Manuscript accepted on :28 May 2018
Published online on: 26-06-2018
Plagiarism Check: Yes
Sri Masyeni1, Usman Hadi2, K Kuntaman2 and Yorapermata Dewi1
1Faculty of Medicine and Health Science, University of Warmadewa, JlTerompong 24, Denpasar-Bali,Indonesia.
2Faculty of Medicine, University of Airlangga, JlMayjen Prof. Dr. Moestopo 47, Pacar Kembang, Surabaya, Kota SBY, Jawa Timur, Indonesia.
Corresponding Author E-mail: masyeniputu@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1449
Abstract
The role of microRiboNucleic Acids (miRNA), a small-non coding RNA has been associated with immune regulation in various viral infectionincluding dengue infection. The microRNA will bind a specific protein target in order to encourage an explosive expression of various cytokines, known as cytokines storm in Dengue infection.The objective of this study aimed to determine and evaluate themicroRNAs profile expression withinperipheral blood mononuclear cells having been infected with one of the dengue virus serotype.To obtained the PBMCs from a healthy donor, Ficoll density gradient centrifugation was used to isolate the PBMCs and then followed infecting it with a DENV-2 clinical isolate. Prior to PBMCs isolation, the virus has been propagated and having titration to get an optimal virus titer. We conducted the infection at the multiplication of infections 4 PFU/106 cells.MiRCURYLNATMExiqon was utilized on purpose to extract the RNA. Quantitative Real-Time PCR was applied in order for the miRNAs relative expression to be measured. The preliminary result reveals that miR-150, miR-146a, hsa-let-7e expression were increased 1.74 folds, 2 folds, and 1.49 foldsrespectively at 12 hours post-infection on PBMCs upon DENV-2 infection.The expression of microRNAswas discovered to behigher inPBMCsat the time of infection withDENV-2.ThemiRNAs expression in the uninfected PMBCs was lower than that of the miRNA. This high expression of miRNAsin dengue infection may proceedto dengue infection pathogenesis.
Keywords
Dengue; Expression; Infection; MiRNA
Download this article as:| Copy the following to cite this article: Masyeni S, Hadi U, Kuntaman K, Dewi Y. Profiling of MicroRNA Expression within the Cells of Peripheral Blood Mononuclearafter an Infection with Serotype-2 of Dengue Virus: Preliminary Study. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Masyeni S, Hadi U, Kuntaman K, Dewi Y. Profiling of MicroRNA Expression within the Cells of Peripheral Blood Mononuclearafter an Infection with Serotype-2 of Dengue Virus: Preliminary Study. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20882 |
Introduction
The prevalence of DENV infection in Asia-Pacific region has been reported as hyper endemic in which all the serotypes of the virus were discovered circulating with in the countries.1–4 The most dengue presentations mostly mild form, but actually it were identified to be widely varied from asymptomatic, mild dengue fever (DF), and dengue hemorrhagic fever (DHF), including the dengue shock syndrome which is life-threatening (DSS).5 Themild dengue manifestations (DF) are fever accompanied by a severe headache, retro-orbital pain, arthralgia, myalgia, rash and minor hemorrhage such as petechiae, epistaxis or gum bleeding. In contrast with more severe dengue, dengue hemorrhagic fever (DHF) is characterized by all symptom of DF and occurrence of plasma leakage, such as hemoconcentration, ascites, or pleural effusion.5 Severe plasma leakages lead the patients fall to DSS with relatively high mortality rate particularly in the population such as younger, elderly, pregnant, or the patients with pre-existing illnesses. Despite many studies having been conducted, dengue pathogenesis is not fully understood. In addition, several studies have explored the factors to predict the disease progression between DF to DHF or DSS.6–9 Studies in Southeast Asian countries found the issues related to the dengue severity are immune status, genetics of the population, viremia titers, serotypes divergences, and the abundant release of cytokines.8,10,11 The non-neutralizing antibody of theprevious dengue virus infection, dengue serotype-2 found to bethe critical role to getsevere DENV infection.12,16 The role of cytokines in dengue pathogenes is has been related to the process of leakage within the endothelial cell.13–16 The elevated levels of IL-6, IL-10, IFN-γ, MIF, and CCL-4 might be used as a potential predictors to get severe dengue.17–21 The study of cytokines gene expression within human peripheral blood monocyte-derived macrophages having been infected with dengue virus serotype-2 which found IL-8, IL-1b, osteopontin, GRO-a, -b and -g, I-309 genes expression, was significantly larger compared to that of the uninfected.22
The expression of cytokines encoding gene may be controlled by several number of endogenous or exogenous factors. A small, short, non-coding RNA, contains an18-22 nucleotide named micro RNA which has been found to be functional primarily by attaching on the 3’UTR un-translated area of mRNA target, resulting in impediment of translation or mRNA degradation.23 The miRNA has been predicted as a regulator of cytokine gene expression by binding a specific protein, resulting in uncontrolled expression of the cytokine gene. The Role of miRNA in the production of cytokines regulation, cytokine which signals and facilitates the stability of Th1/Th2 polarization,has been studied elsewhere.23,24 The proteinmiRNAs such as miR-146a, miR-30e*, miR150, miR-548g-3p as well as the others are induced by DENV and associated with DENV replication or cytokines induction.25–29 The current study reportsthemiR-150 expression, has-let-7e and miR-30e* in infected PBMCsversusnon-infected with local clinical isolate (Semarang DENV-2 isolate).
