Abdelrahman Ibrahim Abushouk1,2, Aya Ashraf Ali3,4, Ahmed Abdou Mohamed3,4, Loalo'a El-Sherif3,4, Mennat-Allah Abdelsamed4,5, Mohamed Kamal Mohamed4,5, Merhan Kamal Sayed3,4, Nehal Alaa Mohamed3,4, Ahmed Abdelbaset Osman3,4, Sameh M Shaheen1 and Mohamed M. Abdel-Daim6
1Faculty of Medicine, Ain Shams University, Cairo, Egypt.
2NovaMed Medical Research Association, Cairo, Egypt.
3Faculty of Medicine, Minia University, Minia, Egypt.
4Minia Medical Research Society, Minia University, Minia, Egypt.
5Faculty of Clinical Pharmacy, Minia University, Minia, Egypt.
6Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
Corresponding Author E-mail: Abdeldaim.m@vet.suez.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/1413
Abstract
Atrial fibrillation (AF) is a common, sustained tachyarrhythmia, associated with an increased risk of mortality and thromboembolic events. We performed this meta-analysis to compare the clinical efficacy of rate and rhythm control strategies in patients with AF in a meta-analysis framework. A comprehensive search of PubMed, OVID, Cochrane-CENTRAL, EMBASE, Scopus, and Web of Science was conducted, using relevant keywords. Dichotomous data on mortality and other clinical events were extracted and pooled as risk ratios (RRs), with their 95% confidence-interval (CI), using RevMan software (version 5.3). Twelve studies (8451 patients) were pooled in the final analysis. The overall effect-estimate did not favor rate or rhythm control strategies in terms of all-cause mortality (RR= 1.13, 95% CI [0.88, 1.45]), stroke (RR= 0.97, 95% CI [0.79, 1.20]), thromboembolism (RR= 1.06, 95% CI [0.64, 1.76]), and major bleeding (RR= 1.10, 95% CI [0.90, 1.35]) rates. These findings were consistent in AF patients with concomitant heart failure (HF). The rate of rehospitalization was significantly higher (RR= 0.72, 95% CI [0.57, 0.92]) in the rhythm control group, compared to the rate control group. In younger patients (<65 years), rhythm control was superior to rate control in terms of lowering the risk of all-cause mortality (p=0.0003), HF (p=0.003) and major bleeding (p=0.02). In older AF patients and those with concomitant HF, both rate and rhythm control strategies have similar rates of mortality and major clinical outcomes; therefore, choosing an appropriate strategy should consider individual variations, such as patient preferences, comorbidities, and treatment cost.
Keywords
Atrial Fibrillation; Meta-analysis; Rate Control; Rhythm Control
Download this article as:| Copy the following to cite this article: Abushouk A. I, Ali A. A, Mohamed A. A, El-Sherif L, Abdelsamed M. A, Mohamed M. K, Sayed M. K, Mohamed N. A, Osman A. A, Shaheen S. M, Abdel-Daim M. M. Rhythm Versus Rate Control for Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Abushouk A. I, Ali A. A, Mohamed A. A, El-Sherif L, Abdelsamed M. A, Mohamed M. K, Sayed M. K, Mohamed N. A, Osman A. A, Shaheen S. M, Abdel-Daim M. M. Rhythm Versus Rate Control for Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20680 |
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting more than 5% of the worldwide population.1 It is associated with a high risk of thromboembolic events, including stroke, which occurs in about 23% of AF patients, older than 80 years.2,3 Over the last decade, it accounted for about one third of hospital admissions for cardiac arrhythmias4,5 with an increasing prevalence in patients with cardiovascular problems, such as valvular heart disease, heart failure (HF), and coronary artery disease (CAD).6,7
The pharmacological management of AF targets either rate control (maintaining the heart rate at normal levels, using pharmacological agents, such as beta-blockers, non-dihydropyridine calcium-channel blocker, and cardiac glycosides) or rhythm control (restoration of sinus rhythm, using electrical cardioversion and/or antiarrhythmic agents, such as sodium channel blockers and potassium channel blockers) (8). In the past few years, several randomized controlled trials (RCTs) have investigated whether rhythm control is superior to rate control with respect to mortality and cerebrovascular accidents.9-22
Besides the controversial results of these trials, former meta-analyses showed conflicting results, suggesting that rate control is either similar or superior to rhythm control in terms of mortality and stroke rates.23,24 Moreover, recent trials have compared both strategies in different groups of AF patients, including younger and those with concomitant HF.9,12 Therefore, we conducted this systematic review and meta-analysis to update the evidence regarding the optimal control approach for AF.
Methods
This study was conducted following the guidelines of the Cochrane handbook of systematic reviews of interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.25,26 All steps have been prespecified in a published protocol on PROSPERO register of systematic reviews (CRD42016049648).
Literature Search Strategy
We performed a comprehensive search of PubMed, Scopus, Web of Science (ISI), Embase, OVID and Cochrane Central register of controlled clinical trials (CENTRAL), during September 2016, to identify relevant studies. We developed the search strategy for each database using the following terms: “Atrial fibrillation”, “Rate control”, “Beta blockers”, “Calcium channel blockers”, “Antiarrhythmic”, “Cardioversion”, and “Rhythm control” (Supplementary file 1). No publication period or language restrictions were applied during literature search. We also checked the bibliography of included studies and searched the clinical trials registry (Clinicaltrials.gov) for any ongoing trials.
Eligibility Criteria and Study Selection
We included all RCTs that compared the efficacy of rate control versus rhythm control strategies, including non-invasive procedures of electrical cardioversion, in atrial fibrillation patients. We excluded trials on other types of atrial arrhythmia, such as atrial flutter, reviews, non-randomized trials, observational, and studies from which data could not be reliably extracted.
