Mervat M. Halawani1 , Gamal S. Abdul Aziz1
, Gamal S. Abdul Aziz1 , Hanan A. Amin1, 2
, Hanan A. Amin1, 2 , Hesham N. Mustafa1
, Hesham N. Mustafa1 and Amira A. Elhaggagy2
and Amira A. Elhaggagy2
1Anatomy Department, Faculty of Medicine, King Abdulaziz University, KSA.
2Histology Department, Faculty of Medicine, Cairo University, Egypt.
Corresponding Author E-mail: hesham977@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1419
Abstract
The liver is almost unique in its capacity for regeneration after hepatectomy but the exact mechanisms are not yet fully clarified. Antioxidants have been shown to promote liver regeneration after major hepatectomy. The present study evaluated the ameliorative effect of vitamin E administration on the liver regeneration after different periods of partial hepatectomy (PH) in rats. Fifty-six adult male albino rats were divided into three groups: Control sham operated group; partially hepatectomized group which were divided into three subgroups sacrificed at 1day, 3 days and 7days after the operation respectively; Partially Hepatectomized group with vitamin E pretreatment before PH where the rats were given a daily oral dose of vitamin E until the time of sacrifice of the rats. Immunohistochemical detection of proliferating cell nuclear antigen (PCNA) and labeling index were demonstrated. After PH, the PCNA positive hepatocytes and the PCNA labeling indices were significantly high after the 1st day and then much decreased after the 3rd day, to be followed by a slight increase at the 7th day. Vitamin E pretreatment in PH rats resulted in a decrease in PCNA positive cells and its labeling indices in the 1st day with a gradual increase in the 3rd and 7th days. Vitamin E has an inhibitory effect in the first 24 hours on liver regeneration followed by stimulatory effect at the third and seventh days after PH. These data indicated that vitamin E pretreatment has an important role in regulation and enhancement of liver regeneration after PH.
Keywords
Immunohistochemistry; Labeling Index; Vitamin E; Partial Hepatectomy; PCNA;
Download this article as:| Copy the following to cite this article: Halawani M. M, Aziz G. S. A, Amin H. A, Mustafa H. N, Elhaggagy A. A. Immunohistochemical Study of the Ameliorative Effect of Vitamin E on Liver Regeneration after Different Periods of Partial Hepatectomy. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Halawani M. M, Aziz G. S. A, Amin H. A, Mustafa H. N, Elhaggagy A. A. Immunohistochemical Study of the Ameliorative Effect of Vitamin E on Liver Regeneration after Different Periods of Partial Hepatectomy. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20244 |
Introduction
The mammalian liver is the only organ that exhibits an amazing potential to regenerate after surgical resection.1 Liver regeneration is the organized and controlled response of the liver toward tissue damage induced by trauma, infections, toxic agents, or post-surgery resection.2,3 Surgical resection or partial hepatectomy (PH) is performed for the treatment of mass lesions in the liver like primary tumors, metastases and hemangioma or for living donor liver transplantation.4
It is well known that remnant liver after 70% PH can regenerate and restore its original mass within seven to twelve days in rats.5 In the human liver, regeneration period may be six to twelve months.6,7 Liver regeneration is carried out by proliferation of all adult liver cells including hepatocytes, sinusoidal endothelial cells, biliary epithelial, Kupffer and hepatic stellate cells.8 Also, it has been firmly established that mature hepatocytes have an almost unlimited capacity to proliferate, so that the liver can be entirely repopulated by intact hepatocytes that represent 1% of the hepatocyte population.9,11
In humans, when the liver regeneration is impaired, it results in the pathogenesis of liver failure or development of fibrosis.12 which remains poorly understood. The fundamental parameters for liver failure following PH were explained on the basis of functional mass of the remnant liver, the age of the patient and the presence of pre-existing liver disease such as cirrhosis, chronic hepatitis or fatty liver disease. These situations interfere with the normal regenerative pathways or initiate apoptotic pathways, resulting in a functional loss of remaining hepatocytes.13 Also; another mechanism has suggested an increased production of reactive oxygen species (ROS) in liver mitochondria following PH, which can alter the function of the enzymes involved in oxidative phosphorylation.14,17