Materials and Methods
This research was assessed as well asaccepted by Institutional Review Board of the Faculty of Medicine, Udayana University(Approval No: 485/UN.14.2/KEP/2017). The study was an experimental study in regards to peripheral blood mononuclear cells having been infected with dengue virus serotype-2. The DENV-2 was isolated from the local sample (Semarang, Indonesia isolate). Sub-cultured propagating the virus was conducted in C6/36 mosquito cells. In addition,the virus titer was verified through plaque assay as depicted in the prior report.31 Prior to the process of obtaining blood samples being performed, a healthy don or was given written consent. By utilizing Ficoll-Hypaque density gradient centrifugation, PBMCs were placed in isolation. All the PBMCs are re-suspended through identical medium with 10% inactivated fetal calf serum after having been sterilized by using RPMI-1640 medium, Culturing and incubating the PBMCs at 37°C in 5% CO2 for 6-12 hours was the last procedure conducted in the process. Infecting thePBMCs with DENV-2 within medium with the infection multiplicity(MOI) was then performed following the procedure mentioned above. The cells were placed in theincubation at 37°C and sampled at 6, 12 and 24 hours post-infection. Virus growth characteristic in the cell line was determined with a viral kinetic assay in duplications. MiRCURYLNATM Exiqon (Sweden) RNA isolation kit was usedin a single step as described by the kit manufacturer’s instructions in order for the RNA to be able to be totally extracted. The RNA quality and quantity were evaluated with Qubit 3 fluorophotometer. The internal reference genes werenon-coding nuclear RNA (snRNA U6) for miRNA which are small in size. In order to be able to analyze the relative miRNA expression, Quantitative real-time polymerase chain reaction (qRT-PCR) (Biorad, USA) wasapplied in the process. Total RNA of the donor was used for detection of miRNAs has-let-7e, miR-30e*, miR-150, miR-146a and has-miR-4290.
Results
The supernatants were obtained at 6, 12 and 24 hours post DENV-2 infection. At the 12th observation of the observation time, the expression of miR-150was foundup-regulated 1.78 fold swhich are higher than the uninfected sample.The miRNAhsa-let-7e was expressed 1.49 folds at 12th hpost infection in infected samples compared with that of the uninfected sample. The expression of miR-146a was discovered as high as 2 folds in 12th h post infection compared with the uninfected sample. On the other hand, the miRNA, miR-30e* and miR-4290 were not expressed as high as the othermiRNA (data not shown) (Figure 1).The target gene of the miRNA such as suppressor of cytokine signaling (SOCS-1 or SOCS-3) was also evaluated in the current study. In addition, it was detected that the immunological responses of the PBMCs were stimulatedby DENV-2 infection through the qRT-PCR expressionprofiling of genes encoding cytokines and chemokine namely IL-6, IL-8, IP-10 and MIP-1β (data not shown).
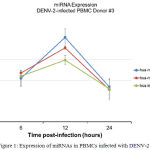 |
Figure 1: Expression of miRNAs in PBMCs infected with DENV-2
|
Discussion
Vascular leakage is a hallmark of severe dengue manifestation which is a leading factor in morbidity and mortality in dengue shock syndrome.15,30 Elevation of several cytokines in dengue infection has been widely correlated with the vascular leakage in which the fluid accumulation on the third space such as pleural effusion, ascites or even shock, may present as the manifestation of plasma leakage.33,34 Control of cytokine overproduction by the protein such as SOCS may be lostdue to the binding of this protein by particular miRNA.Up- regulation of miR-150 has been reported to correlate with low expression of SOCS-1 in the leucocyte patient with DHF.26 In our study, although the expression miR-150was not as high as with another study which was expressed 7.16 folds in the serum samples of DHF than DF (p=0.008).26 This discovery seems to support the involvement of miR-150 within the pathogenesis of DENV infection. This difference across all types of miR-150 expression may possibly be due to the difference in terms of microRNA time points sampling procedure, different DENV infection methods conducted in our research, which is lower compared to the previous study.26 High expression of miR-146a, miR-30e*, miR-150, miR548g-3p in DENV infection which suggests the role important of miRNA in the DENV pathogenesis has been reported.28,35,36
In contrast, the low expression of miR-4290 and miR-30e* in PBMCs (data not shown)is contrasted with the previous study.27,35 The PBMCs are unstable cells(mortal cell) which even reflect closer in-vivo, may explain this discrepancy with high expression of miR-30e* which was conducted in the human monocyte U937 cell line.35 Meanwhile, the expression of miR-146a is supportedbyanother study.36 In this study the highest expression of miR-146a was only 2-fold at the 12 h post infection in PMBCs, meanwhile another study found the expression as high as 9-fold, 10-fold and 10-fold at 12 h, 24 h and 48 h p.i in human monocyte. We do not able to isolate the monocyte from the PBMCs, which may explained why the expression of the miR-146a is not as high as another study in human monocyte. Since the report is a preliminary report, further data need tobe brought into submission and reported in order to support the miRNA role in DENV infection.To summarize all the details, our research emphasizes the respective role of miRNA in DENV in infected PBMCswhich support other discoveries of miRNA expression in order to find out the new DENV infection pathogenesis.
