Manuscript accepted on :28 April 2018
Published online on: 24-05-2018
Plagiarism Check: Yes
Dusan Surdilovic1, Prabhu Manickam Natarajan1, Tatjana Ille2, Shisir Ram Shetty1 and Pooja Adtani1
1College of Dentistry, Gulf Medical University, Ajman, UAE.
2College of Medicine, Gulf Medical University, Ajman, UAE.
Corresponding Author E-mail: surdilovic74@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1440
Abstract
Collagen forms the major organic constituent of human tooth dentin with non-collagen proteins and proteoglycans contributing for minor fragment. Studies involving the nature of non–collagen proteins are comparatively rarer than the other dentinal components. With this background the authors conducted the present study. To identify the specific and unspecific non-collagen proteins in the dentin. Samples of teeth tissue prepared using guanine hydrochloride in natrium acetate and then subjected to polyacrylamide gel electrophoresis. The samples were visualized using Coomassie Blue coloring technique. The pattern of electrophoresis of dentin fraction indicated large protein component. The mass spectrometric analysis results of the two gel parts confirmed the presence of dentin sialoprotein and bone sialoprotein in the dentin fraction. Difference in the molecular mass was evident between the two protien fractions. The authors suggest that since dentin sialoprotein is specific to dentin and not identified in any other tissue, it may be a unique constituent and may play a critical role in formation of dentin.
Keywords
Bone Sialoprotein; Dentin; Dentin Sialoprotein; SDS-PAGE; Osteopontin
Download this article as:| Copy the following to cite this article: Surdilovic D, Natarajan P. M, Ille T, Shetty S. R, Adtani P. Non-Collagen Protein in the Dentin Tissue – the Role in the Process of Dentinogenesis. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Surdilovic D, Natarajan P. M, Ille T, Shetty S. R, Adtani P. Non-Collagen Protein in the Dentin Tissue – the Role in the Process of Dentinogenesis. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20489 |
Introduction
Dentin, as a specific hard dental tissue, is rich in organic substances which make around 20% of its weight and 33%of its volume. Almost 90% of dentin’s organic matrix is collagen, whereas the rest contains non-collagen proteins and proteoglycans with a dominant anionic character, as well as some lipid components.
However, non-collagen organic content of the dentin has not been studied enough. Dentin sialoprotein (DSP) belongs to the group of acidic glycoproteins. This protein was discovered only inside the dentin and its molecular mass of 95kDa was derived in the process of SDS – polyacrylamid gel electrophoresis (PAGE) . The secretion is getting collected from the odontoblast and some dental papilla cells. DSP synthesis is carried out after collagen type I secretion, but prior to the repositioning of the mineralized dentin’s first layer. The role of dentin sialoprotein has not been defined in the process of dentinogenesis. Bone sialoprotein (BSP) can be found in traces inside the dentin. Our project’s results also confirm the presence of low BSP quantity. Bone sialoprotein (BSP) is highly glycolized and sulfatased phosphoprotein, originally isolated in the bones. The process of immunodetection proved that BSP is absent from the soft tissues such as muscles, cartilage, tendons, skin and liver. BSP consists of nearly 300 amino acids and it is rich in glycine and asparagyl.
Osteopontin is a phosphorolyzed glycoprotein containing RGD (arginine-glycine-asparagyl acid) integrin receptor bonding sequence, integrin being responsible for the interaction between a cell and an extracellular matrix and found on the cell membrane surface. This fact connects Osteopontin with the biochemical processes taking place in the extracellular matrix. Osteopontin molecular mass is 44kDa. It is located in the predentin and is not typical of dentin, as it is present in many tissues. Osteopontin is located in the mineralization front region where it has a role in inhibition of forming apatite crystals.
Dentin and its complex ultra structure, molecular content and biochemical processes happening in this tissue have not been enough clarified yet. Therefore, they still present a challenge for science.
Introducing SDS-PAGE electrophoresis enabled the presence of protein in the dentin to be more precise determined, as well as its quality and quantity content.