ROS modulate a variety of signaling pathways that may affect liver regeneration where they are known to mediate cell growth arrest and activate proteins that inhibit the cell cycle.18,19
Therefore, ROS production in the liver remnant is likely to have a role in the negative control of regeneration, where it has been suggested that increased ROS-production affects the early-phase of hepatic regeneration.20,21
Accordingly, the antioxidant activity or the inhibition of the generation of ROS is important in providing protection against hepatic damage in cases of liver regeneration.22 Vitamin E (alpha-tocopherol) is the most important antioxidant in tissues, red cells and plasma.23,24 It has lipid radical scavenging action and had been shown to protect animal tissues against oxidative damage such as lipid peroxidation (LP) both in vitro,25 and in vivo.26
It was suggested that the preoperative administration of vitamin E modulated the cellular immune response and restored impaired liver function following PH, presumably through its antioxidant capacity.27 Previous reports have demonstrated the beneficial effects of vitamin E, which has two important functions in the membrane: as a liposoluble antioxidant that prevents ROS damage in polyunsaturated fatty acids, and also as a membrane stabilizer agent acting against damage caused to phospholipids.28 Vitamin E acts by means of breaking the antioxidant chain that prevents ROS-produced cell membrane damage.29Recently, it was reported that short-term (7-day) antioxidant supplementation attenuates lipid peroxidation and protects against liver injury and dysfunction in an ethanol intoxication model during PH-induced liver regeneration.30
In view of that, a novel pharmacological strategy is needed for protection against liver dysfunction and enhancement of regenerative capacity following major hepatic resection. In the present study, 70% PH model in rats was selected to investigate the immunohistchemical identification of PCNA expression and its labeling indices in proliferating liver cells with the potential benefit of vitamin E to enhance liver regenerative capacity.
Materials and Methods
Animals and Experimental Design
Fifty six adult male Sprague-Dawley albino rats, weighing 190,240 grams, were used in this study. They were obtained from the Animal House of King Fahd Center for Medical Research, KSA. At the beginning of the experiment the animals were kept in separate metallic cages under controlled conditions including temperature of 28 ºC, light (12/12 h light/dark) and humidity 50%-55%. The animals were fed with standard rat chow and tap water ad libitum. This study was approved and registered by the Committee of Animal Investigations in Department of Anatomy, Faculty of Medicine, King Abdulaziz University. During all the steps of the study, the animals were cared at King Fahd Center for Medical Research. After one week of acclimatization, the rats were randomly divided into 3 groups:
Control sham-operated group
Was formed of 8 rats, which were subjected to the same surgical procedure without excision of any liver lobes.
Partially Hepatectomized group
was formed of 24 rats to which PH was done (day 0). These hepatectomized rats were divided to three subgroups of 8 rats each, and were sacrificed at 1day (PH1), 3 days (PH3) and 7days (PH7) after the operation.
Partially Hepatectomized Group with Vitamin E Pretreatment
Was formed of 24 rats to which PH was done (day 0). Three days before the operation, the rats were given a daily oral dose of vitamin E (500 mg/Kg body weight) by oral gavage until the time of sacrifice of the rats (Horvath et al., 2001). These hepatectomized rats were divided to three subgroups of 8 rats each, which were sacrificed at (PHE1), 3 days (PHE3) and 7days (PHE7) after the operation.
Partial Hepatectomy
Two thirds partial hepatectomy (PH) was performed according to the standard technique of Greene and Flecknell,31 and Ramírez-Farías.30 Briefly, the surgery was performed under light ether anesthesia, where the rat was placed in the full supine position with the four limbs fixed to the operating table. Through a 3 cm midline laparotomy incision, the liver was exposed after retraction of the abdominal wall. A silk thread of caliber 3-0 was passed around the left lateral and median lobes and was drawn up as far as possible to their origin where they were excised below the ligation. This is corresponding to a resection of approximately 70% of the whole liver.32 The right and caudate lobes of the liver were left in place in all rats. After closure of the abdominal wall, the rats were left till they became fully conscious and oral feeding was allowed after about three hours.
