Alexander A Upnitskiy
Department of Clinical Pharmacology, Pirogov Russian National Research Medical University (RNRMU), 117997, 1 Ostrovitianov Str., Moscow, Russian Federation.
Corresponding Author E-mail: upal2005@yandex.ru
DOI : https://dx.doi.org/10.13005/bpj/1459
Abstract
The aspects of chronic heart failure treatment in age-related risk group are described in the present article. It is established that the optimal treatment duration is 6-12 months. The authors focused on pharmacotherapeutic part only, excluding active application of invasive treatment and diagnostics methods. The analysis of long-term therapy in three parallel groups defined the role of both blood pressure control and pharmacodynamics specifics of the drugs in clinical positive dynamics of the disease development and reverse heart and vessels remodeling. Indication of spironolactone along with the basic blood pressure control therapy increased tolerance to physical loads, improved quality of life in patients with chronic heart failure (CHF), accelerated recovery rate of the disturbed circadian rhythm of blood pressure, significantly reduced left ventricle dimensions and left atrium dilation reversion, decreased systolic blood pressure in pulmonary artery due to significant improvement of left ventricle diastolic function. Indication of trimetazidine in combination with spironolactone lowered functional class of the disease by New York Heart Association (NYHA) functional classification in 66.7% of cases and increased glomerular filtration rate. Nephroprotective activity of trimetazidine confirmed the fact that 80% of patients were rediagnosed with lower stage of chronic kidney disease after the therapy.
Keywords
chronic heart failure, drug indication. morbidity rate decrease, pharmacotherapy, treatment,
Download this article as:| Copy the following to cite this article: Upnitskiy A. A. Pharmacotherapy of Chronic Heart Failure. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Upnitskiy A. A. Pharmacotherapy of Chronic Heart Failure. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=21041 |
Introduction
Development of diastolic dysfunction (DD), which is considered to be an obligatory compound of CHF with preserved ejection fraction (CHFpEF), is characterized by the processes of left ventricle (LV) active relaxation disorders and increase of its passive stiffness. Abnormality of LV active relaxation, which is an energy-consuming process, can develop due to cardiomyocyte ischemia or cardiomyocyte energy metabolism disorders. Besides, the increase of LV myocardium stiffness results in end-diastolic blood pressure rise and cardiac output reduction, which was shown by the study results on diastolic function, obtained by means of invasive and non-invasive methods of measurement at rest and under physical loads. The substrate of “stiff” myocardium is presented by excessive accumulation of collagen and Sodium ions, that are transported into erythrocytes, along with titin phosphorylation deficit, which results in extracellular matrix stiffness increase. Among other pathophysiologic mechanisms the authors evaluated reduction of peripheral vasodilator reserve and chronotropic response, disorder of left ventricular vascular compliance, diastolic and systolic dyssynchrony and autonomic nervous system dysfunctions (Katsumata, 2017). It is well known that hyperactivation of renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system during CHFpEF contributes to the progression of the disease. Lately, a significant number of studies were focused on molecular disorders, in particular, on decreased activity of nitrogen oxide cascade in the channel of systemic vascular cyclic guanosine monophosphate (cGMP) – protein kinase, which enhances oxidative stress and leads to endothelium dysfunction and microvascular inflammation. Systemic inflammation, which develops not only under cardiovascular pathology, but also under physiologic aging, acts as a trigger of endothelial (ED) and microvascular dysfunction. ED is an important factor of arterial elastic vessel stiffness increase. Along with vascular endothelium alterations, depletion of aorta wall elastin, elastin fibers degradation and fragmentation, associated with excessive collagen fibers content in basal cells and Calcium deposits, play an important role in the formation of aorta stiffness as an extracardiac development factor of CHFpEF during aging and arterial hypertension (AHT). In its turn, increase of aorta stiffness leads to acceleration of pulse wave velocity (PWV) and rise of amplitude and elongation of reflected wave, and contributes to the increase of systolic and pulse BP (Antoniadis, 2017). This mechanism is crucial from the point of view LV hypertrophy development, which is a central morphological substrate of CHFpEF development (Yamamoto, 2017).
Pressure load on myocardium contributes to the slowdown of its relaxation. It should be noted that, presently, there is lack of complex studies that focus on parallel investigation of vascular endothelial function and elastic and stiffness characteristics of arteries in patients with AHT and CHFpEF. The role of associated diseases, including AHT, ischemic heart disease (IHD), diabetes mellitus, atrial fibrillation (AF), obesity, obstructive sleep apnoea and chronic kidney disease (CKD), is unquestionable in pathophysiological cascade of CHFpEF. According to the research data (Petrov, 2017), pulmonary hypertension, chronic kidney failure (CKF), anemia, vascular malformations and AF influence on the outcome of patients with CHFpEF. The level of serum creatinine >2 mg/dl is associated with increased inpatient mortality rate regardless of VLEF (4.8% among patients with CHFpEF; 8.4% among patients with CHFrEF, р<0.0001). The most significant predictors of inpatient mortality rate in both groups were increase of blood urea nitrogen level (BUN) to > 37 mg/dl (OR = 2.53; 95% CI 2.22-2.87) and level of systolic blood pressure (SBP) ≤ 125 mmHg (OR = 2.58; 95% CI 2.33-2.86).
Literature review
The study, conducted by Artuni (Arturi, 2017), evaluated the influence of extracellular matrix content, which was identified invasively, on hemodynamic parameters and disease outcome in patients with CHF of non-ischemic etiology. Regardless of LVEF values, the rate of hospitalizations for decompensation or cardiovascular death among patients with extracellular matrix area > 30.5% within 9-month period of monitoring was statistically higher in comparison with patients, who had extracellular matrix area of < 30.5%. Direct correlations were determined between the area of extracellular matrix and LV end-diastolic blood pressure (EDBP), wedge pressure, average pulmonary pressure and right atrial pressure.
Among echocardiogram parameters the most significant predictor of unfavorable prognosis for the disease was left atrial volume (LAV). Roth et al. (Roth et al., 2017) in their study defined the LAVi > 32 ml/m2 as an independent predictor of cardiovascular events in patient population older than 65 years old. This parameter acted as a predictor of first cardiovascular event along with such echocardiogram parameters as left ventricular mass index (LVMi) > 120 g/m2 and systolic and diastolic dysfunction of LV. Similar data was obtained by echocardiographic substudy of irbesartan indication to patients with CHFpEF (I-PRESERVE), where left atrial (LA) enlargement was a more significant parameter for diagnose specification than dopplerographic results of DD. The importance of LAV measurement is explained by the fact that its enlargement does not depend on heart volume overload and reflects the presence of long-lasting DD.
CHFpEF remains a relevant issue in cardiology due to its unspecified pathogenesis and diagnostics and lack of definite positive treatment results. Issues of CHFpEF diagnostics improvement in geriatric patients are still acute because of high level of polymorbidity. Obligatory diagnostic procedures are laboratory tests, primarily, plasma level of brain natriuretic peptide (BNP), and echocardiographic data that confirms DD diagnosis and its intensity (Nagrecha, 2017).
Clinical signs of CHF include the following common symptoms: dyspnea, peripheral edema, pulmonary rale, tachycardia, hepatomegaly, hypertension in jugular veins and general weakness. The majority of symptoms are associated with Sodium and liquid retention. However, in geriatric patients sensitivity and specificity of these symptoms are insufficient for CHF diagnostics due to comorbid conditions impact. In general, these are the patients with CHFpEF, who are characterized by high level of polymorbidity. The most significant CHFpEF associated diseases are AHT, diabetes mellitus type 2, obesity, CKF, chronic obstructive pulmonary disease (COPD), AF and anemias. Lately, the influence of subclinical hypothyroidism on CHFpEF development is observed. Comparative analysis of 2,429 CHFrEF patients data and 2,167 CHFpEF patients data showed that, on average, CHFpEF patients suffered more often from obesity, AF and AHT, while CHFrEF patients more often had IHD and cardiac valve damages (Doumouras, 2018).
In general, one third of all geriatric patients lack common symptoms and show atypical symptoms, associated with comorbidity. Therefore, the authors performed the analyses of specificity and sensitivity of the symptoms in patients with tentative syndrome of CHF with comorbid diseases. The patients mainly complain on the decrease of working capacity (40%), dyspnea (35%), cognitive impairments (31%) and musculoskeletal disorders (29%). Half of the patients had two or more symptoms identified. According to the results of the performed analysis, the authors defined the independent determinants of heart failure: male sex, age, paroxysmal nocturnal dyspnea, absence of hissing respiration, loss of appetite and low body mass index (BMI) (Piepoli, 2017). Pulmonary rale and peripheral edema are often diagnosed in patients with acute decompensated heart failure, while in patients with chronic decompensated heart failure interstitial edema reduces due to enhancement of lymphatic drainage from lung tissue, which leads to blood pressure increase in LA in absence of pulmonary congestion. Uncontrolled AHT is more often diagnosed in patients with CHFpEF, and pulmonary rales and increased blood pressure in jugular veins are more often diagnosed in patients with CHF with systolic dysfunction. COPD occupies a special place among comorbid diseases that disguise CHF. Dyspnea is observed in one third of patients with CHF. It is considered to be a factor of an increased risk of cardiovascular mortality. Dyspnea is also considered to be a symptom of such diseases as anemia, obesity and neurological diseases (myopathias, anxiety disorders). The sensitivity of this syndrome in diagnostics of CHF is 66%, and specificity is only 52%, while orthopnoea had higher specificity of 85% and nocturnal paroxysmal dyspnea of 76% at lower sensitivity (Kato, 2018).
