistry-Medical Biochemistry Department, Medical Division, National Research Centre (NRC), Cairo, Egypt.
4Internal Medicine, Department of Internal Medicine, 6th October University-Egypt.
Corresponding Author E-mail: mwgnicola@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1469
Abstract
Systemic inflammation describes certain metabolic alterations which are mediated by inflammatory cytokines. Theses occur essentially as a defensive body response towards offending agents such as surplus nutrient staffs. Our aim is to find out the role of inulin as a protective agent against metabolic inflammation. Twenty eight type 2 diabetic females were subjected to the estimation of their serum high sensitivity C-reactive protein, lipopolysaccharides, tumor necrosis factor alpha, adiponectin and HOMA-IR test before and after three weeks of inulin ingestion. There was a significant drop in the level of serum high sensitivity C-reactive protein, lipopolysaccharides, tumor necrosis factor alpha, HOMA-IR and a non-significant rise in serum adiponectin after inulin ingestion. In summary inulin can act as a useful protective agent in systemic inflammation.
Keywords
Adiponectin; Tumor Necrosis Factor Alpha HOMA-IR; High Sensitivity C-reactive Protein; Inulin; Lipopolysaccharides; Metabolic Inflammation;
Download this article as:| Copy the following to cite this article: Nicola W. G, Girgiss M. W, El-Arab A. M. E, Habib D. F, Elnemr M. E, Ahmed N. M, Youness E. R. Role of Inulin in the Protection and Management of Metabolic Inflammation in Humans by. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Nicola W. G, Girgiss M. W, El-Arab A. M. E, Habib D. F, Elnemr M. E, Ahmed N. M, Youness E. R. Role of Inulin in the Protection and Management of Metabolic Inflammation in Humans by. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=21128 |
Introduction
Systemic inflammation primarily describes certain metabolic alterations which occur essentially in obesity, type 2 diabetes mellitus, the metabolic syndrome and their complications.1 However, systemic inflammation underlies other serious diseases including atherosclerosis.2 steatohepatitis, airway diseases, dementia, cancer,3 and intestinal inflammatory diseases namely crohnʹs disease and ulcerative colitis.4
To approach the issue of systemic inflammation, it is preferable to recall some crucial facts. First: the tight functional integration between the neuroendocrine axis and the immune system which is mandatory for the wellbeing and survival of mankind; this triad is the main regulator of the body metabolism. This same triad is involved in the initiation and propagation of the inflammatory pathways3. Second: inflammation is essentially a defensive body reaction towards an offending agent. This reaction can be a local one (the classic type of inflammation) or might be an extensive reaction involving several tissues in the body (the systemic or metabolic inflammation)3.
Literally, the term inflammation denotes the natural defensive reaction undertaken by the body in response to an attacking noxious or injurious agent. The invoking agent might be any of the following: biological (virus or bacteria), mechanical (friction or rubbing), thermal (heat) or chemical (acid, alkali or any caustic material). This type of inflammation is usually of short duration and limited extent (usually local); it shows itself in four cardinal features: hotness, swelling, redness and pain (color, tumor, rubor and dolor). The inflammatory response and its features are provoked by certain mediators secreted by particular cells, mostly in the vicinity of the inflammation.5
In systemic inflammation on the other hand, it is interesting and amazing to know that, the human body perceives excess nutrients as noxious foreign invaders and reacts towards these accordingly3. The defensive inflammatory reaction here, starts more or less like the classic one but differs in: it lacks the cardinal features, the intensity is less acute, the duration is more prolonged and the sequels are harmful. Besides the extent is wider and involves several tissues and organs, hence the name systemic inflammation.3,6
The mouth is not the only inlet to admit the invaders inside the body. The colon is also an important route for the access of special invaders (lipopolysaccharides) inside the body. These are integral components of the cell membrane of gram negative bacteria which inhabit the large intestine. Also, lipopolysaccharides are important components of the endotoxins produced by these bacteria.7 High dietary fat consumption increases serum lipopolysacchrides level. This does not necessarily imply the increase in gram negative bacteria in the large gut.8 The rise in serum lipopolsacchrides level occurs as a result of increased lipopolsacchrides absorption, decreased degradation or increased gut mucous membrane permeability to them.9-11 Rise of serum lipopolysaccharides level increases serum proinflammatory cytokines12-14.