Tissue processing for immunohistochemistry
The rats of each group were sacrificed by an over dose of ether anesthesia 1day, 3days and 7days after partial hepatectomy. Liver samples were taken immediately from the remnant lobes of the liver. The specimens were fixed in 10% formol saline for 48 hours followed by dehydration, clearance and embedding in paraffin. Liver sections (4-6 um thick) were cut and subjected to immunohistochemical staining to assess the proliferative activity and DNA synthesis of liver cells by detecting the proliferating cell nuclear antigen (PCNA). Sections were dewaxed, rehydrated and treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase. Before incubation with the primary antibody, the sections were immersed in 10 mM citric acid buffer (pH 6.0) for 12 minutes. Immunostaining for PCNA was done using a primary anti serum to PCNA (clone PC10, DAKO Corp. Denmark) followed by biotinylated horse antimouse antiserum, avidin-biotin complex and DAB as the chromogen. The sections were finally counterstained with hematoxylin solution before being dehydrated and mounted in Canada balsam. Tonsil and small intestines were used as positive control specimens. On the other hand, one of the liver specimens was used as negative control by omitting the primary antibody. A positive reaction was expressed as a dark brown color in the nuclei of liver cells.
Immunohistochemical Assessment
The data were obtained using Leica Qwin 500 image analyzer computer system (England). The patterns of PCNA immunostaining were evaluated without knowledge of the studied animal group. PCNA labeling index (LI) was estimated for each specimen as the percentage of positively stained liver cells out of all cells for each field. The average PCNA index (LI) in each group was then obtained in 10 fields in each slide. Three slides were taken from each specimen.
Statistical Methods
The mean of PCNA labeling indices was calculated at the end of the estimations. The statistical analysis was performed using analysis of variance (ANOVA). Results were considered significant when probability P was < 0.5. Comparisons between the groups were done using the post-hoc Newman-Keuls test to know which groups are particularly different from each other.
Results
Liver expression of PCNA
The liver regeneration in rats subjected to PH alone or with vitamin E pretreatment was investigated by immunohistochemical identification of PCNA expression in proliferating liver cells. The nuclei of the hepatocytes labeled with anti PCNA showed a positive reaction expressed as a granular or diffuse brown colour indicating the presence of normal cell proliferation. Also, this reaction appeared in the nuclei of cells lining the bile ducts, the endothelial cells and the von-kupffer cells.
Liver Expression of PCNA in Control Rats
The sections showed very few positive liver cells in the hepatic lobules especially around the portal spaces (Fig. 1-A).
Effect of Partial Hepatectomy (PH) on Liver Expression of PCNA
In the liver specimens taken from the rats of PH1 subgroup, almost all nuclei of hepatocytes showed a positive reaction (Fig. 1-B). While those taken from the rats of PH3 subgroup showed a decrease in the number of the PCNA stained nuclei (Fig. 1-C). Again, in the rats of PH7 subgroup, the picture was more or less similar to the PH3 group (Fig. 1-D).
Effect of Pretreatment with Vitamin E on Partial Hepatectomy (PHE) on Liver Expression of PCNA
In the liver specimens taken from the rats of PHE1 subgroup, many hepatocytes showed a positive PCNA immuno-staining, however, the number of positive hepatocytes was decreased when compared to PH1 group (Fig. 1-E). While, in the PHE3 subgroup, many hepatocytes with positive PCNA reaction were increased when compared with the PH3 subgroup (Fig. 1-F). There was an increase in the nuclei of the hepatocytes with positive PCNA reaction in the liver specimens taken from the rats of PHE7 subgroup, when compared to the PH7 subgroup (Fig. 1-G). These results indicated that vitamin E has an inhibitory effect in the first 24 hours followed by stimulatory effect at the third and seventh days on the liver regeneration after PH.