During diagnostics in geriatric patients, it is necessary to consider the relation between CHF and cognitive impairments. Cognitive dysfunction influences on patients attitude to their health condition and treatment compliance, as well as complicates the diagnostics. A higher rate of hospitalization and mortality was observed in the group of patients with cognitive dysfunction. The results of recent studies showed that neuropsycological functions can improve, but still not normalize after the treatment of CHF decompensation.
A more complete blockade of RAAS in patients with CHF is achieved by indication of aldosterone antagonists in combination with ACE inhibitors. Aldosterone, synthesized in adrenal gland cortex zona glomerulosa, is considered to be the most active mineralocorticoid and one of the markers of CHF severity. Mechanisms of increased aldosterone synthesis in patients with CHF include activation of RAAS and angiotensin II, stimulation of heart and vessels volume and osmoreceptors at cardiac output reduction and increase of central venous pressure that leads to stimulation of right atrial and venae cavae baroreceptors. Common activity, exerted by aldosterone, includes Sodium and liquid retention with simultaneous expression of Potassium and Magnesium ions. Circulating and tissue aldosterone contributes to fibroblast activation, collagen synthesis and myocardial and perivascular fibrosis development by stimulation of mineralocorticoid receptors. This, in its turn, leads to LV stiffness increase, myocardial DD enhancement and CHFpEF development (Roche, 2018).
Mineralocorticoid receptor (MR) blockade is considered to be a promising method of CHFpEF treatment due to the pleiotropy of its effects: suppression of inflammation and production of free radicals, regression of left ventricle hypertrophy, improvement of vessel elasticity and myocardial perfusion. Spironolactone, antagonist of mineralocorticoid receptors, improves quality of life and LV diastolic function, which is proved by the Aldo-DHF, ТОРCAT study results. In particular, the study ALDO-DHF (ALDOsterone receptor blockade in Diastolic Heart Failure) investigated the influence of spironolactone 25 mg 12-month therapy in comparison with placebo in 422 patients with CHFpEF on the Е/Е’ parameter and tolerance of physical loads with peak oxygen consumption during bicycle ergometry. After 12-month treatment the patients, that received spironolactone, had their diastolic function significantly improved (degrease of Е/Е’), which was associated with simultaneous decrease of LV mass and level of Nt-рго-BNP under moderate decrease of blood pressure on average by 8/3 mmHg. However, these significant structural and functional alterations in heart did not improve the tolerance of physical loads, disease functional class by NYHA classification and quality of life. Spironolactone registered adverse events included renal function deterioration (in 36% of patients in comparison with 21% of patients in placebo group), higher rate of hyperkalemia, anemia (16% vs 9%) and gynecomastia (4% vs 1%) (De Vecchis, 2017).
The study of spironolactone 50 mg 6-months therapy in 92 patients with CHFpEF showed improvement of diastolic function, which lead to alterations in transmittal flow parameters: wave E speed increase, wave A speed decrease and relation of E/A at simultaneous decrease of wave A speed and time of LV isovolumic relaxation. The specified alterations improved clinical symptomatology and decreased the level of Nt-рго-BNP, which correlated with lowering of disease functional class by NYHA classification.2 However, the evaluation of LVDD was conducted only by transmittal flow analysis, which, to certain extent, limited the significance and interpretation of the obtained results (den Boer, 2017).
The results of TOPCAT study (Treatment Of Preserved Cardiac function heart failure with an Aldosterone anTagonist), that enrolled 3,445 patients, showed significant decrease of hospitalization rate among patients with CHFpEF in the group that received spironolactone. However, the mortality rate did not decrease. During the analysis of region-related subgroups data, primary end-points (cardiovascular death, sudden cardiac arrest with successful resuscitation) in patients from USA, Canada, Argentina and Brasilia were registered 3 times more often in placebo group than in patients, who received spironolactone, unlike in patients from Slovakia and Poland, who did not have any significant differences registered. However, additional decrease of blood pressure and higher rate of hyperkaliemia in this group were not registered either, which questions the correction of eastern European patients inclusion into the study and spironolactone therapy compliance (Pryds, 2017).
It is known that ventricle contraction and active relaxation are energy-dependent processes. Energy metabolism impairment contributes to myocardium contraction and relaxation slowdown. Myocardium function may not always be affected at early stages of heart failure development. However, along with CHF progression, inhibition of oxidative processes in mitochondria is observed. On the one hand, it slows down glucose and fatty acids oxidation, and on the other hand, it enhances glycolysis. Izadi et al. (Izadi et al., 2017) study results showed, that in patients with heart failure, myocardial metabolism was performed by means of trimetazidine glucose oxidation, rather than degradation of fatty acids, which improved contraction function of myocardium and disease prognosis in patients with CHF and systolic dysfunction of LV. The revealed positive effects of trimetazidine, like cells phosphocreatine and adenosine triphosphoric acid levels maintenance, reduction of cells apoptosis, improvement of endothelium function and cells Calcium overload decrease, were observed only in patients with ischemia-induced CHF. According to the results of meta-analysis, conducted by Benz (Benz, 2017), which included 955 patients from 17 randomized controlled clinical studies, showed that trimetazidine indication to patients with CHF significantly improved both clinical status (elongation of loading tests duration and improvement of FC) and systolic myocardium function. After long-term trimetazidine therapy the specified effects were associated with decrease of mortality, cardiovascular complications development and hospitalization rates. According to the data, presented by Bektas (Bektas, 2017), apart from the above mentioned positive effects of trimetazidine therapy, patients with CHF also had the levels of BNP, a marker of CHF severity, decreased. Trimetazidine therapy decreased hospitalization rate, but did not influence on general mortality rate. At the same time, multicenter retrospective study, conducted by van Kessen (van Kessel, 2017), revealed the decrease of hospitalization rate and general and cardiovascular mortality rate in patients, who received trimetazidine in comparison with placebo.
The reviewed studies described positive influence of trimetazidine therapy in geriatric patients with CHFpEF: Improvements of morphofunctional heart status and magistral arteries and kidneys condition (Murphy, 2017). The lack of data on possible positive effects of spironolactone (mineralocorticoid antagonist) and trimetazidine (cardioprotector) combination therapy in geriatric patients with CHFpEF defined one of the main tasks of the present study.
Materials and methods
Patients with CHFpEF were distributed into three groups according to the treatment plan with further monitoring in 6 and 12 months. All the patients received similar treatment based on the recommendations on diagnostics and treatment of CHF, which included indication of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) in case of intolerance of ACE inhibitors – 100%, β-blocker– 67.8%, diuretic – 79.5 %, Calcium antagonist – 45.5 % in maximum tolerated doses. In group B (n=30) the patients received only basic therapy, which had not changed minimum 3 months before the study entry and remained unchanged during the study, in group S (n=30) the patients, in addition to the basic therapy, received spironolactone (25 mg OD), in group S+T (n=30) the patients, in addition to the basic therapy, received spironolactone (25 mg OD) and trimetazidine (35 mg BID). By the time of hospitalization, the basic therapy in the study groups was similar (Table 1). Average age of the patients was also similar and was equal to 67.2±2.4 years old in group B, 71.2±1.3 years old in group S and 70.1±1.8 years old in group S+T.
Table 1: Comparative characteristics of antihypertensive drugs indicated to the patients with CHFpEF, %.
| Drugs | Group B (n=30) | Group S (n=30) | Group S+Т (n=30) |
| ACE inhibitor (perindopril) | 66.7 | 73.3 | 70.0 |
| ARB-II (valsartan) | 33.3 | 26.7 | 30.0 |
| β-blocker (nebivolol/bisoprolol) | 66.7 | 70.0 | 66.7 |
| Diuretic (indapamide) | 83.3 | 76.7 | 80.0 |
| Calcium antagonist (amlodipine) | 46.6 | 46.6 | 43.3 |
| Spironolactone 25 mg | 0 | 100 | 100 |
| Trimetazidine 35 mg BID | 0 | 0 | 100 |
Patients with CHFpEF (n=90) were distributed into three groups depending on their treatment plan with further monitoring in 6 and 12 months. All the patients received similar pathogenetic therapy based on recommendations on diagnostics and treatment of CHF, which included indication of ACE inhibitors (perindopril, mean dose 6.8±0.2 mg OD) – 70%, or ARB-II in case of ACE inhibitors intolerance (valsartan, mean dose 189.0 ± 24.6 mg OD) – 30.0%, β-blocker (bisoprolol/ nebivolol in maximum tolerated dose) – 67.8 %, diuretic (indapamide 1.3 mg ± 0.1 mg OD) – 79.5%, Calcium antagonist (amlodipine 4.2 ± 0.1 mg OD) – 45.5%. By the time of study entry, all the patients had received basic therapy in similar doses and drugs combinations. In group B (n=30) the patients received only basic therapy, which had not changed minimum 3 months before the study entry and remained unchanged during the study, in group S (n=30) the patients, in addition to the basic therapy, received spironolactone (25 mg OD), in group S+T (n=30) the patients, in addition to the basic therapy, received spironolactone (25 mg OD) and trimetazidine (35 mg BID). When patient BP met target values, an extended range of spironolactone indication was introduced, i.e. the doses of other antihypertensive drugs, except for spironolactone, were selectively reduced as needed. Average age of the patients in study groups was similar and was equal to 69.2 ± 2.4 years old in group B, 71.2 ± 1.3 years old in group S and 70.1 ± 1.8 years old in group S+T (р>0.05 for all the groups).