Obesity is a third and constant supplier of excess nutrients. Lipopolysaccharides are highly expressed in adipose tissue of obese and diabetic subjects.15 Besides, the different adipose tissue compartments of the body especially the more active central one (omental fat), are under a state of continuous turnover. The products of their lipolysis reach the liver and the general circulation hence to all tissue cells of the different organs.16 These cells become stuffed with fatty acids, triglycerides and their metabolites (diacylglycerol and ceramide) producing what is called lipotoxicityz.17-19 Lipotoxicity is deleterious to the cardiovascular system and to most organs of the body.18,19 Beside the ample supply of invaders from the adipose tissue, adiposity is associated with increased inflammatory cytokine production20 and reduced production of the protective adipokine (adiponectin).21
Inulin, a naturally occurring oligosaccharide of plant origin is known to stimulate certain strains of the colonic microbiome, namely the bifidobacteria. These latter can predominate over the gram negative strains of bacteria and suppress their action.22,23
The aim of the study is to find out how inulin type-prebiotic can fair in the issue of metabolic inflammation in human beings. This entails the estimation of serum high sensitivity C-reactive protein as a monitor of systemic inflammation. Also to determine serum level of lipopolysaccharides and tumour necrosis factor alpha (TNF-α) as proinflammatory compounds, together with serum adiponectin level as protective adipokine.
Materials and Methods
Subjects
28 type 2 diabetic female patients were selected from the governmental hospitals of Cairo-Egypt.
Inclusion criteria: obese (body mass index > 30), type 2 diabetic females, middle aged or above (40-65 years), hypertensives or not.
Exclusion criteria
Endocrine or metabolic disorders, hormonal treatment or contraceptive pills.
Organ failure (liver, kidney, heart or lung) or malignancy.
Local or systemic infection.
Ethical Committee Approval
The study was approved by the ethical committee of the National Research Centre (NRC), Cairo, Egypt. Certificate number 15011.
Consent
All patients gave their signed consent for participation.
Methods
Inulin fructans type-prebiotic was given to the patients as an add on therapy to their conventional antidiabetic treatment. Four grams of inulin were given with milk to each patient daily; 2 grams in the morning and 2 grams in the evening for twenty one consecutive days. This dose was chosen empirically taking in consideration that a small dose of inulin can exert a bifidogenic effect.24
Inulin specifications: Inulin A.R (C6H10O5) N ALPHA-CHEMIKA Mumbai. 400002 (INDIA) An ISO: 9001: 2000 Certified company.
All patients were subjected to the following before and after the period of inulin ingestion.
A- Full history B- Thorough clinical examination C- Laboratory investigations:
Determination of Fasting Serum High Sensitivity C- Reactive Protein (Hs-CRP).
This was done quantitatively by immunoenzymometric chemiluminescence assay after Yudkin JS.25 Kit used “Acculite – CLIA Microwells.” hs-CRP Test System. Product Code: 3175-300. Monobind Inc. Lake Foreset, CA 92630, USA.
Fasting Serum Lipopolysaccharides Determination
This was determined by ELISA technique after Ruiz et al.26 Kit used: Human Lpopolysaccharides (LPS) ELISA Kit from WKEA MED SUPPLIES CORP 206 Building 6, Chenguang Garden, Qianjin Street. Changchun 130012 China.
Fasting Serum Tumour Necrosis Factor Alpha (Tnfα) Determination
TNF-α was determined using the ELISA technique after Taylor PC.27 Kit used: Human TNF-α ELISA kit from ASSAYPRO LLC 3400 Harry S Truman Blvd St. Charles, MO 63301.
Fasting Serum Adiponectin Determination
Adiponectin was determined using ELISA technique afte Tsao et al.28 Kit used: Human Adiponectin ELISA kit fro ASSAYPRO. Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301.
HOMA Determination
Insulin resistance (IR) was assessed using homeostasis model assessment (HOMA) from fasting serum glucose and fasting serum insulin level, after Mathiews et al.29
The Equation
Insulin resistance (HOMA-IR) = fasting glucose (mg/dl) x fasting insulin (m I.U./ml)/ 405.