Three reviewers independently screened the retrieved titles and abstracts for matching our criteria. Then, the eligible abstracts underwent further full-text screening for eligibility to meta-analysis. Unrelated or duplicate reports were removed and multiple reports for the same trial were linked together as one study. All disagreements were solved by discussion between the reviewers.
Data Extraction
Data was extracted from included studies by one reviewer and checked by another one. The extracted data included the following: a) baseline characteristics of enrolled patients, b) risk of bias assessment domains, and c) main outcomes including the incidence of all-cause mortality, cardiovascular mortality, arrhythmic mortality, stoke or transient ischemic attack (TIA), systemic embolism, heart failure or worsening of heart failure, major or life threatening bleeding, re-hospitalization and subsequent myocardial infarction (MI).
Risk of Bias Assessment
Two independent reviewers assessed the risk of bias in included trials, using the Cochrane risk of bias (ROB) assessment tool.25 This tool is designed to detect six types of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential sources of bias. The authors classified included studies in each domain as of low, high, or unclear risk of bias. The risk of publication bias was assessed, using funnel plot-based methods, whenever 10 or more studies reported on the same outcome.27
Data Synthesis
The statistical analyses were performed using the RevMan software (version 5.3 for windows), provided by the Cochrane Collaboration. Under the fixed effect model, dichotomous data were pooled as risk ratios (RRs) with their 95% confidence interval (CI), using the Mantel Haenszel (M-H) method. The existence of heterogeneity was assessed by Chi-square test and its extent was measured by I-square test. In case of significant heterogeneity (Chi-square p < 0.1), the analysis was conducted under the random effects model. Sensitivity analysis was performed to resolve significant heterogeneity and to ensure that our results were not affected by the weights of individual studies. We also conducted subgroup analyses to compare both strategies in patients with HF or under 65 years of age (by pooling studies in which the mean age was less than 65 years).
Results
Literature Search and Screening Process
A comprehensive database search retrieved 3879 unique records. Following title and abstract screening, 74 full-text articles were retrieved for assessment of eligibility to meta-analysis. Finally, we included 12 RCTs (14 full text articles: 8451 patients) that compared rate control to rhythm control in AF patients9-22 (Figure 1). Table 1 displays a summary of the used drugs and main findings of included studies and Table 2 shows baseline characteristics of enrolled patients in these studies.
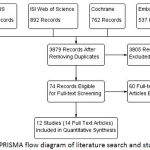 |
Figure 1: PRISMA flow diagram of literature search and study selection.
|
Table 1: shows a summary of the used drugs and main findings of included trials.
| Study ID | Sample Size | Rate control arm | Rhythm control arm | Patient inclusion criteria | Follow up duration | Conclusions |
| Beta-blockers (βB) and digitalis | Electrical cardioversion, amiodarone and either sotalol or dofetilide | Patients with left ventricular (LV) ejection of ≤ 35%, a history of symptomatic heart failure (HF) [NYHA class II or IV] within the past 6 months. | 19± 37 months | Rhythm control does not reduce cardiovascular mortality, as compared with a rate-control strategy. | ||
| AF CHF | 1376 | |||||
| βB, digitalis, and calcium-channel blockers (CCB) | Amiodarone or sotalol | Patients with recurrent AF who were at least 65 years of age or who had other risk factors for stroke or death. | 6 years | Rhythm-control strategy offers no survival advantage over the rate-control strategy which has a lower risk of adverse drug effects. | ||
| AFFIRM | 4060 | |||||
| Digoxin and βB | Amiodarone | Patients aged >18 years of age with persistent AF and chronic symptomatic HF with evidence of systolic dysfunction on echocardiography. | 14 months | Rhythm-control strategy in patients with AF and HF provides more improvement to quality of life (QoL) and LV function, when compared with rate control strategy. | ||
| CAFÉ II | 61 | |||||
| Diltiazem | Amiodarone | All patients with rheumatic heart disease who had AF. | 12 months | In patients with rheumatic AF, rhythm-control is superior to rate control in improving exercise capacity, QoL, and possibly mortality. | ||
| CRAFT | 144 | |||||
| βB, CCB, or both | Amiodarone | Patients with postoperative AF that persisted for more than 60 minutes or recurrent episodes of AF during the index hospitalization (≤7 days after surgery). | 60 days | Both rhythm and rate-control strategy are associated with similar complications and mortality rates, as well as comparable rates of persistent AF, 60 days after onset. | ||
| Gillinov et al. 2016 | ||||||
| 523 | ||||||