Results and Discussions
Patients from all the groups had positive clinical effect in the course of treatment: overall health improvement, decrease of dyspnea, lower limbs edema resolution, increase of tolerance to physical loads based on the results of SMWT test. Thus, the distance of 6-minute walk for all the patients increased after 6 months of therapy. In terms of quantity, the most significant increase was observed in group S (by 10%) and S+Т (by 9.5%) in comparison with group B (by 5.7%). Increase of 6-minute walk distance was registered also after 12 months of therapy (by 13.4% in comparison with the initial data in group S, by 15.1% in group S+T), which demonstrated significant difference in comparison with group B, where the patients received only basic therapy, and where the increase of the distance length was 2 times lower (by 6.8%) (Fig. 1).
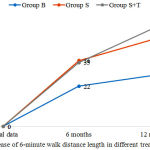 |
Figure 1: Increase of 6-minute walk distance length in different treatment groups |
Symptomatic relief and well-being improvement in patients with CHF are important therapeutic targets in their treatment. Especially, this concerns geriatric population, because in their case, the priority of treatment purposes switches to improvement of life quality. Due to the indicated treatment, the life quality of patients significantly improved in all the three study groups. In 6 months significant reduction of MPQOL score (Medical parameters of quality of life) was observed in comparison with the initial data: in group B, that received only basic therapy, by 15.4%, in group S by 27.7% and in group S+T by 24.2%. The significance of this improvement was considerably higher in patients who were additionally indicated spironolactone and spironolactone in combination with trimetazidine (Table 2). Similar dynamics was observed in 12 months of treatment, the reduction of MPQOL score was equal to 25.0 %, 36.8 % and 33.2 % in group B, S, S + T, respectively. The analysis of intergroup differences showed that reduction of MPQOL score was more significant in groups, where spironolactone and spironolactone in combination with trimetazidine extended range of indication was implemented, in comparison with the basic therapy in 6 and 12 months of treatment (p<0.05). There were no differences observed between groups S and S+T at these stages of monitoring. The identified dynamics of life quality improvement was associated with significant improvement of patients functional status. They had the increase of walk distance registered by SMWT test during 12-month therapy in groups, where extended spironolactone indication plan was implemented. The increase of 6-minute walk distance by 2 times after 12 months of therapy was registered in groups S and S+T in comparison with the distance increase in group B (Table 2).
Distance increase dynamics during SMWT test in groups S and S+T remained during all the period of observation. On the contrary, in group B, where the patients received only basic therapy, significant increase of the walk distance was registered after 6 months of therapy, however, further it sharply reduced. It should be noted that during some studies the results of SMWT test did not change significantly under the influence of spironolactone (mineralocorticoid receptor antagonist). However, during cardiorespiratory loading test, used for evaluation of spironolactone influence on functional status of patients with CHFpEF, significant improvement was registered, which correlated with reduction of diastolic dysfunction severity by E/E’ parameter and growth of peak oxygen consumption during the therapy. Significant reduction of the disease functional class, which is the main parameter of CHF severity evaluation, by 40% was observed only when trimetazidine was additionally indicated, and led to FC lowering by NYHA classification in 66.7% of patients (Table 2). At the same time, patients in group S showed only some tendency towards lowering of FC by NYHA classification by 10% from the initial level. Positive changes in patients functional status and quality of life can be partly associated with BP decrease.
Table 2: Dynamics of blood pressure, functional status and quality of life in patients who received basic therapy (group B) and basic therapy in combination with spironolactone (group S) and with spironolactone and trimetazidine (S+T)
| Parameter | Group B | Group S | Group S+T | ||||||
| Initial data | Δ0-6 | Δ0-12 | Initial data | Δ0-6 | Δ0-12 | Initial data | Δ0-6 | Δ0-12 | |
| SPB, mmHg | 155.4±2.8 | -12.0±1.9* | -14.6±2.0* | 154.1±2.4 | -16.5±2.0* | -21.5±2.8*# | 152.7±2.6 | -14.5±2.0* | -18.1±2.5*# |
| DBP, mmHg | 89.3±1.7 | -5.7±1.6* | -7.9±1.2* | 90.3±1.8 | -9.0±1.5* | -11.0±1.4* | 88.0±1.9 | -7.5±1.4* | -9.3±1.8* |
| SMWT, m | 380.7±13.5 | 21.7±8.3* | 26.0±8.7* | 363.1±13.5 | 36.4±9.2* | 48.8±3.9*# | 361.0±12.9 | 34.3±6.2* | 54.6±8.0*# |
| FC, units | 1.9±0.1 | -0.1±0.1 | -0.1±0.1 | 2.0±0.1 | -0.2±0.1 | -0.2±0.1 | 2.1±0.1 | -0.3±0.1* | -0.4±0.1* |
| MPQOL, score | 39.6±3.0 | -6.1±1.9* | -9.9±2.4* | 46.5±2.7 | -12.9±1.9*# | -17.1±2.6*# | 38.8±3.1 | -9.4±1.9*# | -12.9±2.3*# |
Note:
* – p<0.05 in comparison with the initial data of the respective group
# – p<0.05 in comparison with the respective parameters in group B
As it can be seen in Table 2, significant reduction of both BP variable values (office SBP and DBP) was observed in all the groups. In 6 months of treatment decrease of SBP was registered by 7.7% in group B, by 10.7% in group S and by 9.5% in group S+T in comparison with the initial data in the respective groups. Along with that, during spironolactone therapy and spironolactone and trimetazidine combination therapy, more significant decrease of SBP was registered in 12 months of therapy (by 4.6% and 2.5%, respectively, in comparison with group B, all р<0.05), which can be explained by pleiotropy of spironolactone effects. DBP values had similar changes in groups of comparison after 6 and 12 months of monitoring.
Regression of left ventricle hypertrophy was observed in all the groups during the therapy, but it reached statistically significant values only after 12 months of monitoring. In group B, where the patients received only basic therapy, 6-months monitoring showed no statistically significant changes in LVM (Table 3). Evaluation of basic therapy influence in 12 months of therapy showed that regression of LV hypertrophy was around 4.0% and reached the level of statistically significant reduction (р<0.05).
Table 3: Dynamics of structural and functional heart status parameters in the course of basic therapy in 6 and 12 months of therapy (group B)
| Parameter | Initial data | Δ0-6 | Δ0-12 |
| LVM, g | 261.5±8.1 | -9.3±7.6 | -10.4±4.4* |
| LVMi, g/m2 | 130.1±4.9 | -4.5±3.4 | -5.0±2.1* |
| IST, mm | 12.6±0.3 | -0.4±0.3 | -0.7±0.3* |
| LVPW, mm | 11.4±0.3 | 0.2±0.3 | -0.1±0.3 |
| LVIWT | 0.46±0.01 | -0.01±0.01 | -0.02±0.01 |
| EDD, mm | 49.9±0.9 | 0.4±1.0 | 0.6±0.8 |
| ESD, mm | 30.1±1.3 | 0.9±1.4 | -1.3±1.4 |
| EDV, ml | 96.0±5.6 | -0.7±3.7 | 6.1±3.4 |
| ESV, ml | 38.5±3.1 | 0.8±2.3 | 0.2±2.3 |
| LVEF, % | 60.3±1.5 | 2.2±1.4 | 1.5±1.5 |
| LAV, ml | 56.6±3.4 | -1.0±2.5 | -1.3±2.8 |
| LAVi, ml/m2 | 27.7±1.5 | -0.5±1.3 | -0.8±1.4 |
| Е/А, CU | 0.84±0.05 | 0.01±0.04 | 0.08±0.05 |
| DT, ms | 226.1±11.1 | -5.8±4.1 | -10.3±5.0* |
| IVRT, ms | 109.0±4.6 | -7.0±6.0 | -5.0±5.3 |
| Е/E’, units | 10.5±0.6 | -1.3±0.7 | -1.2±0.7 |
| RA, mm | 31.2±0.6 | -0.8±0.6 | -0.3±0.5 |
| mPAP, mmHg | 25.8±1.4 | 2.0±1.6 | 1.6±1.5 |
Note: * – p<0.05 in comparison with initial data
** – p<0.001 in comparison with initial data of the respective group
LVM – left ventricular mass, LVMi – left ventricular mass index, IST – interventricular septum thickness, LVPW – left ventricle posterior wall thickness, EDD – end-diastolic dimension, ESD – end-systolic dimension, EDV – end-diastolic volume, ESV – end-systolic volume, LVEF – left ventricular ejection fraction, LVIWT – left ventricle interior wall thickness, LAV – left atrial volume, LAVi – left atrial volume index, DT – deceleration time, IVRT – isovolumic relaxation time, LVSBP – left ventricular systolic pressure, mPAP – mean pulmonary arterial pressure
At the same time, additional treatment with spironolactone for 12 months decreased LVM and LVMi by 8.9% (р<0.001) in comparison with initial data, which was significantly different in terms of quantity in comparison with group B (Table 4). The most significant positive dynamics after 12 months of treatment was registered in group S+T, where LVM and LVMi changes were equal to 11.0% (р<0.001), which significantly exceeded the dynamics of the respective parameters in group B (Table 5). There was no difference registered in the development of LV hypertrophy in groups S and S+T.