Fasting Serum Insulin Estimation
This was quantitatively estimated using an enzyme immunoassay method according to National Committee for Clinical Laboratory Standards (National Committee for Clinical Laboratory Standards, 1998).30 Manufacturer of the kit used: immunospect corporation 7018 Owensmouth Ave. Suit 103 Canoga Park, CA, 91303.
Fasting serum glucose determination
This was estimated by an enzymatic colorimetric method. The principal is enzymatic oxidation of glucose by glucose oxidase enzyme, according to Tietz.31
Kit used from Egyptian Company for biotechnology (S.A.E.) Obour City Industrial Area. block 20008 piece 19A Cairo. Egypt.
Statistical Methods
The collected data were coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp., Chicago, USA, 2013.
Descriptive statistics were done for quantitative data as minimum & maximum of the range as well as mean±SD (standard deviation) for quantitative parametric data. Inferential analyses were done for quantitative variables using paired t-test in cases of two dependent groups with parametric data. The level of significance was taken at P value < 0.050 is significant, otherwise is non-significant.
Results
Mean fasting serum level of high sensitivity C-reactive protein (HsCRP) dropped significantly after inulin ingestion from 43.5±21.5 mg/dl to 35.2±19.5 mg/dl with P <0.001, table (1) and figure (1).
Mean fasting serum level of lipopolysaccharides and TNFα dropped significantly after inulin ingestion from 43.7±21.3 pg/ml to 38.8±17.9 pg/ml and from 17.6±2.3 pg/ml to 14.1±1.9 pg/ml respectively with P <0.001, table (1) and figure (2).
Mean fasting level of adiponectin increased after inulin ingestion from 27.5±1.7 ug/ml to 27.6±1.1 ug/ml with P 0.845, table (1) and figure (3).
Mean fasting HOMA(IR) dropped significantly after inulin ingestion from 11.1±8.2 to 7.2±5.0 with P <0.001, table (1) and figure (4).
Table 1: Shows the values of HsCRP, Lipopolysaccharides, TNFα, Adiponectin and HOMA (IR) before and after inulin ingestion period
| Before | After | ^Change |
#P |
||||
| Mean±SD | Range | Mean±SD | Range | Median (IQR) | Range | ||
| HsCRP (mg/dL) | 43.5±21.5 | 14.0–83.0 | 35.2±19.5 | 8.0–80.0 | -7.0 (-9.0–-4.3) | -30.0–-1.0 | <0.001* |
| Lipopolysacharides (pg/mL) | 43.7±21.3 | 16.2–87.3 | 38.8±17.9 | 15.0–68.5 | -3.3 (-7.5–-1.7) | -20.9–12.3 | <0.001* |
| TNFα (pg/mL) | 17.6±2.3 | 14.8–21.6 | 14.1±1.9 | 11.0–18.7 | -3.0 (-4.3–-2.5) | -6.8–-1.6 | <0.001* |
| Adiponectin (µg/mL) | 27.5±1.7 | 22.3–29.0 | 27.6±1.1 | 24.7–28.9 | -0.3 (-0.9–0.5) | -2.5–5.2 | 0.845 |
| HOMA (IR) | 11.1±8.2 | 2.5–32.1 | 7.2±5.0 | 2.1–18.7 | -3.5 (-5.4–-1.6) | -13.4–2.7 | <0.001* |
N=28, ^Negative values indicate reduction, #P-value of paired t-test, *Significant
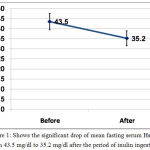 |
Figure 1: Shows the significant drop of mean fasting serum HsCRP from 43.5 mg/dl to 35.2 mg/dl after the period of inulin ingestion.
|
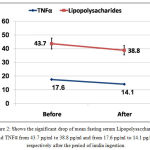 |
Figure 2: Shows the significant drop of mean fasting serum Lipopolysaccharides and TNFα from 43.7 pg/ml to 38.8 pg/ml and from 17.6 pg/ml to 14.1 pg/ml respectively after the period of inulin ingestion.
|
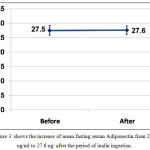 |
Figure 3: shows the increase of mean fasting serum Adiponectin from 27.5 ug/ml to 27.6 ug/ after the period of inulin ingestion.