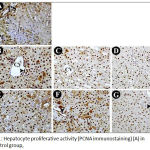 |
Figure 1: Hepatocyte proliferative activity (PCNA immunostaining) (A) in the control group,
|
Immunostained hepatocytes are seen mainly around PS; (B) in PH1, strong immunostaining in almost all nuclei of hepatocytes; (C) in PH3; and (D) in PH7, a slight decrease in number of the immunostained hepatocytes compared to PH1; (E) in PHE1, decreased number of the immunostained hepatocytes compared to PH1; (F) in PHE3 and in (G) PHE7 increased number of the immunostained hepatocytes compared to PH3 and PH7 respectively. Note the presence of positively immunostained nuclei of the endothelial cells and von-kupffer cells lining the blood sinusoids (arrowheads). CV: central vein; PS: portal spaces. (Original mag. x400)
PCNA labeling index (LI) (Table 1, Fig. 2):
The PCNA labelling index (LI) of the control group was very minimal, being 7± 1.2. However, in the rats subjected to partial hepatectomy, it was significantly elevated as compared to the control group to reach a maximal peak (90 ± 3.5) in the PH1 subgroup. In the PH3 subgroup, the LI decreased to be 60 ± 4.2 and then it slightly elevated in the PH7 subgroup to be 65 ± 3.7. In the partial hepatectomy with vitamin E pretreatment subgroups, the PCNA LI was notably decreased after 1 day (75 ± 1.5) when compared to the partial hepatectomy subgroup at the same period (65 ± 3.7). However, at 3 and 7 days after the surgery, the LI was significantly increased when compared to the corresponding partially hepatectomized subgroups to be 86.6 ± 3.3 and 78 ± 4.1 respectively.
Table 1: Comparison of PCNA labeling index (LI) in all studied groups:
| Group | Control | PH1 | PH3 | PH7 | PHE1 | PHE3 | PHE7 |
| PCNA LI | 7±1.2 | 90±3.5* | 60±4.2* | 65±3.7* | 75±1.5# | 86.6±3.3# | 78±4.1# |
The * indicates significant when compared to control (P < 0.05).
The # indicates significant when PHE subgroup compared to corresponding PH subgroup (P < 0.05).
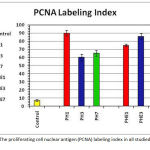 |
Figure 2: The proliferating cell nuclear antigen (PCNA) labeling index in all studied groups.
|
Discussion
Major liver resection sometimes causes fatal hepatic failure and hepatobiliary surgeons are still concerned about the risk of post-resectional liver failure.33,35 The incidence of post-resectional liver failure ranges anywhere, between 0 and 32%. In the past decade, mortality after partial hepatic resection ranged from 0 to 5% and although the cause of death after partial hepatic resection is multifactorial, post-hepatectomy liver failure seems to be the main cause (18–75%).36,38 Therefore, the rationale of this work was to study the time course events of liver regeneration at different periods after major resection using PCNA immune-staining in a rat model of PH and to find improved therapeutic strategies for the regulation of this regenerative process.
The hepatic regenerative capacity is most clearly seen after surgical removal of liver mass; this model which is referred to as PH, was introduced by authors,39 and it is unquestionably the best studied liver regeneration model due to its simplicity of design and reproducibility. After 70% PH is performed, the removed lobes do not re-grow; instead there is compensatory, hyperplastic growth of all residual cells until the size of the liver retains its size.40 Also, it was mentioned that the different liver cell types do not divide simultaneously but show different kinetics in DNA synthesis.8,41
Moreover, liver regeneration after PH in rats is a useful experimental model to investigate the regulatory mechanisms of cellular proliferation in response to the resection. This process starts within a few minutes and leads to an increased synthesis of DNA followed by a first wave of mitosis, after a “latent phase” of about 16 hours.42,44 The studies about PH have shown that initiation of the regenerative response depends on many growth and transcription factors.4,45 Under normal conditions, the hepatocytes have a very low regeneration rate; however, PH triggers a rapid proliferation of the hepatocytesthat tends to compensate the parenchymal loss and restoring the original hepatic mass.46
In this study, it was found that after 70% PH of a rat’s liver, the PCNA positive hepatocytes and the PCNA labeling index were very high in the first day and then decreased in the third day, to be followed by slight increase at the seventh day after the operation. These results of PCNA expression were consistent with the observations of Lai and Chen.47 who mentioned that the DNA synthesis increased abruptly to peak at 24 h, the mitotic index reached a maximum at.48 h, and the remaining lobes doubled in size by 48 h, attaining weight around 90% of the normal liver by 72 h after the surgical procedure. On the other hand, the rate of DNA synthesis correlates with cell proliferation.8 Therefore, monitoring DNA synthesis also indirectly indicates the ability of the liver to regenerate.5
In the present study, vitamin E pretreatment significantly decreased liver regenerative capacity during the 1st day as indicated by decreased PCNA labeling indices, which was followed by increase in these parameters at the 3rd and 7th days of PH when compared with corresponding groups of PH without vitamin E. At 24 hours after PH, the proliferating liver represented a wide immune-positivity for the nuclear presence of PCNA protein, which was observed in most of the liver cells. However, animals subjected to PH and pretreated with vitamin E clearly showed a decreased PCNA expression at 24 hours after surgery. In accord, it was reported that high concentrations of ROS inhibit proliferation and even encourage apoptosis or necrosis.48 In the regenerating liver an increased antioxidant capability leads to a low level of oxidative stress occurs during liver regeneration, correlating with the magnitude of hepatic loss and hepatocellular proliferation.49 Antioxidant treatment with vitamin E has been shown to inhibit liver regeneration after partial hepatectomy.50 In this study by Trejo-Solis et al., a similar delay of hepatocellular proliferation was observed as in the present study and vitamin E application entailed a striking reduction of liver mass recovery.