Table 4: Dynamics of structural and functional heart status parameters in the course of additional therapy with spironolactone in 6 and 12 months of therapy (group S)
| Parameter | Initial data | Δ0-6 | Δ0-12 |
| LV mass, g | 249.1±16.9 | -10.0±8.2 | -22.1±7.9**# |
| LVMi, g/m2 | 126.8±7.4 | -5.0±3.8 | -11.2±3.7**# |
| IST, mm | 13.1±0.5 | -1.4±0.6* | -1.6±0.5* |
| LVPW, mm | 10.8±0.4 | -0.5±0.4 | -0.4±0.3 |
| LVIWT | 0.46±0.03 | -0.03±0.01* | -0.03±0.01* |
| EDD, mm | 46.6±1.8 | 1.2±2.0 | 1.2±1.8 |
| ESD, mm | 28.0±1.6 | -0.5±1.5 | 0.6±2.0 |
| EDV, ml | 83.4±6.7 | 5.2±7.8 | 3.1±4.4 |
| ESV, ml | 35.7±3.0 | -1.0±2.5 | -3.0±1.7 |
| LVEF, % | 60.8±1.6 | 1.5±1.6 | 2.2±1.8 |
| LAV, ml | 68.0±4.1 | -7.6±2.1* | -9.3±2.4**# |
| LAVi, ml/m2 | 34.6±1.7 | -3.9±1.2** | -4.7±1.2**# |
| Е/А, CU | 0.82±0.08 | 0.03±0.04 | 0.04±0.05 |
| DT, ms | 245.7±10.9 | -42.8±10.6*# | -24.9±8.5*# |
| IVRT, ms | 122.1±7.4 | -18.5±8.0* | -7.8±10.5 |
| Е/E’, units | 10.7±0.6 | -1.9±1.0 | -2.3±0.8*# |
| RA, mm | 30.1±0.7 | -0.4±0.9 | -0.8±0.5 |
| mPAP, mmHg | 29.7±1.9 | -1.8±2.6 | -4.6±2.1*# |
Note: * – p<0.05 and ** – p<0.001 in comparison with initial data;
# – p<0.05 in comparison with the respective parameters in group B
Regression of VL hypertrophy was due to IST reduction, which was observed in groups with additional spironolactone therapy (S and S+T) after 6 months of treatment (for all р<0.05), while in the group with only basic therapy this effect was observed after 12 months of treatment.
Initial values of LVPW and LVIWT parameters, that were calculated based on LVPW and EDD parameters, were similar in groups of comparison. However, additional therapy with spironolactone and spironolactone combination with trimetazidine decreased the values of LVIWT (p<0.05), which was not registered in the group with only basic therapy.
Analysis of the parameters, that characterize linear dimensions of LV (EDD, ESD) and volumetric values (EDV, ESV), did not reveal any significant intergroup differences at all the stages of monitoring, which is a common sign of heart remodeling in patients with CHFpEF (Table 3, 4, 5). The parameter of global contractility LVEF did not change in the course of the specified treatment plans. As it is shown in Table 3, in group B there was insignificant reduction of LAV by index-related parameter (LAVi) registered by 1.7% in 6 months of therapy and by 2.3% in 12 months of therapy in comparison with the initial data (р>0.05). Statistically significant decrease of LAVi was registered only in groups S and S+T. The dynamics of changes was significant in comparison with the initial data and was equal to 11.3% in group S (Table 4) and 9.9% in group S+T (Table 5) after 6 months of therapy.
Table 5: Dynamics of structural and functional heart status parameters in the course of additional therapy with spironolactone and trimetazidine (group S+T) in 6 and 12 months of therapy (group S+T)
| Parameter | Initial data | Δ0-6 | Δ0-12 |
| LV mass, g | 259.9±13.1 | -13.0±7.3 | -28.6±7.7**# |
| LVMi, g/m2 | 133.7±6.0 | -6.5±3.8 | -14.7±4.1**# |
| IST, mm | 12.7±0.5 | -0.8±0.4 * | -1.4±0.4* |
| LVPW, mm | 11.8±0.5 | -0.4±0.3 | -0.7±0.5 |
| LVIWT | 0.47±0.02 | -0.01±0.01 | -0.02±0.01* |
| EDD, mm | 49.3±1.0 | -0.1±1.0 | 0.3±0.9 |
| ESD, mm | 28.1±1.0 | 0.7±0.8 | 1.8±1.0 |
| EDV, ml | 88.9±5.3 | -0.1±3.8 | 1.4±5.0 |
| ESV, ml | 34.8±3.2 | -2.2±2.3 | -2.4±3.1 |
| LVEF, % | 62.2±1.5 | 1.4±1.6 | 1.9±1.6 |
| LAV, ml | 56.5±3.1 | -7.6±2.2** | -8.8±2.3**# |
| LAVi, ml/m2 | 29.3±1.6 | -2.9±1.0** | -4.4±1.1**# |
| Е/А, CU | 0.96±0.06 | -0.06±0.05 | -0.12±0.05*# |
| DT, ms | 222.2±11.5 | -16.8±5.2**# | -20.9±6.8**# |
| IVRT, ms | 111.1±4.6 | -3.5±5.9 | -5.9±0.9** |
| Е/E’, units | 10.9±0.5 | -2.0±0.7* | -2.8±0.5**# |
| RA, mm | 30.5±0.5 | -0.1±0.6 | -0.3±0.5 |
| mPAP, mmHg | 28.6±1.3 | -1.4±1.1 | -4.2±1.8*# |
Note: * – p<0.05 and ** – p<0.001 in comparison with initial data;
# – p<0.05 in comparison with respective parameters in group B.
After 12 months of the therapy, further decrease of LAVi by 13.6% and 15.0% was observed in comparison with initial data in the respective groups (Table 4 and 5). Analysis of intergroup differences showed that reduction of LAV and its index-related value in groups S and S+T reached the level of statistical significance in comparison with the group, that received only basic treatment, during 12 months of the therapy. To analyze the influence of different treatment plans on the regression of LA hypertrophy (LAVi>34 ml/m2), which is considered to be a structural parameter of DD according to modern recommendations on diagnostics and treatment of CHF, the authors estimated the share of patients in each treatment group and their quantitative changes at the stages of control. Thus, primary examination of patients in the group B showed that the specified parameter was registered in 20% of patients and remained unchanged during 6 and 12 months of the treatment. This was different from group S, where the share of patients with increased LAVi changed from 66.7% to 40.0% after 6 months of therapy (χ2=4.28; р<0.05) and to 26.7% after 12 months of therapy (χ2=9.64; р<0.001). Similar dynamics was observed in group S+T, where LAVi >34 ml/m2 was registered only in 33.3% of patients during primary examination and in 16.7% of patients after 6 months of treatment (χ2=2.22; р>0.05), decreasing to 6.7% after 12 months of treatment (χ2=6.67; р<0.001).
Evaluation of different treatment plans influence on LV diastolic function (DF) did not reveal any significant changes in Е/А parameter, except for its significant decrease in group S+T after 12 months of therapy (р<0.001) (Testa, 2017).
The analysis of temporary parameters of LVDF in the studied groups showed the increase of isovolumic relaxation time (IVRT) initial values and lack of significant influence on this parameter in groups B and S. In group S+T statistically significant reduction of IVRT was registered by 5.3% after 12 months of treatment (р<0.001). Time parameter of LVDF, DT interval, significantly decreased in groups S and S+T after 6 and 12 months of treatment, reaching intergroup difference with group B. Similarly, significant reduction of E/E’ parameter was observed only in groups with additional spironolactone therapy. It should be noted that statistically significant dynamics of E/E’ parameter changes was registered after 6 months of therapy in group S+T and after 12 months of therapy in groups S and S+T (р<0.05) with lack of significant differences between the specified treatment plans and the basic therapy. In 80.0% of the examined patients I type DD was registered; II type DD was registered only in 20.0% of patients. Hence, reduction of E/A value in group S+T patients after 12 months of treatment was not a sign of DD progression, but was associated with transformation of the patients share with more severe DD by the type of pseudonormalization into a milder degree of DD – delayed relaxation. According to the analysis results, the most sensitive DD parameter, which changed significantly in the course of additional spironolactone therapy and spironolactone and trimetazidine combination therapy, was Е’. In group B the share of patients with Е’ >9 cm/s was equal to 86.7% during initial testing and did not change significantly in the course of therapy. However, in group S significant reduction of patients share with the specified DD parameter by 66.7% was observed in comparison with the initial data (χ2=26.8; р<0.01), and in group S+T – by 63.4% (χ2=26.4; р<0.01) after 12 months of treatment.