The Aim
The Aim of the Research is To
Identify the specific and unspecific non-collagen proteins in the dentin using the SDS-PAGE electrophoresis abilities
Present the molecular and biological importance of the detected proteins in the dentin, in the process of dentinogenesis, using analysis of the obtained results and comparing them with the data from literature.
Material and Methods of Research
Preparation of Samples
100g of teeth were first frozen in liquid nitrogen and then crushed in particles to powder consistence using a hydraulic press. The frozen tooth powder mass was treated with guanine hydrochloride in 50mM natrium acetate, with 5,8pH value. The aim of this procedure was removal of cell remnants and macromolecules not derived from dentin.1,2
SDS-PAGE
SDS-PAGE (SDS – polyacrylamide gel electrophoresis) is an electrophoretic method of polypeptides’ separation based on the difference in their molecular masses. This method is used as an individual procedure of protein electrophoresis’ analysis and it is very important in the initial research of protein groups in a given sample. SDS-PAGE also represents another dimension of 2D electrophoresis, thus achieving more precise separation and characterization of macromolecules in the sample.
SDS-PAGE electrophoresis is performed in polyacrylamide gels containing sodium dodecyl sulfate. Gels used in this procedure are being prepared in labs right before the experiment begins. Polyacrylamide gels are polymerized solutions of acrylamide and bis-acrylamide. The acrylamide itself creates linear polymers, whereas bis-acrylamide enables cross connections among polyacrylamide chains. In that way, a gel is being polymerized, having a role of a sieve, in which the bigger pores are close to the gel top and the pore size is permanently and gradually decreased as approaching to the lower edge of the gel.3,4,5
During the SDS-PAGE electrophoresis, all the sample proteins initially placed in the inert gel for setting samples (stacking gel) and on the top of the separation polyacrylamide gel (running gel), migrate towards the anode (negative electrode) on the bottom of the gel.6
During PAGE, the level of SDS-treated protein migrations is determined by the protein molecular mass. The proteins are filtered through the gel ‘sieve’ and heavier proteins stay trapped in the higher gel parts, while the lighter ones go lower, where the gel pores’ diameter gets smaller. The gels are prepared inside a machine for PAGE electrophoresis, by Amersham Biotech Company. Preparation of the 14% separatory gel is made using the following prescription: 1,875ml of deionised water; 1,25ml of 1,5M Tris HCl with pH8,8; 50 micro liters of 10% SDS; 1,75ml of 40% acrylamide; 1,75ml of 40% Bis-acrylamide; 50 micro liters of 10% ammonium persulfate and 25 micro liters of TEMED. 5% inert gel for setting samples is prepared as following: 1,2ml of deionised water; 0,5ml of 1,5M Tris HCl with pH 6,8; 20 micro liters of 10% SDS; 0,25ml of 40% acrylamide/ Bis-acrylamide; 20 micro liters of 10% ammonium persulfate and 10 micro liters of TEMED.
Prior to the application in the gel, dentin samples are boiled in a dish with water about 5 minutes after the boiling point. This procedure is necessary due to the complex protein structure which requires denaturation of the polypeptide chains. 20 micro liters of merkaptoeptanol is added to the samples, with a role of breaking the disulfate bonds in the protein (Berkelman and Stensted, 1998).
Control samples with familiar molecular masses (MW markers) are placed in the bordering gel columns, thus enabling the comparison between the known facts and the examined unknown samples.
Visualization of Samples
Coomassie Blue coloring technique has been largely applied in the process of protein visualization in SDS-PAGE. Coomassie Blue Staining Protocol involves soaking of the gel into a solution of 50% ethanol and 10% of vinegar acid for a period of time of at least an hour.7,8
What follows is rinsing of the gel in water and then its developing in 0,04% of formalin (35% formaldehyde in water) and 2% of natrium carbonate while intensely stirring the gel. Treated gels, being colored using Silver Staining technique, are kept on 4°C in a 1% vinegar acid solution, up to the moment of the result analysis.9
Visualization of the electrophoresis results being done, gel points are chosen (after coloring, proteins become visible as dark stains), cut out from the gel and directed to the mass spectrometric analysis.
Mass spectrometric procedure includes analyses and verification of the obtained results (traces of specific proteins or protein groups on the gel) by determining the amino acid protein chains existing in the gel part. Determination of amino acid sequences is performed using the process of Edman’s degradation, which allows the isolation of protein in significantly small amounts.
