I. Gede Widhiantara1, Pakajiraporn Arunngam2 and Ferbian Milas Siswanto1
1Department of Biology, Faculty of Health, Science and Technology, Dhyana Pura University, Badung, Indonesia.
2Department of Biomedical Chemistry, School of Science and Technology, Kwansei Gakuin University, Sanda, Japan.
Corresponding Author E-mail: ferbianms@undhirabali.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1473
Abstract
Caesalpinia bonducella F. (Leguminosae) is widely used medicinal plant which contains flavonoid, tannin, saponin, and a potent antioxidant activity. However, the antihyperglycemic effect of the seed of C. bonducella is remained to be evaluated. This study used 24 male Wistar albino rats that were induced for type 2 diabetes with streptozotocin (STZ) and nicotinamide (NA). The rats were divided into three groups: the distilled water-treated group (NC group), glibenclamide-treated group (10 mg/kg/d, oral; PC group), and C. bonducella seed extract-treated group (500 mg/kg/d, oral; T group). Blood glucose and plasma insulin measurements were done after 14 days of treatment. The results showed that the postprandial blood glucose (PPBG) level of both PC and T groups were decreased significantly (p < 0.01 for both), whereas in NC group, the PPBG level was rising (p < 0.01). Glibenclamide was found to be more effective to decrease the PPBG level than C. bonducella seed extract (p < 0.01). The post-test fasting insulin level of T group was higher than other groups (p < 0.05). In summary, our results suggest that ethanolic extract of C. bonducella seed possesses antidiabetic activity against experimentally-induced type 2 diabetes.
Keywords
C. Bonducella Seed; Diabetes; Insulin; Postprandial Glucose; Rat
Download this article as:| Copy the following to cite this article: Widhiantara I. G, Arunngam P, Siswanto F. M. Ethanolic Extract of Caesalpinia Bonducella f. Seed Ameliorates Diabetes Phenotype of Streptozotocin-Nicotinamide-Induced Type 2 Diabetes Rat. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Widhiantara I. G, Arunngam P, Siswanto F. M. Ethanolic Extract of Caesalpinia Bonducella f. Seed Ameliorates Diabetes Phenotype of Streptozotocin-Nicotinamide-Induced Type 2 Diabetes Rat. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=21182 |
Introduction
The aging process is characterized by the emergence of degenerative diseases due to the declining normal physiological function. Type 2 diabetes mellitus (T2DM) is the most common age-related degenerative disease, as the incidence and prevalence of this disease increase with age (Cowie et al., 2006). The T2DM is a metabolic disease characterized by elevated blood glucose levels resulting from defects in insulin secretion, insulin action, or both (Committee and Classification, 2010). The cause of this disease is multifactorial, with several risk factors including genetic, free radicals, hormonal, diet, unhealthy lifestyle and lack of physical activity (Wu et al., 2014).
Several modern medicines have been widely used to treat diabetes, including insulin, sulphonylurea, biguanide, and glitazones (Chaudhury et al., 2017). Most of the diabetes management are primarily intended to control blood glucose levels within normal range so that complications of the disease does not occur. The oral agent management of T2DM is the most common, and in some settings, they are the first option when drug treatment is required. Sulfonylurea class (such as glibenclamide) is the class of drug that has been used widely at large scale becoming the most common treatment since 2007 (Camelo Castillo et al., 2014). However, this synthetic drug can cause adverse side effects such as nausea, vomiting, hematological and dermatological reactions, obstructive jaundice, hyponatremia, weight gain, epigastric pain, lactic acidosis and liver disease (Sola et al., 2015). Nevertheless, the benefits of this drugs far outweigh the possible side effects, then this pharmacological therapy remains the first choice for treating diabetes. The diabetes management without side effect remains a challenge that needs to be further investigated.
Diabetes treatment using herbs has become a worldwide alternative, including in Indonesia. Various types of herbs have been identified to have excellent benefits as a treatment for many diseases. Caesalpinia bonducella F. seed is believed to have many health benefits, as it has been used in traditional Ayurvedic medicine for a long time. C. bonducella derived from Caesalpiniaceae family has also reported having a therapeutic effect as anthelmintics, antibacterial, antipyretic and analgesic, and lately known to treat diabetes (Chakrabarti et al., 2003; Kannur et al., 2006; Parameshwar et al., 2002). C. bonducella seed benefits in traditional medicine have been widely recognized empirically. However, the scientific evidence of this plant both to decrease blood glucose levels and increase fasting insulin levels is necessary.