In each of the studied groups the authors registered a share of patients with the signs of moderate pulmonary hypertension, which was equal to 36.6% (11 patients), 46.6% (14 patients) and 60.0% (18 patients) in groups B, S and S+T, respectively. In the course of basic therapy there were no significant changes in mPAP observed, while in groups with additional spironolactone and additional spironolactone and trimetazidine combination therapy, a significant decrease in this parameter was registered (by 15.5% and by 14.7%) after 12 months of therapy.
The importance of influence on enllarged LA, which is considered to be a marker for DD severity, is confirmed by the observation studies data, where LAVi >34 ml/m2 acted as an independent predictor of both cardiovascular and overall mortality, manifested heart failure development, atrial fibrillation and ischemic stroke, regardless of associated LV hypertrophy. Thus, decrease of LAVi in the course of treatment with spironolactone and its combination with trimetazidine requires further detailed investigation in order to determine the probable prognosis modified influence of spironolactone in patients with CHFpEF.
The improvement of diastolic function (by the dynamics of Е/Е’, Е’ and DT parameters) resulted in mPAP decrease only in groups S and S+T by 4.6±2.3 mmHg and 4.2±1.8 mmHg, respectively, after 12 months of therapy. RV dimensions, measured from parasternal access by the heart long axis, were similar in all the comparison groups and did not change under the treatment. However, in 60.0% of patients from group B the authors registered insignificant excess of RV >30.0 mm, which was identified in 33.3% of patients in group S and in 20.0% of patients in group S+T.
The importance of this parameter positive dynamics, associated with pulmonary hemodynamics, was confirmed by the conducted studies that demonstrated the role of pulmonary hypertension and enlargement of RV as survival limiting factors in patients with CHFpEF.
Geriatric patients diurnal blood pressure profile is characterized by circadian rhythms disorders, increase of morning rising rate and variability of blood pressure. One of the prospective observation studies, which enrolled 516 patients with CHF, was focused on evaluation of overall mortality and rate of cardiovascular events development (median of observation was 20.9 months) in relation to diurnal blood pressure profile. It was determined that in patients with CHFpEF, circadian rhythm of “non-dipper” BP type increased the possibility of unfavorable events development by 2.3 times in comparison with patients with CHFrEF.
According to the data obtained by Japanese researchers, patients with CHFpEF had higher rates of BP and higher rates of “night-peaker” blood pressure circadian rhythms disturbances in comparison with patients with CHFrEF.
After 6 months of basic therapy, significant decrease of office blood pressure variables was registered (Table 6). Along with it, SBP levels did not reach target values (<140 mmHg) in 63.3% of patients that received basic therapy, and DBP levels (<90 mmHg) in 40.0% of patients. Similar trending changes were observed during evaluation of 24-hour blood pressure parameters monitoring (ABPM): parameter SBPad significantly decreased after 6 months of therapy, while DBPad significantly changed only after 12 months of therapy. Mean pulse blood pressure (PBPad) parameter values significantly decreased at all the stages of treatment control.
Table 6: Dynamics of BP and ABMP parameters in the course of basic therapy
| Parameter | Initial data | After 6 months of therapy | After 12 months of therapy |
| SBPof, mmHg | 155.4±2.8 | 143.4±2.2* | 140.8±1.8* |
| SBPad, mmHg | 143.0±3.0 | 135.4±2.4* | 130.2±2.0* |
| SBPd, mmHg | 146.6±3.1 | 138.3±2.6* | 136.1±2.0* |
| SBPn, mmHg | 136.2±3.6 | 124.4±2.2* | 121.4±2.1* |
| DBPof, mmHg. | 89.3±1.7 | 83.6±1.4* | 81.5±1.3* |
| DBPad, mmHg | 76.9±2.0 | 75.0±1.4 | 72.5±1.2* |
| DBPd, mmHg | 81.3±1.8 | 79.4±1.3 | 78.3±1.2 |
| DBPn, mmHg | 71.5±2.2 | 69.0±1.3 | 66.6±1.0* |
| PBPad, mmHg | 64.9±2.1 | 57.9±1.8* | 56.7±1.6* |
| HRd, bpm | 65.9±1.8 | 71.4±1.4 | 72.1±1.2 |
| HRn, bpm | 58.8±1.5 | 61.9±1.8 | 59.4±1.1 |
| TI SBPd, % | 57.8±4.9 | 49.9±4.0* | 40.1±6.1* |
| TI SBPn, % | 67.0±5.0 | 46.7±4.1* | 46.7±7.1* |
| TI DBPd, % | 27.4±4.7 | 20.6±2.7 | 18.4±2.1* |
| TI DBPn, % | 37.1±6.0 | 28.1±4.6 | 15.3±2.2* |
Note: * – p<0.05 in comparison with initial data
The most significant office SBP and DBP decrease (by 16.5±2.0 and 9.0±1.5 mmHg, respectively) was registered in the group with additional spironolactone therapy after 6 months of treatment, while only in 6 (20.0%) patients SBP values exceeded the target ones, and in 2 (6.7%) patients threshold values of DBP were registered, which showed significant tendency towards decreasing in the course of further therapy (Table 7).
Table 7: Dynamics of BP and ABMP parameters in the course of additional spironolactone therapy
| Parameter | Initial data | After 6 months of therapy | After 12 months of therapy |
| SBPof, mmHg | 154.1±2.4 | 137.6±1.8* | 132.6±1.5*# |
| SBPad, mmHg | 141.0±3.5 | 130.2±2.4* | 129.9±2.0* |
| SBPd, mmHg | 144.3±3.7 | 135.7±2.5* | 134.7±2.3* |
| SBPn, mmHg | 137.1±3.9 | 123.8±2.8* | 117.0±2.7* |
| DBPof, mmHg | 90.3±1.8 | 81.3±1.2* | 79.3±1.0* |
| DBPad, mmHg | 78.0±1.6 | 74.5±2.4 | 70.5±1.5* |
| DBPd, mmHg | 81.2±3.4 | 80.5±2.7 | 75.0±1.7* |
| DBPn, mmHg | 73.8±2.2 | 68.9±2.2 | 65.9±1.4* |
| PBPad, mmHg | 62.6±1.7 | 57.2±1.2* | 56.2±1.8* |
| HRd, bpm | 68.2±3.1 | 71.3±3.2 | 67.3±1.9 |
| HRn, bpm | 60.5±2.3 | 62.0±1.6 | 60.6±1.7 |
| TI SBPd, % | 58.7±4.8 | 45.7±3.8 | 39.8±4.9* |
| TI SBPn, % | 68.1±5.9 | 43.0±4.0* | 36.3±5.4* |
| TI DBPd, % | 31.4±8.2 | 25.6±6.4 | 13.3±1.9* |
| TI DBPn, % | 38.2±7.9 | 33.0±6.9 | 14.5±2.4* |
Note: * – p<0.05 in comparison with initial data
# – p<0.05 in comparison with respective parameters from group B
Target BP levels were not achieved in patients, who had additional combination therapy with spironolactone and trimetazidine, with the rate similar to the one in patients, who had additional spironolactone therapy: by SBP level in 5 (16.6%) patients after 6 and 12 months of therapy and by the level of DBP in 6 (20.0%) patients after 6 months of therapy, i.e. decreased by 2 times after 12 months of therapy (Table 7). Target level based BP analysis in geriatric patients with BP <150/90 mmHg showed that in each of the studied groups a share of patients remained, whose BP did not meet target levels. There were 9 (30%) patients in group B with only basic therapy, while there were only 2 (6.7%) patients in group S and 1 (3.3%) patient in group S+T.
It is known that in patients with arterial hypertension, who do not receive therapy, average diurnal level of SBP is by 4-15 mmHg lower and level of DBP is by 3-9 mmHg lower than the values, obtained after single BP measurements in the clinics. In geriatric patients more significant differences are observed, which can be different by 22 mmHg for SBP and 10 mmHg for DBP from average diurnal levels. Average nighttime SBP and DBP levels were lower by 14 mmHg and 13 mmHg than the respective average daytime levels. This data indicates on complementarity of these methods and the necessity to measure office BP and to conduct ABPM.