| βB, nondihydropyridine CCB; digoxin | Propafenone, disopyramide, or sotalol | AF patients, aged 50 to 75 years of age, known to have AF for at least 7 days, but not for 2 years. | 1.7 ± 0.4 years | There were no significant differences in major clinical endpoints between rate and rhythm control groups. | ||
| HOT CAFE | ||||||
| 205 | ||||||
| βB, CCB, and digitalis | Propafenone, | Patients with persistent AF with no contraindications to anticoagulation. | 578 days | Rhythm control was associated with fewer adverse events than rate control. However, no difference was showed with respect to mortality and cardiovascular morbidity. | ||
| J-RHYTHM | 885 | disopyramide, | ||||
| flecainide, | ||||||
| aprindine, | ||||||
| pirmenol, | ||||||
| bepridil, and | ||||||
| amiodarone | ||||||
| Digoxin and metoprolol | Cardioversion and amiodarone | Patients ≥ 18 years of age who had persistent AF lasting more than 48 hours and nonischemic LV dysfunction diagnosed by echocardiography (EF < 50%). | 3 years | Rhythm-control is preferable in patients with non-ischemic HF and AF with lower mortality and improved exercise capacity. | ||
| Ockun et al. 2004 | ||||||
| 154 | ||||||
| Diltiazem | Amiodarone | Patients, aged 18 to 75 years, presenting with symptomatic | 1 year | Rhythm-control improved exercise tolerance, yet with more frequent hospital admissions than rate-control. Otherwise, both strategies had similar clinical results. | ||
| persistent AF of between 7 days and 360 days duration. | ||||||
| PIAF | 252 | |||||
| Digitalis, a nondihydropyridine CCB, and a βB alone or in combination | Sotalol, class IC antiarrhythmic drugs and amiodarone | Patients with recurrent persistent AF, in whom oral anticoagulation was not contraindicated. | 2.4 years | Rhythm control strategy is associated with increased cardiovascular morbidity and mortality in persistent AF patients. | ||
| RACE | 522 | |||||
| βB, digitalis, CCB, or atrioventricular node ablation ± pacemaker implantation | Class I antiarrhythmic agents or sotalol, βB and/or | Patients aged ≥ 18 years, with ≥ 1 of the following criteria: AF for 4 weeks; left atrial size ≥ 45 mm; LV ejection fraction ≤ 45%, or 1 prior cardioversion with arrhythmia recurrence. | 12 to 36 months | No differences between the two treatment strategies were recorded in all clinical endpoints, except for rehospitalizations. | ||
| amiodarone. | ||||||
| STAF | 200 | |||||
| Digoxin, verapamil and metoprolol | Amiodarone | Patients aged ≥ 18 years who had HTN and persistent AF for > 48 hours. | 21 ± 39 in rate control group and 40 ± 19 in rhythm control group | Rate control is an acceptable strategy in hypertensive AF patients although rhythm control has beneficial effect on exercise capicity. Both strategies have similar rates of mortality and embolic events. | ||
| Yildiz et al. 2008 | 221 |
Abbreviations: AF: atrial fibrillation, AFFIRM: Atrial Fibrillation Follow-up Investigation of Rhythm Management study, AF CHF: Atrial Fibrillation and Congestive Heart Failure study, BB: Beta-Blockers, CAFÉ-II:controlled study of rate versus rhythm control in patients with chronic AF and HF, CHF: congestive heart failure, HF: Heart failure, HOT CAFÉ: How to Treat Chronic Atrial Fibrillation study, J RHYTHM: Japanese Rhythm Management Trial for Atrial Fibrillation, NYHA: New York Heart Association, PIAF: Pharmacological Intervention in Atrial Fibrillation study, QoL: Quality of life, RACE: Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation study, RHD: rheumatic heart disease, STAF: Strategies of Treatment of Atrial Fibrillation study.
Table 2: shows baseline characteristics of enrolled patients in included studies.
| Age (Mean ± SD) | Comorbidities | Patients in sinus rhythm at the end | ||||||||
| Study ID | Study Arm | Sample size (N) | Males (%) | Anticoagulation | ||||||
| Hypertension N (%) | Heart Failure (Class II-IV) | Valvular Disease | Coronary Heart Disease | |||||||
| Rate Control | 694 | 67 ± 11 | 85 | 319 (46%) | 215 (31%) | 34 (5%) | 333 (48%) | 624 (90%) | — | |
| AF CHF | Rhythm Control | 682 | 66 ± 11 | 78 | 334 (49%) | 218 (32%) | 34 (5%) | 327 (48%) | 586 (86%) | — |
| Rate Control | 2027 | 69.8 ± 8.9 | 56.4 | 1045 (51.6%) | 475 (23.4%) | 98 (4.8%) | 497 (24.5%) | 1722 (85%) | 701 (34.6%) | |
| AFFIRM | Rhythm Control | 2033 | 69.7 ± 9 | 72.1 | 1018 (50.1%) | 464 (22.8%) | 100 (4.9%) | 562 (27.6%) | 1423 (70%) | 1272 (62.6%) |
| Rate Control | 31 | 72.2 ± 8.3 | 81 | 22 (72%) | 31 (100%) | — | 17 (55%) | 31 (100%) | 27 (90%) | |
| CAFÉ II | Rhythm Control | 30 | 72 ± 5.4 | 87 | 20 (68%) | 30 (100%) | — | 13 (44%) | 30 (100%) | 19 (66%) |
| Rate Control | 48 | 38.4 | 41.7 | — | 34 (70.8%) | 48 (100%) | — | 48 (100%) | — | |
| CRAFT | Rhythm Control | 48 | 39.5 | 47.9 | — | 37 (77%) | 48 (100%) | — | 48 (100%) | 29 (69.1%) |
| Gillinov et al. 2016 | Rate Control | 262 | 69.2 ± 9.8 | 75.2 | 193 (73.7%) | 35 (13.4%) | 140 (53.4%) | 50 (19.1%) | 112 (42.7%) | 220 (84.2%) |
| Rhythm Control | 261 | 68.4 ± 8.4 | 76.2 | 198 (75.9%) | 33 (12.6%) | 148 (56.7%) | 48 (18.4%) | 113 (43.3%) | 227 (86.9%) | |
| Rate Control | 101 | 61.4 ± 17.6 | 62.4 | 60 (59.4%) | 53 (52.4%) | 15 (14.8%) | 38 (37.6%) | 74.30% | 56 (53.8%) | |