Conflict of Interest statement
There is no conflict of interest.
Acknowledgements
We thank all team for the great support of this study. For technical assistance, we would like to thank Benediktus Yohan, M. Biomed and R. Tedjo Sasmono, PhD in Dengue Laboratorium of Eijkman Institute Jakarta. This study was supported by Faculty of Medicine and Health Science, University of Warmadewa.
Funding Source
This work was funded by Indonesian Kemenristek Dikti and University of Warmadewa.
References
- Balmaseda A, Hammond SN, Pérez L, et al., Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74(3):449-456. doi:74/3/449 [pii]
- Khan SA, Dutta P, Borah J, Chowdhury P, Doloi P.K, Mahanta J. Dengue outbreak in an Indo-Myanmar boarder area: Epidemiological aspects and risk factors. Trop Biomed. 2013;30(3):451-458.
- Megawati D, Masyeni S, Yohan B, et al., Dengue in Bali: Clinical characteristics and genetic diversity of circulating dengue viruses. PLoS Negl Trop Dis. 2017;11(5). doi:10.1371/journal.pntd.0005483
CrossRef - Nisalak A, Endy TP, Nimmannitya S, et al., Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68(2):191-202. doi:10.1016/j.ijid.2012.05.319.
CrossRef - Who. Dengue and severe dengue. WHO Fact Sheet. 2012:1-4. doi:10.1111/1469-0691.12442
CrossRef - Fox A, Hoa L.N.M, Simmons C.P, et al., Immunological and viral determinants of dengue severity in hospitalized adults in Ha Noi, Viet Nam. PLoS Negl Trop Dis. 2011;5(3).
doi:10.1371/journal.pntd.0000967
CrossRef - OhAinle M, Balmaseda A, Macalalad AR, et al., Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med. 2011;3(114). doi:10.1126/scitranslmed.3003084
CrossRef - Vaughn D.W, Green S, Kalayanarooj S, et al., Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2-9. doi:10.1086/315215
CrossRef - Hammond SN, Balmaseda A, Pérez L, et al., Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73(6):1063-1070. doi:73/6/1063 [pii]
- Clyde K, Kyle JL, Harris E. Recent Advances in Deciphering Viral and Host Determinants of Dengue Virus Replication and Pathogenesis. J Virol. 2006;80(23):11418-11431. doi:10.1128/JVI.01257-06
CrossRef - Hung NT, Lan NT, Lei HY, et al., Association between sex, nutritional status, severity of dengue hemorrhagic fever, and immune status in infants with dengue hemorrhagic fever. Am J Trop Med Hyg. 2005;72(4):370-374. doi:72/4/370 [pii]
- Martina B.E.E, Koraka P, Osterhaus A.D.M.E. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564-581. doi:Doi 10.1128/Cmr.00035-09
CrossRef - Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, et al., Natural history of plasma leakage in dengue hemorrhagic fever: A serial ultrasonographic study. Pediatr Infect Dis J. 2007;26(4):283-290. doi:10.1097/01.inf.0000258612.26743.10
CrossRef - Chaturvedi UC, Agarwal R, Elbishbishi E.A, Mustafa A.S. Cytokine cascade in dengue hemorrhagic fever: Implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28(3):183-188. doi:10.1016/S0928-8244(00)00163-2
CrossRef - Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr Infect Dis J. 2012;31(12). doi:10.1097/INF.0b013e31826fd456
CrossRef - Rathakrishnan A, Wang SM, Hu Y, et al., Cytokine Expression Profile of Dengue Patients at Different Phases of Illness. PLoS One. 2012;7(12). doi:10.1371/journal.pone.0052215
CrossRef - Ferreira R.X, de Oliveira S.A, Gandini M, et al., Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta Trop. 2015;149:138-147. doi:10.1016/j.actatropica.2015.04.023
