Laila A. Baqasi1,2, Huda A. Qari1,2, Nihal Al-Nahhas3, Reem H. Badr3, Wafaa K. Taia3, Rehab A. El-Dakak3 and Ibrahim A. Hassan2,3
1Department of Biology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
2Centre of Excellence in Environmental Studies (CEES), King Abdulaziz University, 21589 Jeddah, Saudi Arabia.
3Department of Botany, Faculty of Science, Alexandria University, 21589 El Shatby, Alexandria, Egypt.
Corresponding Author E-mail: ihassan_eg@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1450
Abstract
Growth, yield, protein content, net photosynthetic rates, stomatal conductance and amino acid profiles were determined in wheat (Triticum aestivum L) plants in response to 50 ppb O3 during the growing season. This concentration is similar to the concentrations of O3 in ambient air. O3 decreased photosynthetic rates (24%) and stomatal conductance (25%), which were reflected in lower growth and yield in terms of number of grains and 100 grain weight. Scanning electron microscopy showed a collapse in the epidermal cells adjacent to stomata that led to stomatal closure and consequently reductions in stomatal conductance. The significance of O3-induced impairment of growth, yield and alteration in amino acid contents are discussed. To the best of our knowledge, this is the first study reporting impact of ozone on protein content, amino acids and yield of wheat in Saudi Arabia.
Keywords
Growth; Ozone; Physiology; Wheat; Yield
Download this article as:| Copy the following to cite this article: Baqasi L. A, Qari H. A, Al-Nahhas N, Badr R. H, Taia W. K, El-Dakak R. A. Hassan I. A. Effects of Low Concentrations of Ozone (O3) on Metabolic and Physiological Attributes in Wheat (Triticum Aestivum L.) Plants. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Baqasi L. A, Qari H. A, Al-Nahhas N, Badr R. H, Taia W. K, El-Dakak R. A. Hassan I. A. Effects of Low Concentrations of Ozone (O3) on Metabolic and Physiological Attributes in Wheat (Triticum Aestivum L.) Plants. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20789 |
Introduction
Jeddah suffers from serious air pollution problems due to emissions from different sources such as industrial activities, oil refiners and desalination plants. There is very poor legislation regarding emissions from old cars in streets as well from factories, which cause environmental hazards. Levels of heavy metals, gaseous air pollutants and particulate emissions far exceed internationally acceptable standards.1-5
Ozone concentrations in Jeddah were recorded to be between 40 – 70 ppb6.6-8 These concentrations are high enough to affect many plant processes, such as photosynthesis, transpiration, nutrient uptake, senescence, changes in metabolic, biochemical and physiological processes, resulting in significant effects on crop growth and yield in Europe and the USA.9
Wheat is a nutritious and versatile crop, it has been known to be sensitive to O3 Worldwide.9-11 Chronic exposure to O3 alters physiological processes leading to reduction in growth and yield of economic crops.1,8 However, very little is known about its sensitivity in the Middle East. The information on impact of O3 on plants in Saudi Arabia are extremely scanty and fragmented,7,8 although high concentrations of O3 were recorded.6
Amino acids are key elements in the balance between catabolism and biosynthesis of various compounds. Amino acids represent a source of energy, some amino acids are directly linked to antioxidant biosynthesis12
Wheat plant was chosen due to its economic importance, nutritional value and its compact size and rapid growth.
The aim of the present study was to clear our understanding related to molecular response of wheat (Triticum aestivum L) plants to chronic low levels of O3.
Materials and Methods
Plant Samples, Growth Conditions and Experimental Design
Grains of wheat were washed with distilled water before soaking and were soaked in a continuously aerated distilled water in room temperature for 24 h in darkness to remove any pesticides. Then, 10-15 seeds were sown plastic pots (18 cm height x 16 cm diameter) filled with multipurpose compost at about 1cm from the surface. Each pot was watered with tap water until the first leaf expanded. Then plants were transferred to four closed fumigation chambers; 2 chambers were receiving 50 ppb O3 for 8 h d-1 (9:00 am – 5:00 pm) during Jan 2017 – March 2017 (Fig. 1).
 |
Figure 1: Fumigation chambers: (a) Filter Air (FA) chamber. (b) Ozone.
|
Non-destructive harvest
Visible injury symptoms
Foliar injury symptoms were assessed carefully, every week of each treatment by counting the number of injured leaves and estimating the percentage of each leaf’s area showing injury (on a score of 0 ‘no injury’ to 5 ‘100% injury’).1,3,8
Gas Exchange Measurements and Chlorophyll Content
Net CO2 photosynthetic rate (A) and stomatal conductance (gs) were measured using a portable LICOR (IRGA-LICOR-6400, Lincoln, NE, USA). Measurements were carried out the first true foliage leaf on weekly basis.