| HOT CAFÉ | Rhythm Control | 104 | 60.4 ± 7.9 | 68.3 | 72 (69.2%) | 74 (71.1%) | 16 (15.4%) | 52 (50%) | 15.60% | 66 (63.5%) |
| J-RHYTHM | Rate Control | 404 | 64.5 ± 12.3 | 69.6 | 165 (40.8%) | 16 (4%) | 26 (6.4%) | 31 (7.7%) | 240 (59.4%) | 177 (43.9%) |
| Rhythm Control | 419 | 64.9 ± 10.3 | 69 | 187 (44.6%) | 14 (3.3%) | 20 (4.8%) | 30 (7.2%) | 252 (60.1%) | 305 (72.7%) | |
| Ockun et al. 2004 | Rate Control | 84 | 58 ± 12 | 68 | 45 (54%) | — | 0 (0%) | 0 (0%) | 100 (100%) | 36 (42.9%) |
| Rhythm Control | 74 | 61 ± 10 | 66 | 33 (47%) | — | 0 (0%) | 0 (0%) | Discontinued after the 1st month of cardioversion. | 33 (47%) | |
| Rate Control | 125 | 61 ± 9 | 74 | 67 (54%) | — | 19 (15%) | 33 (26%) | 125 (100%) | 9 (10%) | |
| PIAF | Rhythm Control | 127 | 60 ± 10 | 72 | 56 (46%) | — | 22 (17%) | 26 (20%) | 127 (100%) | 45 (56%) |
| Rate Control | 256 | 68 ± 9 | 63 | 110 (43%) | 131 (51%) | 46 (18%) | 74 (29%) | 96% | 26 (10%) | |
| RACE | Rhythm Control | 266 | 68 ± 8 | 64 | 146 (55%) | 130 (49%) | 43 (16%) | 69 (26%) | 86% | 103 (39%) |
| Rate Control | 100 | 66.2 ± 7.6 | 68 | 62 (62%) | — | 16 (16%) | 53 (53%) | 100 (100%) | 9 (9%) | |
| STAF | Rhythm Control | 100 | 65.3 ± 9.4 | 59 | 63 (63%) | — | 10 (10%) | 34 (34%) | 100 (100%) | 38 (38%) |
| Rate Control | 66 | 57 ± 11 | 50 | 66 (100%) | — | 0 (0%) | 0 (0%) | 100 (100%) | 0 | |
| Yidiz et al. 2008 | Rhythm Control | 155 | 61 ± 9 | 48.3 | 155 (100%) | — | 0 (0%) | 0 (0%) | Discontinued after the 1st month of cardioversion | 62 (40%) |
Abbreviations: AFFIRM: Atrial Fibrillation Follow-up Investigation of Rhythm Management study, AF CHF: Atrial Fibrillation and Congestive Heart Failure study, CAFÉ-II:controlled study of rate versus rhythm control in patients with chronic AF and HF, HOT CAFÉ: How to Treat Chronic Atrial Fibrillation study, J RHYTHM: Japanese Rhythm Management Trial for Atrial Fibrillation, PIAF: Pharmacological Intervention in Atrial Fibrillation study, RACE: Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation study, STAF: Strategies of Treatment of Atrial Fibrillation study.
Risk of Bias Assessment
All included studies had a low risk of selection (random sequence generation), attrition, and reporting biases, except for AFFIRM trial20,21 (which did not clarify their randomization method), as well as CAFÉ II (16) and RACE15,22 (which had an unclear risk of reporting bias). None of the included studies achieved or reported on blinding of patients and personnel or blinding of outcome assessors. Figure 2 shows a summary of the results of ROB assessment for each included study and the details of authors’ judgements are illustrated in Supplementary file 2.
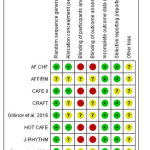 |
Figure 2: Risk of bias summary for included randomized trials
|
Clinical Outcomes
All-Cause Mortality
The pooled effect estimate of 12 studies (8451 patients),9-22 under the random-effects model, showed no significant difference between rate and rhythm control group (RR = 1.13, 95% CI [0.88, 1.45], p = 0.32) in terms of all-cause mortality. Pooled studies were heterogeneous (p = 0.04, I2 = 46%) Figure 3a. Heterogeneity was best resolved (p = 0.21, I2 = 24%) by excluding the study by Okcun et al (2004), while the overall estimate remained non-significant (p = 1). A symmetrical funnel plot showed no evidence of publication bias.
Cardiovascular Mortality
The pooled effect estimate of seven studies (6676 patients)9,11,12,15,16,19,20 showed comparable rates of cardiovascular mortality (RR = 1, 95% CI [0.88, 1.14], p = 0.97) between both groups. Pooled studies were homogenous (p = 0.69, I2 = 0%); Figure 3b.
Arrhythmic Mortality
The pooled effect estimate of five studies (6410 patients) 9,12,15,19,20 showed no significant difference (RR = 1.12,95% CI [0.91,1.38], p = 0.28) between both groups in terms of arrhythmic mortality. Pooled studies were homogenous (p = 0.80, I2 = 0%); Figure 3c.
 |
Figure 3: Forest plots of risk ratios for A) All-cause mortality, B) Cardiovascular mortality, and C) Arrhythmic mortality.
|
Stoke/TIA
The overall risk ratio of 10 included studies (8138 patients)9-15,17,18,20-22 did not favor either of the two groups (RR = 0.97, 95% CI [0.79, 1.20], p = 0.77) in terms of the incidence of stroke/TIA. Pooled studies were homogenous (p = 0.27, I2 = 19%). A symmetrical funnel plot showed no evidence of publication bias; Figure 4a.
Systemic Embolism
The overall risk ratio of nine studies (6929 patients) 9-11,13-15,18-22 showed no significant difference (RR = 1.06, 95% CI [0.64, 1.76], p = 0.83) between both groups regarding the risk of systemic embolism. Pooled studies were homogenous (p = 0.41, I2 = 3%); Figure 4b.
Heart Failure/Worsening of Heart Failure
The pooled risk ratio of nine included studies (7933 patients),9,10,12-15,17,18,20 under the random-effects model, showed no significant difference (RR = 1.04, 95% CI [0.79, 1.38], p = 0.76) between both groups in terms of development or worsening of HF. Pooled studies were heterogeneous (p = 0.09, I2 = 42%). Heterogeneity was best resolved (p = 0.41, I2 = 3%) by excluding the study by Okcun et al (2004), while the pooled estimate remained non-significant (p = 0.78); Figure 4c.