Materials and Methods
Animals
Twenty-four 8-week-old experimentally naïve male Wistar albino rats with an average initial body weight of 190 ± 13 g (Animal House of Faculty of Veterinary Medicine, Udayana University, Denpasar, Indonesia) were used. Animals were housed under environmentally controlled condition (12 hr light/dark cycle; 22 – 24 °C), food and water were available ad libitum throughout the experiment. Animals were allowed to adjust to a new condition for seven days. The protocols used conformed to guidelines of animal studies and were approved by the committee on the ethics of animal experiments in Faculty of Veterinary Medicine, Udayana University.
Preparation of the Extract and Phytochemical Analysis
The C. bonducella seed extract was prepared according to the previously described method (Lilaram and Ahmed, 2013). In summary, the dried seed was coarsely powdered using a grinder and then extracted with 95% ethanol in the Soxhlet apparatus. The crude extract from the previous step was filtered by using Whatman paper and the solvent was dried by vacuum rotary evaporator under reduced pressure at a maximum temperature of 50 °C. The final fraction was stored at -20 °C until further use.
The crude ethanolic extracts of C. bonducella seed was tested for the presence of alkaloids, phenolic, flavonoids, steroids, tannins, saponins and triterpenoid glycosides were performed using previously described method (Iqbal et al., 2015). The qualitative results are expressed as (+) for the presence and (−) for the absence of phytochemicals. The total phenolic content was performed by the Folin–Ciocalteu colorimetric method expressed in µg GAE/mg (GAE= Gallic Acid Equivalent), and the total flavonoid content was examined using the aluminum colorimetric method expressed in µg CE/mg (CE= Catechin Equivalent) (Iqbal et al., 2015). The free radical-scavenging activity of the C. bonducella ethanolic seed extract was measured in terms of hydrogen donating or radical-scavenging ability using the stable radical 1-1-diphenyl 2-picryl hydrazyl (DPPH) as previously described (BLOIS, 1958).
Streptozotocin-nicotinamide-induced type 2 diabetes
Type 2 diabetes was induced by intraperitoneal injection of 100 mg/kg BW nicotinamide (Sigma Chemical Co, St. Louis, MO, USA) dissolved in 0.9% normal saline followed after 15 minutes by freshly prepared streptozotocin (Sigma Chemical Co, St. Louis, MO, USA) of 45 mg/kg BW dissolved in 0.1 M citrate-phosphate buffer (pH = 4.5). Rats were considered diabetic when their postprandial plasma glucose concentration was greater than 150 mg/dL (Abdellatief et al., 2017).
Experimental Design
After 48 h of diabetes induction, diabetic rats were randomly divided into three groups, containing eight rats in each group as follows: negative control (NC) group that was induced with streptozotocin (STZ)–nicotinamide and treated with oral distilled water, positive control (PC) group that was induced with STZ–nicotinamide and treated with oral Glibenclamide (Sigma Chemical Co, St. Louis, MO, USA) of 10 mg/kg/d, and the treatment (T) group that was induced with STZ–nicotinamide and treated with C. bonducella seed ethanolic extract of 500 mg/kg/d. The rats were orally administered once a day with Glibenclamide and C. bonducella seed ethanolic extract by dissolving in distilled water by intragastric tube. The rats were treated daily for a period of 14 days.
Biochemical Analysis
Plasma glucose concentrations were determined by colorimetric methods after an enzymatic reaction with peroxidase (Randox, CO. Antrim, UK). The plasma insulin levels were determined using a rat insulin ELISA kit (Bioassay Technology Laboratory Shanghai, China) according to the manufacturer’s instructions. Blood was sampled for postprandial glucose measurements at 2 hours after the meal and for fasting insulin measurements at 8 hours after the meal.
Statistical Analysis
Data are presented as group mean ± standard deviation (SD). One way ANOVA with Least Significance Difference (LSD) post hoc test was performed to detect the significant differences between groups. The paired t-test was performed to detect the significant differences between pretest and posttest within the same group. Experimental differences were considered statistically significant if p < 0.05.