|
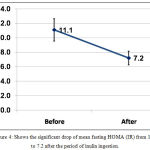 |
Figure 4: Shows the significant drop of mean fasting HOMA (IR) from 11.1 to 7.2 after the period of inulin ingestion.
|
Discussion
Metabolic inflammation is a main culprit in the pathogenesis of a myriad of serious diseases which afflict mankind. The adipose tissue and the liver are the usual sites of the inflammatory process. However, beside the adipocytes and the hepatic cells, a variety of cells of the immune system are also involved including: macrophages, neutrophils, oesinophils, mast cells and T- lymphocytes. Moreover, other cells are implicated namely the vascular endothelial cells, gut mucosal cells and cells from other tissues involved in systemic inflammation.3,15,19,25,32
Systemic inflammation ushers itself by a rise of serum acute phase reactant proteins including C- reactive protein (CRP), which is secreted by the liver25.
However, obesity is the origin and mother which fosters metabolic inflammation through several ways. First: when the adipocytes and their adjacent preadipocytes get engorged with lipids, they increase in size; their mass outweighs their blood supply and become necrotic. These dead cells invite macrophages which infiltrate the adipose tissue together with other cells of the immune system. Second: the interaction of the living adipocytes with the immune system cells, triggers and propagates the process of inflammation through the production of inflammatory mediators.33-36
In fact the adipocytes and the immune system cells share striking key features such as pathogen sensing, phagocytic activity, complement activation and inflammatory mediators secretion. Mediators produced by macrophages are termed cytokines and when secreted by the adipocytes are called adipokines. These mediators are responsible for the activation of the inflammatory signaling network of pathways3.
Third, lipopolysaccharides (LPS) are usually increased in the adipose tissue of obese humans.15,37 These are the precursors of tumour necrosis factor alpha (TNFα)12-14. This cytokine is also over expressed in both the adipose tissue and the muscles of obese individuals.38,39
TNFα stands out as the first impressive link between the triad of obesity, metabolic inflammation and type 2 diabetes mellitus. 40 TNFα plays a critical role in the pathogenesis of type 2 diabetes mellitus, vascular atherosclerosis, inflammatory bowel diseases (IBDs) and other diseases in which systemic inflammation is a causative factor3.
On the level of energy metabolism (obesity, type 2 diabetes mellitus and the metabolic syndrome), TNFα and other inflammatory cytokines are also highly expressed in the adipose tissue and muscle tissue of these subjects. TNFα predominates other cytokines in increasing tissue insulin resistance and impeding insulin action3.
In the cardiovascular system, TNFα is involved in several disease processes, particularly in the initiation and progression of atherosclerosis and its complications including vascular ischemic changes, reperfusion injury and heart failure. Normally the healthy endothelial cells resist the adhesion of blood leucocytes to their surface. When the endothelium is implicated in the process of systemic (metabolic) inflammation, the cytokines vascular cell adhesion molecule-1 (VCAM-1) and TNFα facilitate the adhesion of monocytes to the endothelial cells and their diapedesis into the intima. These monocytes aquire inflammatory properties through engulfing oxidized and glycated blood lipids. These starting steps progress towards the full picture of atherosclerosis and its complications.41-43
TNFα is also, involved in the pathogenesis of autoimmune diseases including rheumatoid arthritis. It is noteworthy that the course of atherosclerosis progresses relentlessly in rheumatoid arthritis patients than in subjects with traditional risk factors.42
Autoimmunity can involve and disrupt the intestinal mucosal cells in two distinct inflammatory bowel diseases (IBD) namely ulcerative colitis (UC) and crohnʹs disease (CD). TNFα plays a key role in the pathogenesis of these two distinct IBD.44,45
In the present study, inulin fiber prebiotic effectively improved several facets in the issue of metabolic inflammation. It significantly reduced the high sensitivity c-reactive protein (HSCRP) which is an important signal that heralds the early steps of inflammation. Again, inulin significantly reduced serum level of lipopolysaccharides which are the main precursors of TNFα. Also, inulin significantly reduced serum TNFα which is an important and a key cytokine in the initiation and provocation of systemic inflammation. This cytokine is produced by a variety of cells whether involved in the process of inflammation such as adipocytes, macrophages, mast cells, lymphoid cells, fibroblasts or other implicated cells like hepatocytes, cardiac myocytes, endothelial cells and neuronal cells. The decreased level of TNFα was accompanied by a significant decrease in HOMA test level. This means a decrease in insulin reistance and improved insulin action.