Destructive Harvest
Biomass and grain yield
As describe in our previous paper3, plants were selected from each treatment and divided into main organs every week throughout the entire course of the experiment for dry matter portioning.
Measurement of Photosynthetic Pigments
0.1g fresh weight of leaves was homogenized with pestle and mortar in in 10 ml acetone. The homogenate was centrifuge for 5 min at 9000 rpm in a cold room (5 0C). The supernatant was used to determine the Photosynthetic pigments (chlorophyll a and chlorophyll b) using UV-spectrophotometer (SHIMADZU 2500).11
Amino Acids Profile
0.1 mg sample was hydrolyzed with 6.0 M HCl at 110 0C for 24 h, then samples were filtered and HCl was evaporated. Hydrolysates were dissolved in citrate-Na citrate buffer (0.1 M, pH 2.2).13 Levels of amino acids were measured by GC-MS (Shimadzu 2010, Japan).
Scanning Electron Microscopy
Leaf tissue was collected at the end of the experiment by taking leaf punches from the center half way between the leaf edge and midvein from the same leaves that were sampled for gas exchange. The leaf sections were fixed in 4% formaldehyde, 50mM PIPES pH 6.8 by vacuum infiltration for 20 min. The tissues were dehydrated and then critical point dried in liquid CO2. Surface images were taken on a JEOL 6060LV scanning electron microscope (JEOL USA, Inc., Peabody, MA, USA).14
Data Analysis
Data were log-transferred prior to analysis to ensure that they were normally distributed. One-way analysis of variance (ANOVA) test was applied to the log-transoformed data, differences between means were tested by Tuckey Test using SPSS statistical package, USA (Package 4, USA).
Results and Discussion
Visible injury symptoms appeared in the form of chlorotic stippling on leaves. Ozone was found to increase number of injured leaves and degree of injury on leaves by 1.2- and 4.5-fold, respectively (Table 1).
Table 1: Effect of O3 on foliar injury symptoms of Wheat leaves
| Parameter | Filtered air (FA) | O3 |
| Number of injured leaves | 18a | 41d |
| Degree of injury | 0.16a | 0.88c |
(Figures not followed by the same letter in each row are significantly different from each other at P ≤ 0.05, n=40).
The effect of O3 on net photosynthetic rates (A) started to appear on the third week of exposure (Fig. 2).
O3 caused significant reductions in A in plants by 17.2, 15.7, 24.8, 20.8 and 20.3% in the weeks 3, 4, 5, 6, 7, and 8 respectively.
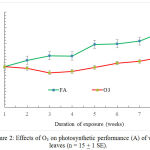 |
Figure 2: Effects of O3 on photosynthetic performance (A) of wheat leaves (n = 15 + 1 SE).
|
O3 caused decreased in stomatal conductance (gs) by 13.9, 12.1, 20.5, 22.7, 22.2 and 23.4% on the third, fourth, fifth, sixth, seventh and eighth week of exposure, respectively (Fig.3)
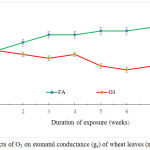 |
Figure 3: Effects of O3 on stomatal conductance (gs) of wheat leaves (n = 15 + 1 SE).
|
Chl b showed no significant response to or O3, while Chl a was decreased by 50% when compared to plants grown in filtered air (P ˂0.01) (Fig. 4).
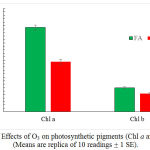 |
Figure 4: Effects of O3 on photosynthetic pigments (Chl a and Chl b). (Means are replica of 10 readings + 1 SE).
|
O3 caused reductions in number of grains/ear. Number of grains/ears, dry mass of grains by 18.6, 17,9% and 2-fold, respectively. A harvest index as indicated by 1000 grain weight, was decreased by 38.3% (Table 2). Moreover, O3 caused decreases in protein content by 29.7% (Tab. 2).