Results and Discussion
Phytochemical Screening of C. Bonducella Seed Ethanolic Extract
Phytochemical analysis and antioxidant activity of C. bonducella were carried out in this research. The preliminary phytochemical screening revealed the presence of alkaloids, phenolics, flavonoids, steroids, tannins, and saponins. The triterpenoid glycosides were not detected in the C. bonducella ethanol extract. The total amount of phenolic content present in C. bonducella seed ethanolic extract was 26.99 µg GAE/mg, and the total flavonoid content was 16.28 µg CE/mg. The DPPH radical-scavenging activity revealed that the IC50 value of C. bonducella seed ethanolic extract was 78.41 μg/ml (Table).
Postprandial Blood Glucose (PPBD) Level
Postprandial hyperglycemia is the most phenotype of type 2 diabetes and a critical therapeutic target for optimizing glycemic control. Our study showed that prior to the treatment, the average of PPBG level between groups was not different (p > 0.05). In contrast, after 14 days of treatment, both the Glibenclamide (PC) group and the Caesalpinia bonducella seed ethanolic extract (T) group had a lower PPBG level compared to distilled water-treated group (p < 0.01 for both). The blood glucose of NC group was elevated by 14 days STZ-nicotinamide treatment by 19.5% (p < 0.01), while both Glibenclamide and ethanol extract of Caesalpinia bonducella seed decreased postprandial blood glucose level of STZ-nicotinamide-induced diabetes rats by 49.3 and 20.3% respectively (p < 0.01 for both) (Fig 1).
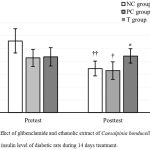 |
Figure 1: Effect of glibenclamide and ethanolic extract of Caesalpinia bonducella F. seed on the postprandial blood glucose (PPBG) level of diabetic rats during 14 days treatment.
|
All the values are expressed as the mean ± SD. **indicates the significant difference when compared to the NC group of the same measurement time (p < 0.01; one way ANOVA with LSD post hoc test). ††indicates the significant difference when compared to pretest counterparts of the same group (p < 0.01; paired t-test).
It is supported by previous research which proved that the hydroalcoholic extract of Caesalpinia bonducella seed decreased blood glucose levels significantly in normal mice, hyperglycemia mice and alloxan-induced diabetic mice (Nadaf, 2017). Another study using a hydroethanolic extract of Caesalpinia bonducella seed showed that it decreased fasting blood glucose levels (Ghosh et al., 2012). The antidiabetic activity of the Caesalpinia bonducella seed extract may be generated from the chemically active compounds content such as alkaloids, flavonoids, tannins, phenols, and saponins. Phytochemical analysis showed that ethanol extract of Caesalpinia bonducella seed containing these substances (Table 1).
Table 1: Phytochemical screening and DPPH radical-scavenging activities of Caesalpinia bonducella ethanolic seed extract.
| Screening | Test | Presence or Amount |
| Alkaloids | Dragendorff’s Test | (+) |
| Phenolic | Kokate’s method | (+) |
| Flavonoids | Kokate’s method | (+) |
| Steroids | Salkowski test | (+) |
| Tannins | Kokate’s method | (+) |
| Saponins | Kokate’s method | (+) |
| Triterpenoid | Salkowski test | (-) |
| Glycosides | Keller-Killiani test | (+) |
| Total Phenol | Folin–Ciocalteu colorimetric method | 26.99 µg GAE/mg |
| Total Flavonoid | Aluminum colorimetric method | 16.28 µg CE/mg |
| Antioxidant activity | DPPH radical-scavenging test | 78.41 μg/ml |
(+) = presence, (-) = absence, GAE = Gallic acid equivalent, CE = Catechin Equivalent
Several pathologies, including type 2 diabetes, are related at some point to a pancreatic cell deregulation. Beta cell apoptosis is a common feature of both type 1 and type 2 diabetes, although the mechanism activating it in each case may be different. Therefore, the antiapoptotic activity of the Caesalpinia bonducella might be involved in beneficial effects against diabetes. Flavonoids are known as a natural antioxidant that protects beta cells from oxidative damage, as well as regenerate damaged-beta cells (Pinent et al., 2008). Several experiments proved that flavonoids can exert antiapoptotic effects in pancreatic beta cells (Han, 2003). Other active compounds contained in Caesalpinia bonducella, saponin, can inhibit the elevation of blood glucose by inhibiting the activity of the α-glucosidase enzyme. Saponin inhibits the absorption of small nutrient molecules such as glucose by inhibiting glucose transporter system (Li et al., 2015). Saponin was reported to inhibit the transport of glucose in the intestine by inhibiting the sodium glucose co-transporter-1 (S-GLUT-1) (Li et al., 2018). Caesalpinia bonducella also contains tannin has also been proven can be used to decrease blood glucose levels by stimulating glucose and fat metabolism. Tannin also has a hypoglycemic activity by inducing phosphorylation of the insulin receptor and Akt, as well as translocation of glucose transporter 4 (GLUT 4) (Liu et al., 2005). Previous research found that the Caesalpinia bonducella seed extract reduces the inflammatory cytokines which are causes insulin resistance in diabetes patient (Shukla et al., 2011).