The results in terms of determined amino acid sequences are then compared with the available database containing the sequences of the existing and described proteins. The analysis of amino acid sequence similarities has been enabled by using a special database with all polypeptide amino acid chains discovered up to the present moment.
Research Results
Polyacrylamide gel samples with the results of dentin fraction electrophoresis show presence of very big protein concentration in this fraction. The mass spectrometric analysis results of the two gel parts confirm the presence of dentin sialoprotein (DSP) and bone sialoprotein (BSP) in the dentin fraction. DSP molecular mass is about 90kDa, whereas BSP is located in the gel with high concentrations at the molecular mass levels ranging from 70 to 75kDa. Picture 1 to 5 show mass spectrometric analyses results presented in BLAST program for identification of amino acid protein sequence.
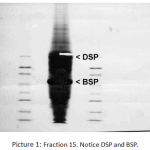 |
Figure 1: Fraction 15. Notice DSP and BSP.
|
 |
Figure 2: Results of mass spectrometer analysis of fraction 15 dentin gel sample shown in BLAST program, for identification of protein amino acid sequence (NCBI).
|
One of the samples was identified as dentin sialoprotein
 |
Figure 3: A result of mass spectrometer analysis of fraction 15 dentin gel sample shown as amino acid sequence (DSP).
|
 |
Figure 4: Results of mass spectrometer analysis of fraction 15 dentin gel sample shown in BLAST program, for identification of protein amino acid sequence (NCBI).
|
One of the samples was identified as bone sialoprotein (BSP II).
 |
Figure 5: A result of mass spectrometer analysis of fraction 15 dentin gel sample shown as amino acid sequence (BSP II).
|
Mass spectrometric analysis of dentin fraction 32, treated in PAGE, reveals presence of acid glycoprotein, Osteopontin, and is of about 44kDa molecular mass. The results are shown in BLAST program of NCBl database (Figures 6 to 8).
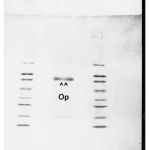 |
Figure 6: Fraction 32. Notice Osteopontin
|
 |
Figure 7: Results of mass spectrometer analysis of fraction 32 dentin gel sample shown in BLAST program, for identification of protein amino acid sequence (NCBI).
|
The sample was identified as Osteopontin
 |
Figure 8: A result of mass spectrometer analysis of fraction 32 dentin gel sample shown as amino acid sequence (Osteopontin).
|
Discussion
The results of the original researches point out to the presence of sialoprotein in the examined dentin fraction (Figure 1). Dentin sialoprotein (DSP), known also as 95kDa glycoprotein, is rich in sialic acid and it is found only in the dentin. As opposed to other glycoproteins, Osteopontin, bone sialoprotein and BAG-75, DSP does not contain phosphates. Similar to other sialoproteins, DSP is rich in aspartame acid, glutamine acid, serine and glycine.¹⁰ 29,6% of carbohydrate content in DSP includes around 9% of sialic acid. DSP is made of a singular polypeptide chain without cysteine or disulfide bonds (Figures 2 and 3) .
Migration of DSP in the process of SDS polyacrylamide gel electrophoresis shows that its molecular mass is about 140 000 in 5-15% gradient gel, but 105 000 in 15% gels.¹¹ Data from modern literature stress the migration level of DSP to be slightly above 94 000, which is a standard value of its molecular mass, and that officially confirmed DSP having a molecular mass of 95kDa.6 These facts fully correlate with the results of our project.
Dentin sialoprotein is an odontoblast product and it secretases into predentin and dentin.12,13 More precise immunohistochemical researches with purified antibodies confirm that SDP is synthesized by the odontoblast and that it is found both in the predentin and dentin. The presence of this protein has not yet been confirmed in the glut, bones, muscles and cartilage.14
DSP role in the process of dentinogenesis is not known. It is supposed that DSP influences the process of mineralization.15,16 Taking into consideration the expression of this protein in odontoblast and odontoblastic cells, DSP may be an important marker of odontoblast phenotype. Induction experiments of reparation dentinogenesis show that cells replacing the damaged odontoblastic layer contain dentin sialoprotein, which is not the case with the neighboring cells14.