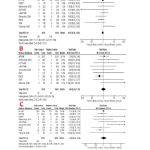 |
Figure 4: Forest plot of risk ratios for A) Stroke/Transient Ischemic Attack, B) Systemic embolism, and C) Development or worsening of heart failure
|
Supplementary file 1 shows the detailed search strategy, used in different electronic databases
| Database | Literature Search Strategy | Results |
| PubMed | ((Atrial fibrillation) OR AF OR (A-fib) OR AFib) AND ((Postoperative OR (cardiac surgery)) OR ((Heart failure) OR (Congestive heart failure) OR HF OR CHF OR (Congestive cardiac failure) OR CCF) AND (((Rate control) OR (Beta blockers) OR (β-blockers) OR (Calcium channel blockers) OR CCB OR (Cardiac glycosides) OR Antiarrhythmic OR Digoxin) AND (Cardioversion OR (rhythm control)))) (“clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH Terms] OR “clinical trial”[All Fields] OR (randomized controlled)) | 427 |
| SCOPUS | (TITLE-ABS-KEY ((atrial fibrillation) OR AF OR AFib) AND TITLE-ABS-KEY ((postoperative OR (Cardiac surgery)) OR ((Heart failure) OR (Congestive heart failure) OR HF OR CHF OR (Congestive cardiac failure) OR CCF) AND (((Rate control) OR (beta blockers) OR (β-blockers) OR (Calcium channel blockers) OR CCB OR (Cardiac glycosides) OR Antiarrhythmic OR Digoxin) AND (Cardioversion OR (Rhythm control)))) AND TITLE-ABS-KEY (Clinical trial)) | 902 |
| ISI Web of Science | (((Atrial fibrillation) OR AF OR (A-fib) OR AFib) AND ((Postoperative OR (Cardiac surgery)) OR ((Heart failure) OR (Congestive heart failure) OR HF OR CHF OR (Congestive cardiac failure) OR CCF) AND (((Rate control) OR (Beta blockers) OR (β-blockers) OR (Calcium channel blockers) OR CCB OR (Cardiac glycosides) OR Antiarrhythmic OR Digoxin) AND (Cardioversion OR (Rhythm control)))) AND (clinical trial)) | 892 |
| Cochrane CENTRAL | (((Atrial fibrillation) OR AF OR (A-fib) OR AFib) AND ((Postoperative OR (Cardiac surgery)) OR ((Heart failure) OR (Congestive heart failure) OR HF OR CHF OR (Congestive cardiac failure) OR CCF) AND (((Rate control) OR (Beta blockers) OR (β-blockers) OR (Calcium channel blockers) OR CCB OR (Cardiac glycosides) OR Antiarrhythmic OR Digoxin) AND (Cardioversion OR (Rhythm control)))) AND ((Clinical trial) OR (Randomized controlled))) | 762 |
| EMBASE | (((Atrial fibrillation) OR AF OR (A-fib) OR AFib) AND ((Postoperative OR (Cardiac surgery)) OR ((Heart failure) OR (Congestive heart failure) OR HF OR CHF OR (Congestive cardiac failure) OR CCF) AND (((Rate control) OR (Beta blockers) OR (β-blockers) OR (Calcium channel blockers) OR CCB OR (Cardiac glycosides) OR Antiarrhythmic OR Digoxin) AND (Cardioversion OR (Rhythm control)))) | 537 |
| OVID | (((Atrial fibrillation) OR AF OR (A-fib) OR AFib) AND ((Postoperative OR (Cardiac surgery)) OR ((Heart failure) OR (Congestive heart failure) OR HF OR CHF OR (Congestive cardiac failure) OR CCF) AND (((Rate control) OR (Beta blockers) OR (β-blockers) OR (Calcium channel blockers) OR CCB OR (Cardiac glycosides) OR Antiarrhythmic OR Digoxin) AND (Cardioversion OR (Rhythm control)))) AND (clinical trial)) | 401 |
Supplementary file 2: Risk of bias assessment for included randomized trials.
| AF CHF | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed with permuted blocks of various sizes and was stratified according to the study center”. |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | “Patients were randomly assigned to either the rhythm control group or the rate control group in an unblinded fashion”. |
| Blinding of outcome assessment (detection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | “Prespecified analyses were performed according to the intention-to-treat principle”. |
| Selective reporting (reporting bias) | Low risk | The study’s pre-specified (primary and secondary) outcomes have been reported in the protocol: NCT00000609. |
| Other bias | Unclear | |
| AFFIRM | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Unclear | The method of randomization is not adequately described. |
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | Unclear | Blinding is not mentioned. However, according to the study protocol/methodology, blinding was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear | |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data were adequately addressed by intention-to-treat analysis. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes were adequately reported. |
| Other bias | Unclear | |
| CAFÉ II | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly allocated (block randomisation with variable block size) to either a rate control or rhythm control strategy”. |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | “The present study was unblinded. Patient and observer bias could have influenced all of the results” |
| Blinding of outcome assessment (detection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | “All analyses were based on the intention-to-treat principle”. |
| Selective reporting (reporting bias) | Unclear | Some important outcomes as bleeding and thromboembolic events were not adequately reported. We could not reach the protocol to identify if these were targeted outcomes. |
| Other bias | Unclear | |
| CRAFT | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “All patients who consented to participate in the study were randomized in an open design to either the rate control group or the rhythm control group in a ratio of 1:2”. |
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | High risk | A part of the study that compared amiodarone to placebo (rhythm control) was double blinded, while the rate control group was not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear | It is not mentioned in the article whether the assessors were blinded to patient allocation. |
| Incomplete outcome data (attrition bias) | Low risk | During the 12-month study period, 10 group I patients (6 in the amiodarone subgroup and 4 in the placebo subgroup) and 8 group II patients were lost to follow-up and were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes were adequately reported. |
| Other bias | Unclear | |
| Gillinov 2016 | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “Patients with new-onset postoperative atrial fibrillation were randomly assigned to undergo either rate control or rhythm control”. |
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | Unclear | Blinding is not mentioned. However, according to the study protocol/methodology, blinding was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear | |
| Incomplete outcome data (attrition bias) | Low risk | “The majority of patients who discontinued or switched therapy did so for protocol- specified clinical reasons (80.0% in the rate control group and 64.5% in the rhythm-control group)”. |