Fasting Insulin Level
Decreasing fasting insulin level is one of the risk factors for diabetes. The mean fasting insulin level before treatment was not different among groups (p > 0.05). Inversely, after the treatment for 14 days, the Caesalpinia bonducella seed ethanolic extract (T) group had the highest fasting insulin level compare to both NC and PC groups (p < 0.05 for both). The fasting insulin level of PC group was comparable to the NC group (p > 0.05). By comparing pretest and posttest value within the group, it was noticed that the fasting insulin level of both NC group and PC group were declined by 38.4 and 23.4% respectively (p < 0.05 for both), after 14 days STZ-nicotinamide treatment with or without Glibenclamide. In contrast, the treatment of ethanol extract of Caesalpinia bonducella seed in T group prevented the decreasing fasting insulin level caused by 14 days of STZ-nicotinamide (p > 0.05) (Fig 2).
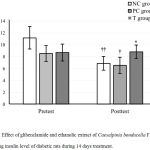 |
Figure 2: Effect of glibenclamide and ethanolic extract of Caesalpinia bonducella F. seed on the fasting insulin level of diabetic rats during 14 days treatment.
|
All the values are expressed as the mean ± SD. **indicates the significant difference when compared to the NC group of the same measurement time (p < 0.01; one way ANOVA with LSD post hoc test). ††indicates the significant difference when compared to pretest counterparts of the same group (p < 0.01; paired t-test).
This research is also supported by the previous study proved that streptozotocin-induced diabetic rats showed an improvement in the activity of hexokinase, glucose-6-phosphate dehydrogenase and glucose-6-phosphatase in the liver which are the biomarker controlled by insulin (Ghosh et al., 2012). Polyphenolic bioactive compounds of Caesalpinia bonducella seed can protect pancreatic beta cells from cytokine-, reactive oxygen species- (ROS-), and glucose-induced toxicity under chronic hyperglycemia conditions (Oh, 2015). They are also able to increase pancreatic beta cell mass (Ogata et al., 2004), as well as improve the insulin production and secretion of the pancreatic beta cell (Chang et al., 2013). Flavonoids improve the insulin granule exocytosis of pancreatic beta cells by modulating Ca2+ metabolism, improve ATP generation in β-cells and provide an increase in the transcriptional activation of insulin-mediated by cyclic adenosine monophosphate (cAMP) signaling (Dias Soares et al., 2017).
Interestingly, the Caesalpinia bonducella seed extract protected the rats from decreasing fasting insulin level caused by STZ-nicotinamide treatment, while the treatment of Glibenclamide failed to do so (Fig 2). This result differs from the fact that as a standard antidiabetic drug, Glibenclamide supposes to increases second-phase insulin secretion at a stimulating blood glucose level (Proks et al., 2002). In spite of decreasing fasting insulin levels by Glibenclamide treatment (Fig 2), the PPBG level was still decreasing (Fig 1). This might be due to the short half-life of sulfonylureas effect on insulin levels, or the sulfonylureas increase the sensitivity of pancreatic beta cells to glucose by decreasing the glucagon (Pfeifer et al., n.d.).