The results of ABPM data analysis in patients with CHFpEF revealed similar above mentioned clinical picture in comparison with office BP values. In all the studied groups increase of average diurnal, daytime and night time values of SBP was registered, which was especially significant in the course of 6 months of additional spironolactone and spironolactone and trimetazidine combination therapies (Table 7, 8). However, these levels did not meet the recommended threshold values. Only after 12 months of therapy average diurnal values of SBP significantly changed in patients, who received spironolactone and spironolactone in combination with trimetazidine in addition to the basic therapy for CHF: SBPad was <135 mmHg, SBPd was <135 mmHg and SBPn was <120 mmHg. It should be noted that in all the patients, regardless of the received therapy, decrease of SBP after 6 and 12 months of treatment was statistically significant. The analysis of the respective parameters of DBP showed that its average diurnal levels did not exceed the recommended 80 mmHg in one of the studied groups and significantly decreased in all the groups only after 12 months of therapy (р<0.05). At the same time, the authors registered insignificant excess of average diurnal (>80 mmHg) and average night time (>70 mmHg) output DBP values. It should be noted that significant decrease of DBPd was registered only after 12 months of therapy in all the three groups. However, additional indication of trimetazidine led to statistically significant decrease of average night time DBP values after 6 months of monitoring (Table 8).
Table 8: Dynamics of office BP and ABMP parameters in the course of additional spironolactone and trimetazidine therapy
| Parameter | Initial data | After 6 months of therapy | After 12 months of therapy |
| SBPof, mmHg | 152.7±2.6 | 138.2±1.6* | 134.6±1.7*# |
| SBPad, mmHg | 138.2±2.8 | 128.3±2.3* | 123.8±2.0* |
| SBPd, mmHg | 141.2±2.5 | 135.6±1.8* | 132.6±1.8* |
| SBPn, mmHg | 130.2±3.1 | 121.5±2.2* | 115.7±2.1* |
| DBPof, mmHg | 88.0±1.9 | 80.5±1.4* | 78.7±1.4* |
| DBPad, mmHg | 76.2±2.0 | 71.2±1.4 | 69.8±1.4* |
| DBPd, mmHg | 79.7±1.8 | 76.6±1.3 | 76.0±1.5 |
| DBPn, mmHg | 70.1±2.1 | 65.8±1.3* | 63.6±1.1* |
| PBPad, mmHg | 61.1±1.6 | 56.7±1.3* | 55.6±1.5* |
| HRd, bpm | 68.8±1.5 | 71.0±1.5 | 70.5±1.9 |
| HRn, bpm | 61.4±1.5 | 61.2±1.2 | 58.8±1.3 |
| TI SBPd, % | 49.2±5.1 | 40.6±4.4* | 33.4±4.0* |
| TI SBPn, % | 60.7±6.8 | 40.5±3.9* | 29.5±4.8* |
| TI DBPd, % | 23.1±4.2 | 16.7±2.5 | 13.1±1.8* |
| TI DBPn, % | 30.1±6.0 | 17.0±3.8* | 12.1±2.2* |
Note: * – p<0.05 in comparison with initial data
# – p<0.05 in comparison with respective parameters in the control group
Target BP level for general population of patients with AHT is determined considering age-related BP increase. There are higher BP threshold values for geriatric patients, while for ABMP parameters evaluation there is no differentiated approach. According to the data, reported by Sega R. et al., average daytime BP values were registered in 800 ambulatory and hospitalized patients with AHT older than 65 years old. They ranged from 128/77 mmHg to 140/78 mmHg. According to other researchers data, average diurnal BP values were equal to 138/82 mmHg in older patients and 147/83 mmHg in elderly patients (over 80 years old). Exclusion of age-related peculiarities during ABMP parameters analysis leads to a certain inconsistency and increase of patients share, whose BP did not achieve target levels, which requires higher reference values than in middle age patients.
Level of average diurnal PBP, which is >55 mmHg, is an important predictor of cardiovascular complications, especially, in elderly patients. Despite the fact that BP control was significant for target values achievement in the majority of patients, the values of PBP remained quite high at the stages of control, although significantly after 6 and 12 months of therapy.
HR values during daytime and night time monitoring remained within the norm in all the patients from all the groups and did not change significantly in the course of treatment.
For quantitative evaluation of increased BP events, the parameters of “pressure load” are used. The authors in the present study used time index (TI) of both BP variables in different time of the day. This parameter characterizes hyperbaric load on target organs more precisely than average BP values, and it is considered “normal” at excessive BP threshold values during daytime and night time <15% and “increased” at >30% [30]. As it is shown in Tables 6-8, TI SBP value exceeded the specified thresholds in all the study groups during primary examination and further at all the stages of control. After the treatment this parameter value significantly decreased in each group. The most significant dynamics of TI DBP (by 13.0%) was registered after 6 months of treatment in group S (Table 7). The analysis of IT PBP showed that its decrease did not reach significant values after 6 months of therapy in group B and in group S. However, additional trimetazidine therapy led to more significant decrease of TI DBP during night time after 6 months of therapy (р=0.04). TI DBP daytime and night time target values were registered after 12 months of treatment in groups with additional spironolactone and trimetazidine therapy (for all р<0.05).
It is known that physiological profile of BP circadian rhythm is expressed as a two-phase curve with decrease of SBP and DBP values by 10-20% at nighttime in comparison with daytime. Overall, patients with CHFpEF often had insufficient decrease of both BP variables (in 21.1% of cases). Along with this, a phenomenon of SBP or DBP “isolated non-dipping” was registered in the studied groups. Diurnal profiles of SBP and DBP in patients with CHFpEF are presented on three diagrams, depending on the treatment plan (Fig. 2-4). Daily index (DI) SBP was reduced in all the groups and was equal to 7.1±1.5% in group B, 5.0±2.3% in group S and 7.7±1.3% in group S+T, which is explained by the share of patients with diurnal “nondipper” BP profile. Initial values of DI DBP were higher and were equal to 12.2±1.5, 10.4±1.8 and 13.0±1.5%.
As it is shown in Fig. 2, in the course of 12-month therapy the share of patients with normal diurnal SBP profile increased. However, the specified changes were statistically significant (χ2=2.5; р>0.05). At the same time, in group S (Fig. 3) the share of patients with diurnal “dipper” BP profile significantly increased and the share of patients with “non-dipper” profile (χ2=4.44; р<0.05). In group S+T (Fig. 4) more significant changes were observed, which influenced on DI SBP rise, which on average exceeded 10.0% in the group and after 12-month therapy was equal to 11.9±1.2%, which was explained by the increase of “dipper” patients share (χ2=5.55; р<0.05) and insignificant increase of “оver-dipper” patients share.
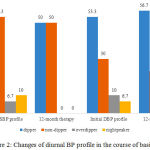 |
Figure 2: Changes of diurnal BP profile in the course of basic therapy |
Under the influence of basic therapy diurnal DBP profiles did not change significantly, except for 2 patients with the most unfavorable “night-peaker” profile, whose profile changed to “non-dipper”, and the share of “overdippers” patients increased by 2 times in comparison with initial data (Fig. 2).
Changes of diurnal DBP profile were mostly significant in group S (Fig. 3). Additional indication of spironolactone decreased the share of “non-dipper” patients almost by 2 times (χ2=4.29; р<0.05) and increased the share of “dippers” by 2.5 times (р<0.01). At the same time, pathologic profiles with excessive increase or decrease of DBP disappeared.
As it is shown in Fig. 4, therapy with additional indication of spironolactone and trimetazidine normalized the circadian DBP profile almost in 2/3 of patients at the final stage of control (р<0.05). However, there were no intergroup differences identified in comparison with basic therapy.
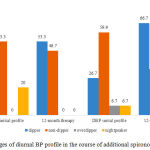 |
Figure 3: Changes of diurnal BP profile in the course of additional spironolactone therapy |
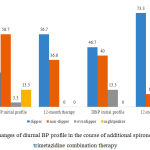 |
Figure 4: Changes of diurnal BP profile in the course of additional spironolactone and trimetazidine combination therapy |
In the course of therapy significant rate decrease of morning SBP rise was registered in all the groups: from 33.6±5.9 to 18.5±1.1 mmHg/h in group B, from 28.4±4.2 to 18.4±1.6 mmHg/h in group S and from 33.1±3.7 to 19.3±2.6 mmHg/h in group S+T. At the same time, morning SBP rise values did not significantly change under the therapy.
Output characteristics of central aortal pressure (CASP, CADP and CPP) were similar in the three study groups in patients with CHFpEF (Tables 9, 10, 11). Basic therapy did not change these parameters statistically significant at the stages of control in 6 and 12 months (Table 9).
CASP decreased by 6.1±0.7 mmHg in comparison with initial data (р 0.05) after 12 months of spironolactone therapy, CAP decreased by 6.1±0.6 mmHg after 6 month of therapy (р< 0.05) and by 6.9±0.8 mmHg after 12 of therapy (р<0.05) (Table 10). Similar dynamics with significant decrease of SBP and PBP was registered in group S+T (Table 11). However, intergroup analysis did not reveal any differences based on the treatment plan.