On the other hand, adiponectin hormone (adipokine) which possesses an insulin sensitizing effect, anti-inflammatory and cardioprotective properties,46 showed an insignificant rise after inulin intake. However, cytokines exert their effect through branching metabolic networks. The strength of each cytokine depends on its position and proximity to this network. The more proximal, the stronger the effect. 3,47Consequently the effect of therapy is not the same on each cytokine. Also, the duration of inulin ingestion in this study was a short one (three weeks).
Conclusions
Inulin type-prebiotic improved several steps in the process of metabolic inflammation. This safe edible fiber can be given as an add on therapy in the different states of systemic (metabolic) inflammation.
Again, we suggest the study of individual metabolic inflammatory diseases separately before and after inulin ingestion, on a large number of patients and for a longer duration. Also, other pathogenic and protective cytokines should be considered in the study together with conclusive parameters in each disease entity e.g. number of coronary artery disease events, cerebral strokes and fatalities in diabetics and patients with cardiovascular disease; Number of bloody diarrhea and endoscopic biopsies in those with inflammatory bowel diseases (IBDs).
Acknolwdgements
Thanks for National Research Center, Cairo, Egypt for financing this work with project number 10010312. Again, thanks to all patients who volunteered to participate in this study.
Conflict of Interest
The authors declare no conflict of interest.
References
- Semenkovich C.F. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813-1822.
CrossRef - Libby P. Review article: Inflammation in atherosclerosis. Nature. 2002;420:868-874.
CrossRef - Hotamisligil G.S. Review Article: Inflammation and metabolic disorders. Nature. 2006;444:860-867.
CrossRef - Munoz F.S, Lopez A.D, Furusho J.K.Y. Role of cytokines in inflammatory bowel disease. World J Gastroentrol. 2008;14(27):4280-4288.
CrossRef - Larsen G.L, Henson P.M. Mediators of inflammation. Annu Rev Immunol. 1983;1:335-359.
CrossRef - Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4-7.
CrossRef - Delzenne N.M, Cani P.D. Gut microbiota and the pathogenesis of insulin resistance. Current Diabetes Reports. 2012;2(1):6-11.
- Cani P.D, Neyrinck A.M, Fava F, Knauf C, Burcelin R.G, Tuohy K.M, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374-2383.
CrossRef - Cani P.D, Possemiers S, Van de W.T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-1103.
CrossRef - Muccioli G.G, Naslain D, Backhed F, Reigstad C.S, Lambert D.M, Delzenne N.M, Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392.
CrossRef - de La Serri C.B, Ellis C.L, Lee J, Hartman A.L, Rutledge J.C, Raybould H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:440-448.
CrossRef - Ghanim H, Abuaysheh S, Sia S.L, Korzeniewski K, Chaudhuri A, Fernandez-Real J.M, et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diab Care. 2009;32:2281-2287.
CrossRef - Amar J, Burcelin R, Ruidavets J.B, Cani P.D, Fauvel J, Alessi M.C, et al.. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219-1223.
CrossRef - Erridge C, Attina T, Spickett C.M, Webb D.J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286-1292.
CrossRef - Delzenne N.M, Cani P.D. Nutritional modulation of gut microbiota in the context of obesity and insulin resistance: Potential interest of prebiotics. Int Dairy J. 2010;20:277-280.
CrossRef - Sam S, Haffner S, Davidson M.H, D’Agostino, Sr. R.B, Feinstein S, et al.. Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particles number and size in type 2 diabetes. Diabetes. 2008;57:2022-2027.
CrossRef - Weinberg J.M. Lipotoxicity. Kidney Inter. 2006;70:1560-2566.
CrossRef - Unger R.H. Minireview: Weapons of lean body mass distribution. The role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159-5165.
CrossRef - Unger R.H. The physiology of cellular liporegulation. Ann Rev Physiol. 2003;65:333-347.
CrossRef - Fontana L, Eagon J.C, Trujillo M.E, Scherer P.E, Klein S. Visceral fat adipokines secretion is associated with systematic inflammation in obese humans. Diabetes. 2007;56:1010-1013.