In general, hyperglycemia lead to the increasing number of free radicals through auto-oxidation of glucose, further leading to oxidative stress and oxidative damage of pancreatic beta cells (Ihara et al., 1999). As we previously proved that Caesalpinia bonducella seed extract possesses a strong antioxidant activity (Table 2), it is also possible that Caesalpinia bonducella seed extract ameliorates diabetes phenotype by preventing the oxidative stress and oxidative damage in pancreatic beta cells thus decreases PPBG level and prevent the decreasing fasting insulin level. In summary, our results suggest that ethanolic extract of Caesalpinia bonducella seed possesses antidiabetic activity against experimentally-induced type 2 diabetes.
Statement of Informed Consent
For this type of study, formal consent is not required.
Conflict of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethics of Animal Experimentation
All procedures performed in studies involving rats as animal models were conducted in accordance with guideline on the welfare of experimental animals and with the approval of the Ethics Committee on the use of animals of Faculty of Veterinary Medicine, Udayana University.
References
- Abdellatief S.A, Beheiry R.R, El-Mandrawy S.A.M. Peppermint essential oil alleviates hyperglycemia caused by streptozotocin- nicotinamide-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017;95:990–999. https://doi.org/10.1016/j.biopha.2017.09.020.
CrossRef - Blois M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958;181:1199–1200. https://doi.org/10.1038/1811199a0.
CrossRef - Camelo Castillo W, Boggess K, Stürmer T, Brookhart M.A, Benjamin D.K, Jonsson Funk M. Trends in Glyburide Compared With Insulin Use for Gestational Diabetes Treatment in the United States, 2000–2011. Obstet. Gynecol. 2014;123:1177–1184. https://doi.org/10.1097/AOG.0000000000000285.
CrossRef - Chakrabarti S, Biswas T.K, Rokeya B, Ali L, Mosihuzzaman M, Nahar N, Khan A.K.A, Mukherjee B. Advanced studies on the hypoglycemic effect of Caesalpinia bonducella F. in type 1 and 2 diabetes in Long Evans rats. J. Ethnopharmacol. 2003;84:41–6.
CrossRef - Chang C.L.-T, Liu H.-Y, Kuo T.-F, Hsu Y.-J, Shen M.-Y, Pan C.-Y, Yang W.-C. 2013. Antidiabetic Effect and Mode of Action of Cytopiloyne. Evidence-Based Complement. Altern. Med. 2013;1–13. https://doi.org/10.1155/2013/685642
CrossRef - Chaudhury A, Duvoor C, Reddy Dendi V.S, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat N.S, Montales M.T, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham C.K, Lohani G.P, Mirza W. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. (Lausanne). 2017;8. https://doi.org/10.3389/fendo.2017.00006
CrossRef - Committee P.P, Classification A. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33:Suppl 1, S11-61. https://doi.org/10.2337/dc10-S011
CrossRef - Cowie C.C, Rust K.F, Byrd-Holt D.D, Eberhardt M.S, Flegal K.M, Engelgau M.M, Saydah S.H, Williams D.E, Geiss L.S, Gregg E.W. Prevalence of Diabetes and Impaired Fasting Glucose in Adults in the U.S. Population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–1268. https://doi.org/10.2337/dc06-0062.
CrossRef - Dias Soares J, Pereira Leal A.B, Silva J, Almeida J.G.S, de Oliveira H. Influence of flavonoids on mechanism of modulation of insulin secretion. Pharmacogn. Mag. 2017;13:639. https://doi.org/10.4103/pm.pm_87_17.