Table 9: Changes in central hemodynamics parameters and arteries elasticity and stiffness status in the course of basic therapy, (M±m)
| Parameter | Initial data | 6 months of therapy | 12 months of therapy |
| CASP, mmHg | 142.6±2.4 | 144.5±2.9 | 139.3±3.2 |
| CADP, mmHg | 82.9±2.3 | 84.9±1.6 | 81.3±2.1 |
| CPP, mmHg | 59.7±3.0 | 59.4±3.0 | 58.1±2.4 |
| PWV, m/s | 9.5±0.2 | 9.4±0.2 | 9.4±0.2 |
| AoSI | 26.3±4.1 | 12.1±1.9* | 12.2±2.7* |
| IMT, cm | 1.06±0.02 | 1.04±0.03 | 1.02±0.02* |
Note: *— р<0.05, **— р<0.001 in comparison with initial data
Parameter PWV, which is the key parameter in regional aorta stiffness evaluation, was similar in all the study groups during evaluation of initial data. In group with basic therapy the dynamics of PWV changes did not reach statistically significant level. At the same time, in groups with additional spironolactone and trimetazidine combination therapy significant and similar decrease of PWV by 0.30±0.05 m/s was registered after 6 months of therapy with further decrease by 0.50±0.07 m/s after 12 months of therapy in comparison with initial data (р<0,05).
Table 10: Changes in central hemodynamics parameters and arteries elasticity and stiffness status in the course of additional spironolactone therapy, (M±m)
| Parameter | Initial data | 6 months of therapy | 12 months of therapy |
| CASP, mmHg | 140.6±3.0 | 136.8±3.2 | 134.5±2.9* |
| CADP, mmHg | 80.3±2.2 | 82.5±2.3 | 80.9±1.6 |
| CPP, mmHg | 60.4±2.4 | 54.3±2.0* | 53.5±2.2* |
| PWV, m/s | 9.4±0.2 | 9.1±0.1* | 8.9±0.2* |
| AoSI | 23.2±3.1 | 17.5±2.9* | 9.3±2.2** |
| IMT, cm | 1.10±0.01 | 1.07±0.02* | 1.05±0.02* |
Note: *— р<0.05, **— р<0.001 in comparison with initial data
AoSI parameter, that characterized local aorta stiffness, significantly decreased in all the study groups after 6 and 12 months of monitoring. The most significant decrease of AoSI parameter was observed in group S – by 2.5 times (р<0.001) and in group S+T – by 3.5 times (р<0.0001) in comparison with the group with basic therapy, where AoSI decrease by 2.2 times was quantitatively the lowest in comparison with initial data (р<0.05).
Evaluation of IMT parameter in groups S and S+T showed statistically significant decrease after 6 and 12 months of monitoring. In group with basic therapy only the decrease of IMT by 0.05±0.01 mm was registered only after 12 months.
Table 11: Changes in central hemodynamics parameters and arteries elasticity and stiffness status in the course of additional spironolactone and trimetazidine therapy, (M±m)
| Parameter | Initial data | 6 months of therapy | 12 months of therapy |
| CASP, mmHg | 136.2 ± 2.6 | 132.8 ± 2.5 | 130.1 ± 2.0* |
| CADP, mmHg | 77.3 ± 1.8 | 79.9 ± 1.6 | 78.5 ± 1.9 |
| CPP, mmHg | 58.5 ± 1.9 | 53.3 ± 1.8* | 52.5 ± 2.2* |
| PWV, m/s | 9.5 ± 0.2 | 9.2 ± 0.1* | 9.0 ± 0.2* |
| AoSI | 28.0 ± 4.4 | 17.4 ± 3.9* | 7.9 ± 1.4** |
| IMT, cm | 1.10 ± 0.02 | 1.00 ± 0.02* | 1.00 ± 0.01* |
Note: *— р<0.05, **— р<0.001 in comparison with initial data
Endothelium vasomotor function, which was evaluated by the rate of EDVD, after primary examination was similar in the groups of comparison and ranged within 2.7% – 3.1% – 3.1% per 60 seconds of reactive hyperemia, increasing to 6.6% – 7.3% – 78% in 120 seconds (Fig. 5-7).
The most significant positive changes in EDVD were observed at early stages of reactive hyperemia: after 6 months of basic therapy EDVD rate rose by 4% in comparison with initial data when evaluated during first 60 seconds, i.e. by 2.5 times, and remained stable by the 12th months of therapy (Fig. 5). EDVD increase rate per 120 seconds during the basic therapy was lower; it was equal to 3.8% in comparison with initial level after 6 months of therapy and did not change significantly after 12 months of monitoring.
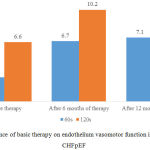 |
Figure 5: Influence of basic therapy on endothelium vasomotor function in patients with CHFpEF
|
In group S EDVD increase by >10% per 120 seconds of reactive hyperemia was registered after 6 and 12 months of therapy. It increased by 4.8% and 5.9%, respectively, in comparison with initial data, which was equal to 7.3% (Fig. 6).
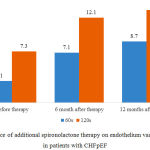 |
Figure 6: Influence of additional spironolactone therapy on endothelium vasomotor function in patients with CHFpEF
|
Note: *— р<0.05, **— р<0.001 in comparison with initial data
Additional spironolactone and trimetazidine combination therapy had the same influence on EDVD as additional spironolactone therapy (Fig. 7).
FIG 7
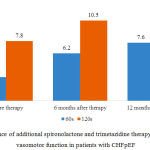 |
Figure 7: Influence of additional spironolactone and trimetazidine therapy on endothelium vasomotor function in patients with CHFpEF
|
Note: *— р<0.05, **— р<0.001 in comparison with initial data
The analysis of therapy impact on EDVD increase rate after 12 months of monitoring showed significant increase of patients share, whose EDVD rate exceeded 10%: in group of additional spironolactone therapy – in 80% (24 patients) in comparison with 20% (6 patients) after primary examination (χ2=21.60; р<0.01) and in 70% (21 patients) in comparison with 20 % (6 patients) after primary examination in group of additional spironolactone and trimetazidine combination therapy (χ2=15.15; р<0.01). At the same time, significant increase of patients share with EDVD rate >10% was not registered in group with basic therapy (χ2=.00; р>0.05). Thus, the proposed treatment plans, that included additional indication of spironolactone, had more significant impact on endothelium vasomotor function in comparison with the basic therapy.
Initial average creatinine levels did not exceed the norm in the examined patients and were equal to 88.0±2.7 µmol/L in group B, 78.1±3.1 µmol/L in group S and 86.2±3.2 µmol/L in group S+T. However, initial values of GFR were reduced in patients in all the study groups (Table 12). CHF class I, characterized by GFR >90 ml/min/1.73m2 identified at primary examination, was registered in 3 patients (10.0%) in group B and in around 7% of patients in groups S and S+T.
Table 12: Alternations in kidneys functional status in the course of therapy
| Parameter | Group B | Group S | Group S+T | |||
| Initial data | After 12 months | Initial data | After 12 months | Initial data | After 12 months | |
| Creatinine, µmol/L | 88.0±2.7 | 85.2±3.4 | 78.1±3.1 | 76.3±2.2 | 86.2±3.2 | 83.4±2.9 |
| GFR, ml/min/1.73m2 | 69.2±2.9 | 72.0±2.8 | 69.5±2.8 | 68.2±2.8 | 62.1±2.4 | 77.0±2.6** |
| CHF class I, number of patients | 3 | 5 | 2 | 2 | 2 | 5 |
| CHF class II, number of patients | 18 | 20 | 22 | 22 | 14 | 23 |
| CHF class III, number of patients | 9 | 5 | 6 | 6 | 14 | 2 |
Note: **— р<0.001 in comparison with initial values of the parameter in the respective group
In groups B and S the examined patients had CHF class II with GFR values from 60 to 89 ml/min/1.73 m2 registered more often: in 18 patients (60.6%) in group B and in 22 patients (73.0%) in group S. At the same time, in group S+T the equal shares of patients had CHF class II and III registered (in 14 patients – 46.5%, respectively). GFR was within the range of 30 – 59 ml/min/1.73m2 in patients in group B (30%) and group S (20%).