Table 2: Effects of O3 on yield parameters and protein content
| Parameter | FA | O3 |
| No. of ears/plant | 3.76* | 3.06** |
| No. of grains/ear | 44.2* | 36.30** |
| 1000 grain weight (g) | 58.5* | 36.10** |
| Dry mass of grains (g) | 4.22* | 2.16** |
| Protein content (%) | 14.23c | 10.01a |
Plants were harvested 70 d after exposure to O3
Proline was the only amino acid (AA) that showed increase in its level due to exposure to O3 by 72.7%. On the other hand, O3 caused decreases in levels of other amino acid analyzed in this study (table.3). The mean contents of phenylalanine (4.78%), isoleucine (3.53%), leucine (6.16%), valine (4.09%), lysine (2.80%) and threonine (2.89%) were found lower in plants fumigated with 50 ppb O3 when compared with those grown in FA (Table. 3).
Table 3: Response of amino acids profile of Wheat to O3.
| Treatment
AA (g 100 g-1 protein) |
FA | O3 |
| Lysine | 3.17b + 0.22 | 2.79a + 0.19 |
| Glycine | 8.01b + 0.99 | 6.97a + 0.24 |
| Threonine | 2.88c + 0.12 | 2.11a + 0.09 |
| Valine | 3.77c + 0.41 | 2.78a + 0.22 |
| Methionine
|
1.12b + 0.11 | 0.78a + 0.21 |
| Phenylalanine | 4.89 + 1.01 | 4.12 + 0.99 |
| Tyrosine | 1.87c + 0.34 | 0.99a + 0.47 |
| Alanine | 2.88b + 0.67 | 2.22a + 0.11 |
| Arginine | 3.79c + .33 | 2.45a + 0.41 |
| Glutamine | 6.41c + 0.56 | 5.11a + 0.43 |
| Proline | 8.06a + 0.87 | 13.92c + 1.27 |
The scanning electron micrographs demonstrate that leaf surfaces of intact leaves of wheat plants were greatly modified by exposure to 50 ppb O3. (Fig. 5).
In O3-treated plants, there was a considerable loss of turgor and collapse of epidermal cells adjacent to stomata.
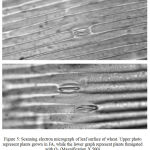 |
Figure 5: Scanning electron micrograph of leaf surface of wheat.
|
Upper photo represent plants grown in FA, while the lower graph represent plants fumigated with O3 (Magnification X 500).
The concentrations of O3 used in the present study is similar to ambient O3 levels recorded in Jeddah.1 This concentration is known to alter physiology of plants and reduce yield of economic crops worldwide. The extent of economic losses of O3 to crops in this country has been poorly evaluated.3,9
The present investigation showed clearly that chronic exposure to low doses of O3 (50 ppb 8hd-1 for 70 days) had negative effects on growth, biomass accumulation, and yield. Analyses of metabolic processes were performed in order to perceive O3 toxicity at early stages.
The reductions in photosynthetic rates (A) are in agreement with our previous work.3 Moreover, is in agreement with the general view reported in literature.14-17 Stomatal conductance (gs) was also reduced due to exposure to either ambient air or 50 ppb O3. The reduction in gs could lead to reduction in absorption of CO2 and reduction in photosynthesis and finally in the growth and yield.3,18
The stomatal closure in wheat can be also attributed to increased concentrations of CO2 in intercellular spaces (Ci) especially since the inhibition in photosynthetic rates was followed by inhibition in stomatal conductance. Therefore, the results of present study (supported with SEM micrographs) indicate that the changes in stomatal conductance in response to O3 could be portioned into a direct effect of O3 on the epidermal tissue and indirect effect via changes in photosynthetic process.
Growth and yield parameters and protein content showed inhibition in response to O3 and this is in agreement with the results of other resercers.19-21. The Changes in dry matter partitioning has an important implication under arid climates, where soil moisture deficit is a major factor limiting crop yield, and where O3-induced diversion of resources to the shoot at the expanse of the root could increase the sensitivity of plants to drought and exacerbate nutritional disorders. Nevertheless, the results of the present study were consistent in the major findings and this gives further confidence to the interpretation.
The effect of O3 on protein and amino acid contents is very complicated, and needs further investigations using more crops. However, O3 causes reductions in AA and protein which is reflected in poor growth and lower yield.
Conclusions
The present study indicated that O3 stress caused a number of morphological and physiological changes in the wheat plants, including decreased shoot length, root fresh and dry weight and shoot fresh and dry weight and 1000-grain weight.