CrossRef - Ghosh D, Chatterjee K, De D, Jana K, Ali K, Bera T. Antihyperglycemic and antioxidative effects of the hydro-methanolic extract of the seeds of Caesalpinia bonduc on streptozotocin-induced diabetes in male albino rats. Pharmacognosy Res. 2012;4:57. https://doi.org/10.4103/0974-8490.91044
CrossRef - Han M.-K. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp. Mol. Med. 2003;35:136–9. https://doi.org/10.1038/emm.2003.19
CrossRef - Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–932. https://doi.org/10.2337/diabetes.48.4.927
CrossRef - Iqbal E, Salim K.A, Lim L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. – Sci. 2015;27:224–232. https://doi.org/10.1016/j.jksus.2015.02.003
CrossRef - Kannur D.M, Hukkeri V.I, Akki K.S. Antidiabetic activity of Caesalpinia bonducella seed extracts in rats. Fitoterapia. 2006;77:546–549. https://doi.org/10.1016/j.fitote.2006.06.013
CrossRef - Li B, Terazono Y, Hirasaki N, Tatemichi Y, Kinoshita E, Obata A, Matsui T. Inhibition of Glucose Transport by Tomatoside A, a Tomato Seed Steroidal Saponin, through the Suppression of GLUT2 Expression in Caco-2 Cells. J. Agric. Food Chem. 2018;66:1428–1434. https://doi.org/10.1021/acs.jafc.7b06078
CrossRef - Li Y, Zhang T, Cui J, Jia N, Wu Y, Xi M, Wen A. Chikusetsu saponin IVa regulates glucose uptake and fatty acid oxidation: implications in antihyperglycemic and hypolipidemic effects. J. Pharm. Pharmacol. 2015;67:997–1007. https://doi.org/10.1111/jphp.12392
CrossRef - Lilaram Ahmed R.N. Effect of ethanolic seed extract of Caesalpinia bonducella pregnant female albino rats. Asian Pacific J. Reprod. 2013;2:85–89. https://doi.org/10.1016/S2305-0500(13)60124-4
CrossRef - Liu X, Kim J, Li Y, Li J, Liu F, Chen X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J. Nutr. 2005;135:165–71.
CrossRef - Nadaf R. A study of hypoglycemic effect of Caesalpinia bonduc extract on alloxan induced diabetic albino rats. Int. J. Basic Clin. Pharmacol. 2017;6:2153. https://doi.org/10.18203/2319-2003.ijbcp20173735.
CrossRef - Ogata, T., Li, L., Yamada, S., Yamamoto, Y., Tanaka, Y., Takei, I., Umezawa, K., Kojima, I., 2004. Promotion of beta-cell differentiation by conophylline in fetal and neonatal rat pancreas. Diabetes 53, 2596–602.
CrossRef - Oh, Y.S., 2015. Plant-Derived Compounds Targeting Pancreatic Beta Cells for the Treatment of Diabetes. Evidence-Based Complement. Altern. Med. 2015, 1–12. https://doi.org/10.1155/2015/629863
CrossRef - Parameshwar, S., Srinivasan, K.K., Rao, C.M., 2002. Oral Antidiabetic Activities of Different Extracts of Caesalpinia bonducella Seed Kernels. Pharm. Biol. 40, 590–595. https://doi.org/10.1076/phbi.40.8.590.14656
CrossRef - Pfeifer, M.A., Halter, J.B., Judzewitsch, R.G., Beard, J.C., Best, J.D., Ward, W.K., Porte, D., n.d. Acute and chronic effects of sulfonylurea drugs on pancreatic islet function in man. Diabetes Care 7 Suppl 1, 25–34.
- Pinent, M., Castell, A., Baiges, I., Montagut, G., Arola, L., Ardévol, A., 2008. Bioactivity of Flavonoids on Insulin-Secreting Cells. Compr. Rev. Food Sci. Food Saf. 7, 299–308. https://doi.org/10.1111/j.1541-4337.2008.00048.x.
CrossRef - Proks, P., Reimann, F., Green, N., Gribble, F., Ashcroft, F., 2002. Sulfonylurea Stimulation of Insulin Secretion. Diabetes 51, S368–S376. https://doi.org/10.2337/diabetes.51.2007.S368
CrossRef - Shukla, S., Mehta, A., Mehta, P., BAJPAI V.K, 2011. Evaluation of comparative antidiabetic effects of ethanolic extracts of Caesalpinia bouncucella and Stevia rebaudianain normal and alloxan-induced experimental rats. Rom. Biotechnol. Lett. 16, 6187–6199.
- Sola, D., Rossi, L., Schianca, G.P.C., Maffioli, P., Bigliocca, M., Mella, R., Corlianò, F., Fra, G.P., Bartoli, E., Derosa, G., 2015. State of the art paper Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 4, 840–848. https://doi.org/10.5114/aoms.2015.53304.
CrossRef - Wu, Y., Ding, Y., Tanaka, Y., Zhang, W., 2014. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 11, 1185–1200. https://doi.org/10.7150/ijms.10001.
CrossRef