Bone sialoprotein (BSP) is only found in traces inside dentin10. Our results also prove its presence in small quantities. BSP is highly glycolized and sulfated phosphoprotein, originally isolated in the bones. Immunodetection proved that BSP is not present in soft tissues such as muscles, cartilage, tendons, skin and liver.17,18 BSP consists of around 300 amino acids and is rich in glycine and asparagine (Figure 4 and 5).
The results we got on the BSP molecular mass match the data from literature. The molecular mass of BSP varies between 70 000 and 80 000 and it is obtained by the protein migration in the process of polyacrylamide gel electrophoresis.19 BSP and its mRNK synthesis in odontoblasts have been demonstrated in a series of studies.20 In the dentin, BSP is localized in odontoblasts and their tags, as well as in the peritubular dentin.21 Protein expression is tied to the mineralized tissues – bone, dentin and cement, but also to the hypertrophic chondrocytes and placental trophoblasts.22
Even though the role of bone sialoprotein in the tissue mineralization has not been clearly stated, it was proven that BSP induces creation of hydroxyapatite crystals in the system of agarose gel, when low concentrations of calcium ions and phosphates are present 25. Some facts from literature suggest that BSP can have an initiation role of growing hydroxyapatite crystals in mineralized tissues21.
Comparative data analysis from the literature and the original project results confirms the presence of Osteopontin in the organic dentin matrix (Figure 6). Osteopontin is phosphorized glycoprotein with molecular mass of around 44kDa. It consists of approximately 280-300 amino acids (depending on the mammal type). It is rich in aspartame acid, serine and glutamine acid18. The protein also contains sulfates,24 especially tyrosine sulfate (Figures 7 and 8).
Experiments in the field of immunolocalization show that Osteopontin secretion is formed out of preosteoblasts, osteoblasts and fresh osteocytes.25 However, Osteopontin is also located in odontoblasts, serum, neuroepithelial cells of the inner ear and in the bone marrow cells.26 It is familiar that Osteopontin gets synthesized in osteoblasts during early stages of bones mineralization. Alkaline phosphatase is also noticed in the early stage of differentiation of cells which participate in the mineralization process21.
A conclusion can be drawn that these two proteins may act as markers of differentiated osteoblasts. In connection to this hypothesis, a question may be raised on whether Osteopontin and alkaline phosphatase may also act as markers of odontoblasts’ differentiation. Osteopontin is found in the organic matrix of dentin²⁷ and predentin.²⁸
The role of Osteopontin in mineralization processes is not known. Certain observations point to three possible roles that Osteopontin might have in the processes of mineralization. Due to its appearance in preosteoblasts and osteoblasts prior to formation of the first minerals, this protein could be a cell adhesion factor10. Other possible function of Osteopontin, as with other phosphoproteins, could be induction of mineralization in bones, dentin and cement28. Finally, the third observed function of Osteopontin is its bonding to osteoclasts in the process of broken bones recovery.29
Conclusion
Dentin sialoprotein (DSP) is a specific protein in the dentin. It is a product of secretory activity of mature odontoblasts. It presents a reliable phenotype marker of odontoblasts. The mere fact of DSP being specific for the dentin and not being found in any other tissue, implicates its unique, critical and essential importance in dentinogenesis.
BSP and Osteopontin discovered in the research are not specific for the dentin and are produced both by odontoblasts and osteoblasts. Bone sialoprotein may have a role in initiation of the growth of hydroxyapatite crystals in the mineralized tissues. Osteopontin and alkaline phosphatase could be markers of odontoblasts’ differentiation.
Analyzing the presented features of these unspecific proteins, it can be concluded their presence in the process of dentinogenesis is necessary.
References
- Wendel M, Sommarin Y, Heinegard D. Bone matrix proteins: Isolation and characterization of a novel cell-binding keratan sulfate proteoglycan from bovine bone. J. Cell. Bio. 1998;141:839-847.
CrossRef - Deutscher M.P. Guide to protein purification. Methods enzymology. 1990;182:1-894.