Also, other investigators reported that the nuclear amount of PCNA, quantified by Western blot assays, showed the same profiles with either PH or vitamin E administration.44,49,51,52 They added that PH induced a progressive enhanced PCNA expression starting at 6 hours and reaching a maximal peak at 24 hours after surgery, being practically normalized at 48 hours after PH. Based on the observation that vitamin E may contribute toward the maintenance of a critical balance between possible positive and negative effects of ROS in active proliferating cells.44
On the basis of the findings from this work, it could be concluded that vitamin E has an inhibitory effect in the early stages (24 h) of the liver regeneration after PH followed by stimulatory effect at the third and seventh days after PH. Furthermore, these data indicated that vitamin E pretreatment in cases of major liver resection is more efficient in liver regeneration protection against PH. This effect is probably caused by stabilization of biological membranes produced by this vitamin, which is in agreement with recent reports of Kirimlioglu et al. and Ramírez-Farías et al.30,53
Conflict of Interest
There is no conflict of interest.
References
- Khan A .Z, Mudan S .S. Liver regeneration mechanisms, mysteries and more. ANZ J Surg. 2007;77:9-14.
CrossRef - Morales-Gonzalez A. J, Gutierrez-Salinas J, Yanez L, Villagomez-Rico C, Badillo-Romero J, Hernandez-Munoz R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration role of route and timing of administration. Digestive diseases and sciences. 1999;44:1963-1974.
CrossRef - Morales-Gonzalez A .J, Jimenez-Garcia F. L, Guiterrez-Salinas J, Sepulveda J, Leija-Salas A, Hernandez-Munoz R. Effects of ethanol administration on hepatocellular ultrastructure of regenerating liver induced by partial hepatectomy. Digestive diseases and sciences. 2001;46:360-369.
CrossRef - Bedirli A, Kerem M, Pasaoglu H, Erdem O, Ofluoglu E, Sakrak O. Effects of ischemic preconditioning on regenerative capacity of hepatocyte in the ischemically damaged rat livers. J Surg Res. 2005;125:42-48.
CrossRef - Malik R, Mellor N, Selden C, Hodgson H. Triiodothyronine enhances the regenerative capacity of the liver following partial hepatectomy. Hepatology.2003;37:79-86.
CossRef - Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Annals of surgery. 1987;206:30-39.
CrossRef - Francavilla A, Polimeno L, Barone M, Azzarone A, Starzl TE. Hepatic regeneration and growth factors. J Surg Oncol Suppl. 1993;3:1-7.
CrossRef - Michalopoulos G.K, DeFrances M.C. Liver regeneration. Science. 1997;276:60-66.
CrossRef - Rhim J.A, Sandgren E .P, Degen J .L, Palmiter R .D, Brinster R .L. Replacement of diseased mouse liver by hepatic cell transplantation. Science.1994;263:1149-1152.
CrossRef - Rhim J.A, Sandgren E .P, Palmiter R .D, Brinster R .L. Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:49:42-4946.
CrossRef - Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. The American journal of pathology. 1997;151:1273-1280.
- Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. Journal of hepatology. 2008;48:993-999.