| Selective reporting (reporting bias) | Low risk | The study prespecified the outcomes in a published protocol (NCT02132767). |
| Other bias | Unclear | |
| HOT CAFE | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “The randomization of eligible patients was performed by per-muted block design with equal allocation and was stratified centrally by a steering center of chair and department of cardiology” |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | The HOT CAFE study was a prospective, randomized, open |
| Blinding of outcome assessment (detection bias) | High risk | multicenter clinical trial |
| Incomplete outcome data (attrition bias) | Low risk | “The trial was conducted on an intention-to-treat basis”. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes were reported. |
| Other bias | Unclear | |
| J-RHYTHM | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned to either the rate control or rhythm control treatment group”. |
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | High risk | “The endpoint representing patient acceptance in our study may be biased by the impressions of both patients and physicians who were unblinded to treatment strategy”. |
| Blinding of outcome assessment (detection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | “The average follow-up period was 578 days, and 62 patients dropped out (7%) during the study: rate control group, n=38; rhythm control group, n=24 “. |
| Selective reporting (reporting bias) | Low risk | The study prespecified the outcomes in a published protocol (Umin-CTR No. C000000106). |
| Other bias | Unclear | |
| ÖKÇÜN et al. 2004 | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “The patients were randomized to either the rhythm control group or rate control group” |
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | Unclear | Blinding is not mentioned. However, according to the study protocol/methodology, blinding was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear | |
| Incomplete outcome data (attrition bias) | Low risk | Data were adequately reported for all participants. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes were reported. |
| Other bias | Unclear risk | |
| PIAF | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned (randomisation codes generated per centre, allocation of patients in blocks of six)”. |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | “PIAF was designed as an open, randomised pilot study to |
| Blinding of outcome assessment (detection bias) | High risk | compare two different treatment strategies”. |
| Incomplete outcome data (attrition bias) | Low risk | Only 6 patients were lost to follow up. |
| Selective reporting (reporting bias) | Unclear | Some important outcomes as bleeding and thromboembolic events were not adequately reported. We could not reach the protocol to identify if these were targeted outcomes. |
| Other bias | Unclear | |
| RACE | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “522 patients (256 with hypertension) were randomized to rate or rhythm control” |
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | Unclear | Blinding is not mentioned. However, according to the study protocol/methodology, blinding was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear | |
| Incomplete outcome data (attrition bias) | Low risk | Data were adequately reported for all participants. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes were reported. |
| Other bias | Unclear | |
| STAF | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “Patients were randomized to one of two treatment |
| Allocation concealment (selection bias) | Low risk | strategies (randomization codes computer generated per study center, blocks of 10 patients, and randomization by calling the study center)”. |
| Blinding of participants and personnel (performance bias) | High risk | “The Strategies of Treatment of Atrial Fibrillation (STAF) study was an open, randomized” |
| Blinding of outcome assessment (detection bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Data were adequately reported for all participants. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes, including mortality and thromboembolic events, were reported. |
| Other bias | Unclear | |
| Yidiz et al. 2008 | Risk of bias | Reason/Quotation |
| Random sequence generation (selection bias) | Low risk | “The patients were randomly assigned to either the rhythm |
| control group or rate control group”. | ||
| Allocation concealment (selection bias) | Unclear | The method of allocation concealment is not mentioned. |
| Blinding of participants and personnel (performance bias) | Unclear | Blinding is not mentioned. However, according to the study protocol/methodology, blinding was not possible. |
| Blinding of outcome assessment (detection bias) | Unclear | |
| Incomplete outcome data (attrition bias) | Low risk | Data were adequately reported for all participants. |
| Selective reporting (reporting bias) | Low risk | All-important outcomes, including mortality and thromboembolic events, were reported. |
| Other bias | Unclear |
Major/Life Threatening Bleeding
A pooled analysis of 10 included studies (8138 patients)9-15,17,18,20-22 did not favor either of the two groups (RR = 1.10, 95% CI [0.90, 1.35], p = 37) in terms of major bleeding. Pooled studies were homogenous (p = 0.37, I2 = 7%). A symmetrical funnel plot showed no evidence of publication bias.
Rehospitalization
A pooled analysis of seven included studies (6701 patients)9-12,17,19,20 showed that rehospitalization rates were significantly lower in the rate control group (RR = 0.72, 95% CI [0.57, 0.92], p = 0.009), compared to the rhythm control group. Pooled studies were significantly heterogeneous (p < 0.00001, I2 = 88%) that removal of any included study by the Leave-One-Out method could not resolve such heterogeneity.
Subsequent Myocardial Infarction
Pooling data from two RCTs (5436 patients)12,20,21 showed no significant difference (RR = 0.86, 95% CI [0.64, 1.17], p = 0.34) between rate and rhythm control groups regarding the risk of subsequent MI. Pooled studies were homogenous (p = 0.34, I2 = 0%).
Subgroup Analysis
Heart Failure Patients (Grade II – IV)
Subgroup analysis of data from three trials (1637 patients)9,12,16 collected from patients with grade II to IV HF, showed no significant difference between both groups in terms of all-cause mortality (RR = 1.05, 95% CI [ 0.90, 1.22]), cardiovascular mortality (RR = 0.99, 95% CI [0.83, 1.18]), stroke/TIA (RR = 0.85, 95% CI [039, 1.82]), development/worsening of HF (RR = 0.98, 95% CI [0.87, 1.11]), and rehospitalization rates (RR = 0.72, 95% CI [0.34, 1.49]). Except for rehospitalization (Chi-square p = 0.0002), pooled studies were homogenous in all outcomes (p > 0.1).