CrossRef - de-Oliveira-Pinto L.M, Gandini M, Freitas L.P, et al., Profile of circulating levels of IL-1Ra, CXCL10/IP-10, CCL4/MIP-1β and CCL2/MCP-1 in dengue fever and parvovirosis. Mem Inst Oswaldo Cruz. 2012;107(1):48-56. doi:10.1590/S0074-02762012000100007
CrossRef - Houghton-Triviño N, Salgado DM, Rodríguez JA, Bosch I, Castellanos JE. Levels of soluble ST2 in serum associated with severity of dengue due to tumour necrosis factor alpha stimulation. J Gen Virol. 2010;91(3):697-706. doi:10.1099/vir.0.012971-0
CrossRef - Bozza FA, Cruz OG, Zagne SMO, et al., Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis. 2008;8:1-11. doi:10.1186/1471-2334-8-86
CrossRef - Chen L-C, Lei H-Y, Liu C-C, et al., Correlation of serum levels of macrophage migration inhibitory factor with disease severity and clinical outcome in dengue patients. Am J Trop Med Hyg. 2006;74(1):142-147. doi:10.4269/ajtmh.2006.74.142
CrossRef - Moreno-Altamirano M.M, Romano M, Legorreta-Herrera M, Sanchez-Garcia F.J, Colston M.J. Gene expression in human macrophages infected with dengue virus serotype-2. Scand J Immunol. 2004;60(6):631-638. doi:10.1111/j.0300-9475.2004.01519.x
CrossRef - Bartel D.P. MicroRNA Target Recognition and Regulatory Functions. Cell. 2009;136(2):215-233. doi:10.1016/j.cell.2009.01.002.MicroRNA
- Xiao C, Rajewsky K. MicroRNA Control in the Immune System: Basic Principles. Cell. 2009;136(1):26-36. doi:10.1016/j.cell.2008.12.027
CrossRef - Hukowska-Szematowicz B, Tokarz-Deptuła B, Deptuła W. MicroRNA (miRNA) and the immune system. Cent J Immunol. 2012;37(4):387-390. doi:10.5114/ceji.2012.32730
CrossRef - Chen R.F, Yang K.D, Lee I.K, et al., Augmented miR-150 expression associated with depressed SOCS1 expression involved in dengue haemorrhagic fever. J Infect. 2014;69(4):366-374. doi:10.1016/j.jinf.2014.05.013
CrossRef - Zhu X, He Z, Hu Y, et al., MicroRNA-30e* Suppresses Dengue Virus Replication by Promoting NF-κB–Dependent IFN Production. PLoS Negl Trop Dis. 2014;8(8).
doi:10.1371/journal.pntd.0003088
CrossRef - Wen W, He Z, Jing Q, et al., Cellular microRNA-miR-548g-3p modulates the replication of dengue virus. J Infect. 2015;70(6):631-640. doi:10.1016/j.jinf.2014.12.001
CrossRef - Castrillón-Betancur J.C, Urcuqui-Inchima S. Overexpression of miR-484 and miR-744 in vero cells alters Dengue virus replication. Mem Inst Oswaldo Cruz. 2017;112(4):281-291. doi:10.1590/0074-02760160404
CrossRef - Ouyang X, Jiang X, Gu D, et al., Dysregulated serum miRNA profile and promising biomarkers in dengue-infected patients. Int J Med Sci. 2016;13(3):195-205. doi:10.7150/ijms.13996
CrossRef - Yohan B, Kendarsari R.I, Mutia K, Bowolaksono A, Harahap A.R, Sasmono R.T. Growth characteristics and cytokine/chemokine induction profiles of dengue viruses in various cell lines. Acta Virol. 2014;58(1):20-27.
CrossRef - Rothman A.L. Dengue: Defining protective versus pathologic immunity. J Clin Invest. 2004;113(7):946-951. doi:10.1172/JCI200421512
CrossRef - Chaturvedi U.C. Shift to Th2 cytokine response in dengue haemorrhagic fever. Indian J Med Res. 2009;129(1):1-3.
- Jain A, Chaturvedi U.C. Dengue in infants: An overview. FEMS Immunol Med Microbiol. 2010;59(2):119-130. doi:10.1111/j.1574-695X.2010.00670.x
CrossRef - Qi Y, Li Y, Zhang L, Huang J. MicroRNA expression profiling and bioinformatic analysis of dengue virus-infected peripheral blood mononuclear cells. Mol Med Rep. 2013;7(3):791-798. doi:10.3892/mmr.2013.1288
CrossRef - Wu S, He L, Li Y, et al., MiR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. J Infect. 2013;67(4):329-341. doi:10.1016/j.jinf.2013.05.003.
CrossRef