CrossRef - Helling TS. Liver failure following partial hepatectomy. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2006;8:165-174.
CrossRef - Guerrieri F, Vendemiale G, Grattagliano I, Cocco T, Pellecchia G, Altomare E. Mitochondrial oxidative alterations following partial hepatectomy. Free radical biology & medicine. 1999;26:34-41.
CrossRef - Carnovale C .E, Scapini C, Alvarez M .L, Favre C, Monti J, Carrillo M .C. Nitric oxide release and enhancement of lipid peroxidation in regenerating rat liver. Journal of hepatology. 2000;32:798-804.
CrossRef - Hernandez-Munoz R, Sanchez-Sevilla L, Martinez-Gomez A, Dent MA. Changes in mitochondrial adenine nucleotides and in permeability transition in two models of rat liver regeneration. Hepatology. 2003;37:842-851.
CrossRef - Alexandris I.H, Assimakopoulos S.F, Vagianos C .E, et al. Oxidative state in intestine and liver after partial hepatectomy in rats. Effect of bombesin and neurotensin. Clin Biochem.2004;37:350-356.
CrossRef - Finkel T, Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239-247.
CrossRef - Barnouin K, Dubuisson M .L, Child E .S. et al H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J Biol Chem. 2002;277:13761-13770.
CrossRef - Koch O.R, Pani G, Borrello S. et al Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol Aspects Med. 2004;z25:191-198.
- Nishitani Y, Matsumoto H. Ethanol rapidly causes activation of JNK associated with ER stress under inhibition of ADH. FEBS letters. 2006;580:9-14.
CrossRef - Shakya A.K, Sharma N, Saxena M, Shrivastava S, Shukla S. Evaluation of the antioxidant and hepatoprotective effect of Majoon-e-Dabeed-ul-ward against carbon tetrachloride induced liver injury. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2012;64:767-773.
CrossRef - Burton G, Ingold K.U. Vitamin E application of the principles of physical organic chemistry to the exploration of its structure and function. Accounts of Chemical Research.1986;19:194-201.
CrossRef - Burton GW, Traber MG. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annual review of nutrition. 1990;10:357-382.
CrossRef - Hennig B, Boissonneault G.A, Chow C.K, Wang Y, Matulionis DH, Glauert HP. Effect of vitamin E on linoleic acid-mediated induction of peroxisomal enzymes in cultured porcine endothelial cells. J Nutr. 1990;120:331-337.
CrossRef - Kaneko T, Nakano S, Matsuo M. Protective effect of vitamin E on linoleic acid hydroperoxide-induced injury to human endothelial cells. Lipids. 1991;26:345-348.
CrossRef - Horvath M.E, Gonzalez-Cabello R, Blazovics A.et alEffect of silibinin and vitamin E on restoration of cellular immune response after partial hepatectomy. J Ethnopharmacol. 2001;77:227-232.
CrossRef - Bradford A, Atkinson J, Fuller N, Rand R.P. The effect of vitamin E on the structure of membrane lipid assemblies. Journal of lipid research. 2003;44:1940-1945.
CrossRef - Brigelius-Flohe R, Traber M.G. Vitamin E: function and metabolism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1145-1155.
CrossRef - Ramirez-Farias C, Madrigal-Santillan E, Gutierrez-Salinas J, et al. Protective effect of some vitamins against the toxic action of ethanol on liver regeneration induced by partial hepatectomy in rats. World journal of gastroenterology: WJG. 2008;14:899-907.
CrossRef - Greene A.K, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99-102.
CrossRef - Braulio V.B, Kouyoumdjian M, Zucoloto S, Figueiredo F, Borges D.R. Plasma-kallikrein clearance during liver regeneration after partial hepatectomy in the rat. Liver. 1998;18:371-377.
CrossRef - Ekinci S, Karnak I, Tanyel F.C.et alHepatic lobectomies in children: experience of a center in the light of changing management of malignant liver tumors. Pediatric surgery international. 2006;22:228-232.
CrossRef - Tannuri U, Tannuri C. A, Coelho C. M, Mello S .E, Santos dos A.S. Effect of the immunosuppressants on hepatocyte cells proliferation and apoptosis during liver regeneration after hepatectomy – molecular studies. Pediatric transplantation. 2008;12:73-79.