Age under 65 years old
Interestingly, when pooling data from younger patients (four studies, 681 patients),14,17–19 the overall risk ratio showed a higher risk of all-cause mortality (RR = 3.18, 95% CI [1.71, 5.92]), HF (RR = 3.84, 95% CI [1.57, 9.37]), and major bleeding (RR = 5.07, 95% CI [1.29, 19.90]) in the rate control group, compared to the rhythm control group. However, both groups were comparable in terms of stroke (RR = 1.26, 95% CI [0.61, 2.85]) and systemic embolism rates (RR = 2.90, 95% CI [0.71, 11.89]). Pooled studies in all outcomes were homogenous (p > 0.1).
Sensitivity Analysis
All the effect-estimates remained robust when we removed the two largest studies (AF CHF and AFFIRM), except for all-cause mortality. Upon removal of AF CHF and AFFIRM trials, which reported a non-significant increase in all-cause mortality in the rhythm control group, the effect estimate favored rate control over rhythm control (RR = 1.66, 95% CI [1.15, 2.39], p = 0.006) regarding this particular outcome. The detailed results of sensitivity analysis are shown in Table 3.
Table 3: Results of sensitivity analysis after excluding the largest two studies (AF CHF and AFFIRM)
| Outcome | Risk Ratio | 95% Confidence Interval | P value | Number of included studies | Chi-Square P value | I-Square | |
| All-cause Mortality | 1.66 | [1.15, 2.39] | 0.006 | 10 | 0.25 | 21% | |
| Cardiovascular Mortality | 1.18 | [0.71, 1.98] | 0.52 | 5 | 0.51 | 0% | |
| Arrhythmic Mortality | 1.12 | [0.51, 2.46] | 0.78 | 3 | 0.6 | 0% | |
| Stroke/Transient ischemic attack | 0.88 | [0.60, 1.29} | 0.51 | 8 | 0.15 | 35% | |
| Systemic embolism | 0.98 | [0.54, 1.78] | 0.95 | 8 | 0.34 | 12% | |
| Heart Failure | 1.14 | [0.84, 1.53] | 0.4 | 7 | 0.03 | 58% | |
| Major Bleeding | 1.27 | [0.85, 1.89] | 0.25 | 8 | 0.3 | 16% | |
| Rehospitalization | 0.62 | [0.52, 0.73] | <0.00001 | 5 | <0.00001 | 86% | |
Abbreviations: AFFIRM: Atrial Fibrillation Follow-up Investigation of Rhythm Management study, AF CHF: Atrial Fibrillation and Congestive Heart Failure study
Discussion
Our meta-analysis of 12 studies showed no significant difference between rate and rhythm control groups in terms of mortality rates and other major clinical outcomes (including bleeding and thromboembolic events), except for rehospitalization rate, which was significantly higher in the rhythm control group. Across the five studies9,12,15,19,20 that investigated arrhythmic, cardiovascular, and all-cause mortality, cardiovascular mortality represented 63.8% of all-cause mortality, while arrhythmic death represented 45.5% of cardiovascular and 29% of all-cause mortality.
Although restoring a physiological cardiac rhythm is hypothesized to lower the risk of mortality and embolic events, our analysis shows that neither strategy was superior to the other in these regards. A possible explanation is that the survival benefit of rhythm control is likely to be negated by the non-cardiac side-effects of anti-arrhythmic drugs.28,29
The higher frequency of rehospitalization in the rhythm control group may be explained by the possible occurrence of dysrhythmias, as a complication of anti-arrhythmic drugs and the need to perform cardioversion in a monitored environment.20,30 This higher rate of rehospitalization can be translated to a higher treatment cost in the rhythm control group, which is confirmed by real-world data from observational studies.31–33
We performed a subgroup analysis for AF patients with concomitant CHF because this comorbidity affects more than 50% of AF patients and its interaction with AF means that none of them can be optimally managed without treating the other.9,12 Except for rehospitalization rate, mortality and clinical outcomes’ results were similar to those of the main analysis. This may be explained by the fact that these patients require frequent hospitalization for management of CHF, regardless the method of AF control.
Interestingly, rhythm control strategy was associated with lower rates of mortality, HF, and major bleeding than rate control in younger patients (mean age below 65 years), probably by delaying the progression to permanent AF, which has a higher rate of complications23: This finding is supported by real life data from the RECORDAF registry (Registry on Cardiac Rhythm Disorders AF), established following the AFFIRM trial.34
Postoperative AF occurs in 20% to 50% of patients following cardiac surgery.35,36 An included study by Gillinov et al. showed no significant difference between rate and rhythm control strategies in terms of mortality and complication rates in postoperative patients.10 Additionally, about 17 to 18% of rheumatic patients develop AF.8 The included CRAFT trial showed that rhythm control was superior to rate control in rheumatic heart patients in terms of reducing mortality and improving quality of life and exercise capacity.17
Strength Points
Compared to the formerly mentioned meta-analyses,23,24 our analysis included a larger number of trials, with a fairly higher sample size. We performed subgroup analyses for younger patients and those with HF and conducted a sensitivity analysis to ensure that our results were not affected by the weights of individual studies. Unlike previous meta-analyses, we performed a thorough risk of bias assessment and investigated for publication bias, whenever appropriate.
Limitations
All included trials were open-label studies because the nature of electric cardioversion in the rhythm control group prevents proper blinding and applying a fake electrical cardioversion protocol is ethically controversial and would interfere with the results of other outcomes, such as rehospitalization rate. The main weight of our analysis comes from the two largest trials (AF CHF and AFFIRM); therefore, we performed a sensitivity analysis by excluding these trials to overcome this limitation. We did not assess the impact of either strategy on quality of life outcomes because these data were poorly reported in included studies. Future trials should further investigate the effect of other comorbidities, such as stroke and left ventricular dysfunction on the treatment outcomes. We are aware of few ongoing studies, comparing both strategies, in different categories of AF patients, such as AFARC-LVF trial (NCT02509754) and RAFT-AF trial (NCT01420393).