Freedman ANOVA test was used for evaluation of different treatment plans on kidneys functional status, in particular, on GFR parameter, which was calculated by the formula CKD-EPI. Certain differences were registered in the dynamics of the GFR parameter in the course of treatment (Fig. 8-10). No significant changes in GFR were registered in group B after 12 months of therapy, which influenced on the distribution of patients by CHF classes: class I – in 5 patients (16.5%), class II – in 20 patients (67.0%) and class III – in 5 patients (16.5%), which did not differ significantly from the initial distribution (Fig. 8).
 |
Figure 8: Evaluation of changes in GFR by CKD-EPI after 6 (ЕРІ_2) and 12 (ЕРІ_3) months of basic therapy
|
In group S average creatinine levels and GFR did not reach statistically significant changes and, thus, the distribution of patients by CHF classes did not change after 6 and 12 months of (Fig. 9, 10).
 |
Figure 9: Evaluation of changes in GFR by CKD-EPI after 6 (ЕРІ_2) and 12 (ЕРІ_3) months of additional 25 mg spironolactone therapy
|
 |
Figure 10: Evaluation of changes in GFR by CKD-EPI after 6 (ЕРІ_2) and 12 (ЕРІ_3) months of additional spironolactone and trimetazidine combination therapy
|
At the same time, in group S+T the increase of GFR from 62.1 ± 2.4 to 68.7 ± 2.1 ml/min/m2 was registered after 6 months of therapy and to 77.0±2.6 ml/min/m2 after 12 months of therapy, which was significantly different (р<0.001). This positive dynamics in GFR contributed to the increase of patients share with CHF class I and II (χ2=5.71; р<0.05) and to the respective change of patients share with CHF class III after 12 months of therapy (χ2=12.27; р<0.01).
Thus, Freedman ANOVA test revealed the differences in different treatment plans impact on kidneys functional status. It is possible that increase of GFR (by 14.9±0.8 ml/min/1.73m2), observed only during spironolactone and trimetazidine combination therapy, confirms positive influence of trimetazidine on kidneys functional status.
Conclusion
Clinical efficiency of 12-month therapy with additional indication of spironolactone and spironolactone in combination with trimetazidine was proved by significantly higher increase of 6-minute walk distance (by 13.4%, 15.1% and 6.8%, respectively) and decrease of score by MLHFQ (Minnesota Living With Heart Failure Questionnaire) (11.8% and 8.2%, respectively). This was accompanied by reduction of office and average diurnal BP, restoration of BP circadian rhythm and decrease of SBP morning rise rate.
Additional indication of spironolactone and spironolactone in combination with trimetazidine resulted in significant decrease of LVMi (by 11.2±3.7 and 14.7±4.1 g/m2, respectively) in comparison with the basic therapy by 5.0±2.1 g/m2 (р<0.05). Better regression of LVH in the course of therapy with additional indication of spironolactone and spironolactone in combination with trimetazidine in comparison with the basic therapy was associated with significant decrease of LAVi (by 4.7±1.2 ml/m2 and 4.4±1.1 ml/m2), improvement of diastolic function of LV (decrease of Е/Е’ by 2.3±0.8 and 2.8±0.5) and reduction of mPAP (by 4.6±2.1 and 4.2±1.8 mmHg).
In comparison with the basic therapy, additional indication of spironolactone and spironolactone in combination with trimetazidine resulted in significant decrease of systolic and pulse BP in aorta and improvement of aorta functional status: calculate flow rate quantitatively similar reduced (by 0.50±0.07 m/s) and aorta stiffness index decreased (by 59.9% and 71.7%, respectively).
In the course of additional indication of spironolactone and spironolactone and trimetazidine therapy, the increase of EDVD rate was more significant and was associated with its normalization in 80% and 70% of patients (χ2=21.60; р<0.01; χ2=15.15; р<0.01, respectively), unlike during the basic therapy, which resulted in EDVD normalization only in 23.3% of patients (χ2=1.00; р>0.05 in comparison with initial data).
Additional indication of trimetazidine and spironolactone was associated with significant improvement of CHF development: significant decrease of the disease FC by NYHA classification by 0.40±0.01 and transfer of 66.7% of patients to lower FC by NYHA. Significant increase of GFR (by 14.9±0.8 ml/min/1.73m2) only in the course of combined spironolactone and trimetazidine therapy confirmed positive influence of trimetazidine on kidneys functional status.
Acknowledgment
The author(s) received no specific funding for this work.
References
- Antoniadis A.P, Sieniewicz B, Gould J, et al. Erratum to: Updates in Cardiac Resynchronization Therapy for Chronic Heart Failure: Review of Multisite Pacing. Curr Heart Fail Rep. 2017;14(5):384. doi:10.1007/s11897-017-0359-3.
CrossRef - Arturi F, Succurro E, Miceli S, et al. Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure. 2017;57(3):464-473. doi:10.1007/s12020-016-1166-4.
CrossRef - Bektas S, Franssen F.M.E, van Empel V, et al. Impact of airflow limitation in chronic heart failure. Netherlands Hear .J. 2017;25(5):335-342. doi:10.1007/s12471-017-0965-4.
CrossRef - Benz D.C, Pazhenkottil A.P. Cardiac resynchronization therapy in chronic heart failure: Effect on right ventricular function. J. Nucl Cardiol. 2017. doi:10.1007/s12350-017-0953-5.
CrossRef - De Vecchis R, Cantatrione C, Mazzei D. Vasopressin receptor antagonists in patients with chronic heart failure. 2017;42(5):492-497. doi:10.1007/s00059-016-4482-9.
CrossRef - den Boer S.L, Flipse D.H.K, van der Meulen M.H, et al. Six-Minute Walk Test as a Predictor for Outcome in Children with Dilated Cardiomyopathy and Chronic Stable Heart Failure. Pediatr Cardiol. 2017;38(3):465-471. doi:10.1007/s00246-016-1536-y.
CrossRef - Doumouras B.S, Lee D.S, Levy W.C, Alba A.C. An Appraisal of Biomarker-Based Risk-Scoring Models in Chronic Heart Failure: Which One Is Best? .Curr Heart Fail Rep. 2018;15(1):24-36. doi:10.1007/s11897-018-0375-y.
CrossRef - Izadi N, Sabour S. Sudden cardiac death in patients with chronic heart failure: Rule of thumb in prediction studies. J. Nucl Cardiol. 2017;24(5):1829-1830. doi:10.1007/s12350-017-0936-6.
CrossRef - Kato M. The Concept of Heart Failure: Chronic Diseases Accompanied by an Attack of Acute Exacerbation. In: Sato N, ed. Therapeutic Strategies for Heart Failure. Tokyo: Springer Japan. 2018:1-15. doi:10.1007/978-4-431-56065-4_1.
CrossRef - Katsumata M, Hirawa N, Sumida K, et al. Effects of tolvaptan in patients with chronic kidney disease and chronic heart failure. Clin Exp Nephrol. 2017;21(5):858-865. doi:10.1007/s10157-016-1379-0.
CrossRef - Murphy K.M, Rosenthal J.L. Progress in the Presence of Failure: Updates in Chronic Systolic Heart Failure Management. Curr Treat Options Cardiovasc Med. 2017;19(7):50. doi:10.1007/s11936-017-0552-4.
CrossRef - Nagrecha S, Thomas P.B, Feldman K, Chawla N. V. Predicting Chronic Heart Failure Using Diagnoses Graphs. In: Holzinger A, Kieseberg P, Tjoa A.M, Weippl E eds. Machine Learning and Knowledge Extraction. Cham Springer International Publishing. 2017:295-312.
- Petrov V.N, Agaeva E. V, Popovkina O.E, et al. Modifying Effect of Autotransfusion of Mesenchymal Stromal Cells on the Production of Reactive Oxygen Species and Cytokines by Mononuclear Cells in Patients with Chronic Heart Failure. Bull Exp Biol Med. 2017;164(2):233-240. doi:10.1007/s10517-017-3965-x.
CrossRef - Piepoli M.F. Congestive Heart Failure: Stable Chronic Heart Failure Patients. In: Niebauer J, ed. Cardiac Rehabilitation Manual. Cham: Springer International Publishing. 2017:207-226. doi:10.1007/978-3-319-47738-1_10.
CrossRef - Pryds K, Nielsen R.R, Jorsal A, et al. Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res Cardiol. 2017;112(6):67. doi:10.1007/s00395-017-0658-6.
CrossRef - Roche S.L. Medical Therapy for Chronic Right Ventricular Failure in Congenital Heart Disease. In: Friedberg M.K, Redington A.N, eds. Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Cham: Springer International Publishing. 2018:217-231. doi:10.1007/978-3-319-67096-6_16.
CrossRef - Roth S, Fernando C, Azeem S, Moe G.W. Is There a Role for Ivabradine in the Contemporary Management of Patients with Chronic Heart Failure in Academic and Community Heart Failure Clinics in Canada? Adv Ther. 2017;34(6):1340-1348. doi:10.1007/s12325-017-0529-4.
CrossRef - Testa G, Cacciatore F, Bianco A, et al. Chronic obstructive pulmonary disease and long-term mortality in elderly subjects with chronic heart failure. Aging Clin Exp Res. 2017;29(6):1157-1164. doi:10.1007/s40520-016-0720-5.
CrossRef - van Kessel P, de Boer D, Hendriks M, Plass A.M. Measuring patient outcomes in chronic heart failure: psychometric properties of the Care-Related Quality of Life survey for Chronic Heart Failure (CaReQoL CHF). BMC Health Serv Res. 2017;17(1):536. doi:10.1186/s12913-017-2452-4.
CrossRef - Yamamoto H, Beppu S. Left ventricular cardiac hemangioma in a patient with chronic heart failure. J. Med Ultrason. 2017. doi:10.1007/s10396-017-0852-z.
CrossRef