- Wu F.S. Extraction of proteins for SDS PAGE from protease-rich plant tissues. Anal. Biochem. 1984;139:100-103.
CrossRef - Berkelman T, Stenstedt T. 2d elektrophoresis. Amersham pharmacia biotech. 1998.
- Schagger H. Tricine-SDS PAGE for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987; 166:368-379
CrossRef - Begue-Kirn C. et all: Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. Eur J Oral Sci; 106 (suppl 1). 1998;254-259,
CrossRef - Herbert B.R. et all: Improved protein solubility in 2D electrophoresis using reducing agent. Electrophoresis. 1998;19: 845-851.
CrossRef - Granzier H.L.M, Wang K. Gel electrophoresis of giant proteins solubilization and silver-staining of titin and nebulin from single muscle fiber segments. Electrophoresis. 1993;14:56-64.
CrossRef - Westermeier R. Electrophoresis in practice. VCH-Verlag, Weinheim. 1997.
- Butler, W.T. Sialoproteins of bone and dentin. J Biol Buccale. 1991;19:83-89.
- Butler W.T, Bhown M, Tomana M. Characterization of unique dentin gly-coprotein. Chemistry and biology of mineralized connective tissues. Elsevier, New York. 1981.
- Dimuzio M.T, Bhown M, Walton R.K, Butler W.T. Bone and dentin organ cultures. Chemistry and biology of mineralized connective tissues. EBSCO Media, Birmingham. 1985.
- Bronckers A.L.J.J, Lyaru, D.M. Immunohistochemistry of extracellular matrix proteins during various stages of dentinogenesis. Cennective Tiss. Res. 1989;22:65-70.
- D’Souza R.N. et all: Immunolocalization of a 53 kDa dentin sialoprotein, submited for publication. 1991.
- Gorski J. Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralizyation. Calcif. Tissue Int. 1992;50:391-396.
CrossRef - Butler W.T. Dentin Matrix Proteins and Dentinogenesis. Connective Tissue Research. 1995;33:59-65.
CrossRef - Fisher L.W. et all: Proteoglycans of developing bone. J. Biol. Chem. 1983;258:6588-6594.
- Franzen A, Heinegard D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1990;232:715-724.
CrossRef - Fisher L.W, McBride O.W, Termine J.P. Human bone sialoprotein. J. Biol. Chem. 1990;265:2347-2351.
- Chen J, Shapiro H.S, Sodek J. Developmental expression of bone sialoprotein mRNA in rat mineralized tissues. J. Bone Miner. Res. 1992;7:987-997.
CrossRef - Butler W.T, Ritchie H. The nature and functional significance of dentin extracellular matrix proteins. Int. J. Dev. Biol. 1995; 39:169-179.
- Young M.F, Kerr J.M, Ibaraki K. Structure, expression anf regulation of the major noncollagenous matix proteins of bone. Clin. Orthop. Relat. Res. 1992;281:275-294.
- Hunter G.K, Goldberg H.A. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. USA. 1993;90:8562-8565.
CrossRef - Nagata K, Huang Y.H. Demonstration of type III collagen in the dentin of mice. Matrix. 1992;12:448-455.
CrossRef - Mark M.P, Butler W.T. Developmental expression of 44 kDa bone phosphoprotein (osteopontin) in calcifying tissues of rat. Diferentiation. 1988;37:123-136.
CrossRef - Senger D.R, Peruzzi C.A. Elevated expresion of secreted phosphoprotein I. Anticancer. Res. 1989;9:1291-1300.
- Fujisawa R, Kuboki Y. Preferential absorption of dentin and bone acidic proteins on the (100) face of hydroxyapatite crystals. Biochim. Biophys. Acta. 1991;1075:56-60.
CrossRef - Boskey A.L, Spevak L, Paschalis E. Osteopontin deficiency increases mineral content and mineral crystalinity in mouse bone. Calc. Tissue Int. 2002.
- Chellaiah, M.A. et all: Rho-A is critical for osteoclast podosome organisation, motility, and bone resorption. J. Biol. Chem. 2000;275:11993-11998.
CrossRef