Conclusion
In older AF patients and those with concomitant CHF, both rate and rhythm control strategies have similar rates of mortality and major clinical outcomes; therefore, choosing an appropriate therapeutic strategy should consider individual variations such as patient preferences, comorbidities, and treatment cost. Future trials should compare both strategies in younger patients and those with other comorbidities such as stroke and left ventricular dysfunction.
Confilect of Interests
There is no confilect of interests
Funding
There is no funding source
Acknowledgement
The author(s) received no specific funding for this work.
References
- Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, et al., Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. European heart journal. 2006;27(8):949-53.
CrossRef - Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561–4.
CrossRef - Copley DJ, Hill KM. Atrial Fibrillation: A Review of Treatments and Current Guidelines. AACN Adv Crit Care. 2016;27(1):120–8.
CrossRef - Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–354.
CrossRef - Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–47.
CrossRef - Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994;74(3):236–41.
CrossRef - Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8a):2n–9n.
CrossRef - Wyse DG. Pharmacologic approaches to rhythm versus rate control in atrial fibrillation–where are we now? Int J Cardiol. 2006;110(3):301–12.
CrossRef - Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation. Journal of the American College of Cardiology. 2003;41(10):1690.
CrossRef - Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. New England Journal of Medicine. 2016;374(20):1911–21.
CrossRef - Opolski G, Torbicki A, Kosior DA, Szulc M, WozĚ B, KoĹ P, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. CHEST Journal. 2004;126(2):476–86.
CrossRef - Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al., Rhythm control versus rate control for atrial fibrillation and heart failure. New England Journal of Medicine. 2008;358(25):2667–77.
CrossRef - Ogawa S, Yamashita T, Yamazaki T, Aizawa Y, Atarashi H, Inoue H, et al., Optimal treatment strategy for patients with paroxysmal atrial fibrillation. Circulation Journal. 2009;73(2):242–8.
CrossRef - Ökçün B, Yigit Z, Arat A, Küçükoglu MS. Comparison of rate and rhythm control in patients with atrial fibrillation and nonischemic heart failure. Japanese heart journal. 2004;45(4):591–601.
CrossRef - Rienstra M, Van Veldhuisen DJ, Crijns HJGM, Van Gelder IC. Enhanced cardiovascular morbidity and mortality during rhythm control treatment in persistent atrial fibrillation in hypertensives: data of the RACE study. European heart journal. 2007;
CrossRef - Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, et al., A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure:(CAFE-II Study). Heart. 2009;95(11):924–30.
CrossRef - Vora A, Karnad D, Goyal V, Naik A, Gupta A, Lokhandwala Y, et al., Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian heart journal. 2004;56(2):110–6.
- Yildiz A, Yigit Z, Okcun B, Baskurt M, Ortak K, Kaya A, et al., Comparison of rate and rhythm control in hypertension patients with atrial fibrillation. Circulation Journal. 2008;72(5):705–8.
CrossRef - Hohnloser SH, Kuck K-H, Lilienthal J, Investigators P. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. The Lancet. 2000;356(9244):1789–94.
CrossRef - Investigators AFFI of RM. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;2002(347):1825–33.
- Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J, et al., Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109(16):1973–80.
CrossRef - Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al., A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. New England Journal of Medicine. 2002;347(23):1834–40.
CrossRef - Chatterjee S, Sardar P, Lichstein E, Mukherjee D, Aikat S. Pharmacologic Rate versus Rhythm‐Control Strategies in Atrial Fibrillation: An Updated Comprehensive Review and Meta‐Analysis. Pacing and Clinical Electrophysiology. 2013;36(1):122–33.
CrossRef - De Denus S, Sanoski CA, Carlsson J, Opolski G, Spinler SA. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Archives of Internal Medicine. 2005;165(3):258–62.
CrossRef - Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of interventions. The Cochrane Collaboration. 2008.
CrossRef - Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed.). British Medical Journal Publishing Group. 2009;339(17):b2535.
CrossRef - Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.). 1997;315(7109):629–34.
- Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. The Lancet. 1997;349(9053):667–74.
CrossRef - Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. New England Journal of Medicine. 2005;352(3):225–37.
CrossRef - Cordina J, Mead GE. Pharmacological cardioversion for atrial fibrillation and flutter. The Cochrane Library. 2005;
- Hagens VE, Vermeulen KM, TenVergert EM, Van Veldhuisen DJ, Bosker HA, Kamp O, et al. Rate control is more cost-effective than rhythm control for patients with persistent atrial fibrillation—results from the RAte Control versus Electrical cardioversion (RACE) study. European heart journal. 2004;25(17):1542–9.
CrossRef - Marshall DA, Levy AR, Vidaillet H, Fenwick E, Slee A, Blackhouse G, et al. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Annals of Internal Medicine. 2004;141(9):653–61.
CrossRef - Pietrasik A, Kosior DA, Niewada M, Opolski G, Latek M, Kamiñski B. The cost comparison of rhythm and rate control strategies in persistent atrial fibrillation. International journal of cardiology. 2007;118(1):21–7.
CrossRef - Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey J-Y, et al. Real-life observations of clinical outcomes with rhythm-and rate-control therapies for atrial fibrillation: RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation). Journal of the American College of Cardiology. 2011;58(5):493–501.
CrossRef - Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. Journal of the American College of Cardiology. 2008;51(8):793–801.
CrossRef - Nair SG. Atrial fibrillation after cardiac surgery. Annals of cardiac anaesthesia. 2010;13(3):196.
CrossRef