CrossRef - van den, Broek A .M, Olde Damink W. S , Dejong H. C. et al Liver failure after partial hepatic resection definition, pathophysiology, risk factors and treatment. Liver international .official journal of the International Association for the Study of the Liver. 2008;28:767-780.
- Detroz B, baker Sugar H. P,Knol J.A, Petrelli N, Hughes S .K. Causes of death in patients undergoing liver surgery. Cancer treatment and research. 1994;69:241-257.
CrossRef - Bolder U, Brune A, Schmidt S, Tacke J, Jauch K.W, Lohlein D. Preoperative assessment of mortality risk in hepatic resection by clinical variables: a multivariate analysis. Liver transplantation and surgery : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 1999;5:227-237.
CrossRef - Simmonds C, Primrose N.J, Colquitt L .J ,Garden J. O, Poston J. G, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999.
CrossRef - Morales-Gonzalez A. J, Gutierrez-Salinas J, Hernandez-Munoz R. Pharmacokinetics of the ethanol bioavailability in the regenerating rat liver induced by partial hepatectomy. Alcohol Clin Exp Res. 1998;22:1557-1563.
CrossRef - Starzl E. T, Fung J, Tzakis A. et al Baboon-to-human liver transplantation. Lancet. 1993;341:65-71.
CrossRef - Gebhardt R. Different proliferative activity in vitro of periportal and perivenous hepatocytes. Scandinavian journal of gastroenterology. Supplement. 1988;151:8-18.
CrossRef - Fausto N, Mead E .J. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989;60:4-13.
- Michalopoulos K G. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. The American journal of pathology. 2010;176:2-13.
CrossRef - Arias M. I, Wolkoff W.A, Boyer L. J. et al The Liver: Biology and Pathobiology: Wiley. 2011.
- Webber M. E, Fitzgerald J. M, Brown I. P, Bartlett H. M, Fausto N. Transforming growth factor-α expression during liver regeneration after partial hepatectomy and toxic injury, and potential interactions between transforming growth factor-α and hepatocyte growth factor. Hepatology. 1993;18:1422-1431.
CrossRef - Van Noorden J. C ,Vogels M. I, James J. Adaptive sex-dependent changes in the zonation of carbohydrate and lipid metabolism in rat liver lobules after partial hepatectomy. Hepatology. 1994;20:714-724.
CrossRef - Lai S. H, Chen W.J. Alterations of remnant liver carnitine palmitoyltransferase I activity and serum carnitine concentration after partial hepatectomy in rats. J Surg Res. 1995;59:754-758.
CrossRef - Kang K .A, Duncan A. J , Cattran C. D. et al Effect of oral contraceptives on the renin angiotensin system and renal function. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;280:807-813.
CrossRef - Aguilar-Delfin I, Lopez-Barrera F, Hernandez-Munoz R. Selective enhancement of lipid peroxidation in plasma membrane in two experimental models of liver regeneration: partial hepatectomy and acute CC14 administration. Hepatology. 1996;24:657-662.
CrossRef - Trejo-Solis C, Chagoya De Sanchez V, Aranda-Fraustro A, Sanchez-Sevilla L, Gomez-Ruiz C, Hernandez-Munoz R. Inhibitory effect of vitamin e administration on the progression of liver regeneration induced by partial hepatectomy in rats. Lab Invest. 2003;83:1669-1679.
CrossRef - Hammad H, Higashi T, Tateishi N, Hanatani M, Sakamoto Y. Lipid peroxidation in the liver of carcinogen-resistant rats. Biochimica et biophysica acta.1990;1045:99-106.
CrossRef - Arora V, Iversen L. P, Ebadi M. Manipulation of metallothionein expression in the regenerating rat liver using antisense oligonucleotides. Biochemical and biophysical research communications. 1998;246:711-718.
CrossRef - Kirimlioglu V, Kirimlioglu H, Yilmaz S. et al Effect of fish oil, olive oil, and vitamin E on liver pathology, cell proliferation, and antioxidant defense system in rats subjected to partial hepatectomy. Transplantation proceedings. 2006;38:564-567.
CrossRef