CrossRef - Apovian C.M, Bigomia S, Mortt M, Meyers M.R, Ulloor J, Gagua M, et al. Adipose tissue macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654-1659.
CrossRef - Cani P.D, Neyrinck A.M, Fava F, Knauf C, Burcelin R.G, Tuohy K.M, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374-2383.
CrossRef - Cani P.D, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2 driven improvement of gut permeability. Gut. 2009;58:1091-1103.
CrossRef - Kelly G. Inulin-type prebiotics – – a review part 1. Altern Med Rev.Dec. 2008;13(4):315-329.
- Yudkin J.S. C-reactive protein in healthy subjects. Association with obesity, insulin resistance and endothelial dysfunction. A potential role for cytokines originating from adipose tissue. Arteriosclerosis Thrombosis Vascular Biology. 1999;19:972-978.
CrossRef - Ruiz N, Khane D, Silhavy T.J. Transport of lipopolsaccharide across the cell envelope: the long road of discovery. Nature Reviews Microbiology. 2009;7:677-683.
CrossRef - Taylor P.C. Anti-TNF therapy for rheumatoid arthritis and other inflammatory diseases. Mol. Biotechnol. 2001;19(2):153-168.
CrossRef - Tsao T.S. Lodish H.F. Fruebis J. ACRP30, a new hormone controling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213-221.
CrossRef - Mathiews D.R, Hosker J.P, Rudenski A.S Naylor B.A, Treacher D.F, Turner R.C. Homeostasis model assessment: insulin resistance and b–cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28 (7):412-419.
CrossRef - National Committee for Clinical Laboratory Standards. Procedures for the collection of diagnostic blood specimens by venipuncture: approved standards. 4th Ed. NCCLS Document H3-A4, Wayne, PA. 1998.
- Tietz N.W, ed. Clinical guide to laboratory tests. 3rd ed. Philadelphia. Wb saunders. 1995;268-273.
- Sanchez-Munoz F, Dominquez-Lopez A, Yamamoto-Furusho J.K. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. Jul 21 2008;14(27):4280-4288.
CrossRef - Maury E, Brichard S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1-16.
CrossRef - Maury E, Noel L, Detry R, Brichard S.M. In vitro hyperresponsiveness to tumour necrosis factor-α contributes to adipokine dysregulation in omental adipocytes of obese subjects. J Clin Endocrinol Metab. 2009;94:1393-1400.
CrossRef - Gutierrez D.A, Puglisi M.J, Hasty A.H. Impact of increased adipose tissue on inflammation, insulin resistance and dyslipidemia. Current Diabetes Reports-Middle East Edition. 2009;1(4):23-29.
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901-911.
CrossRef - Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diab Care. 2010;33:1925-1932.
CrossRef - Hotamisligil G.S, Arner P, Caro J.F, Atkinson R.L, Spiegelman B.M. Increased adipose tissue expression of tumour necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95:2409-2415 .
CrossRef - Saghizadeh M, Ong J.M, Garvey W.T, Henry R.R, Kern P.A. The expression of TNF-α by human muscle: relationship to insulin resistance. J Clin Invest. 1996;97:1111-1116.
CrossRef - Hotamisligil G.S, Shargill N.S, Spiegelman B.M. Adipose expression of tumour necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
CrossRef - Kleinbongard B, Heusch G, Schulz R. TNF alpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127(3):295-314.
CrossRef - McKellar G.E, McCarey D.W, Sattar N, Mclnnes I.B. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6(6):410-417.
CrossRef - Libby P. Review article. Inflammation in atherosclerosis. Nature. 2002;420:868-874.
CrossRef - Sanchez-Munoz F, Dominquez-Lopez A, Yamamoto-Furusho Jesus K. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14(27):4280-4288.
CrossRef - Sands B.E, Kaplan G.G. The role of TNF alpha in ulcerative colitis. J Clin Pharmacol. 2007;47(8):930-941.
CrossRef - Robinson K, Prins J, Venkatesh B. Clinical review: Adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15(2):221-243.
CrossRef - Wellen K.E, Hotamisligil G.S. Inflammation, stress and diabetes. J Clin Invest. 2005;115:1111-1119.
CrossRef