Exposure to chronic low doses of O3, negatively affected all the response variables studied, indicating, its detrimental effects on vegetative growth, biochemical activities and development. This study highlighted the gap of knowledge about the changes in assimilate partitioning. This warrants further investigation.
Acknowledgements
This work was supported by a Grant from King Abdul Aziz City for Science & Technology (KACST). Grant #1498-37-TA.
References
- Hassan IA, Haiba NS, Badr RH, Basahi JM, Almellebi T, Ismail IM, Taia WK. Effects of ambient ozone on reactive oxygen species and antioxidant metabolites in leaves of pea (Pisum sativum) Plants. Pak. J. Bot. 2017;49(1):47-55.
- Hassan I.A, Cotrozzi L, Haiba N S, Basahi, J, Ismail I, Almeelbi T, Hammam E. Trace elements in the fruits of date palm (Phoenix dactylifera) in Jeddah City, Saudi Arabia. Agrochimica. 2017;61(1):75-93.
- Hassan IA. Physiological and biochemical response of potato (Solanum tuberosum cv. Kara) to O3 and antioxidant chemicals: possible roles of antioxidant enzymes. Ann. App. Bio. 2006;146:134 – 142.
CrossRef - Ismail IM, Basahi JM, Hassan IA. Gas exchange and chlorophyll fluorescence of pea (Pisum sativum) plants in response to ambient ozone at a rural site in Egypt. Sci. of the Tot. Environ. 2014;497–498:585–593.
- Hassan IA, Basahi JM. Assessing roadside conditions and vehicular missions using roadside lettuce plants. Polish J. Environ. Stud. 2013;22(2):75 – 81.
- Hassan IA, Basahi JM, Ismail IM, Habeebullah TM. Spatial distribution and temporal variation in ambient ozone and its associated NOx in the atmosphere of Jeddah City, Saudi Arabia. Aerosol and Air Qual. Res. 2013;13(6):1712–1722.
CrossRef - Basahi JM, Ismail IM, Haiba NS, Hassan IA, Lorenzini G. Assessing ambient ozone injury in olive (Olea europaea) plants by using the antioxidant ethylenediurea (EDU) in Saudi Arabia. Environ. Monit. Assess. 2016;188(6): 371.
CrossRef - Baqasi LA, Qari HA, Hassan IA. Physiological and Biochemical response of winter wheat (Triticum aestivum) to ambient O3 and the antiozonant chemical ethylenediurea (EDU) in Jeddah, Saudi Arabia. Biomedic. Pharmacol. J. 11(1): 45 – 51.
- Feng Z, Kobayashi K, Ainsworth A.; impact of elevated O3 concentration on growth, physiology and yield of wheat (Triticum aestivum): a meta-analysis. Global Change Bio. 2008;14:2696 – 2708.
- Sarwal A. Effect of ozone on some attributes in wheat plants. MSC thesis, King Abdulaziz University, Jeddah, Saudi Arabia; 134 Pp.
- Hatata M, Badr R, Ibrahim M, Hassan IA. Respective and interactive effects of O3, CO2 an drought stress on physiology and antioxidative ability and yield of wheat plants. Current World Environ. 2013;8(3):421 – 429.
CrossRef - Dumont E, Saari SK, Keinänen M, Cohen D, Ningre N, Baldet P, Gibon Y, Jolivet Y, Elina Oksanen E, Le Thiec D. Ozone affects ascorbate and glutathione biosynthesis as well as amino acid contents in three Euramerican poplar genotypes. Tree Physiology. 2014;34:253 –266.
CrossRef - Akgül R, Kizilkaya B, Akgül F, Romance H. Crude protein values and amino acid profiles of some red algae collected from çanakkal, Turkey. J. Geo-Marine Sci. 2015;44(4):1 – 4.
- Hassan IA, Ashmore MR, Bell JNB. Effect of O3 on the stomatal behaviour of Egyptian of radish (Raphanus sativus cv. Baladey) and turnip (Brassica rapa L. cv. Sultani). New Phytol. 1994;128:236-245.
CrossRef - Liu X, Sui L, Huang Y, Geng C, Yin B. Physiological and visible injury responses in different growth stages of winter wheat to ozone stress and the protection of spermidine. Pollut. Res. 2015;6(4):596–604.
CrossRef - Feng Z, Pang J, Kobayashi K, Zhu J, Ort DR. Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Global Change Bio. 2011;17(1):580–591.
CrossRef - Feng Z, Wang L, Pleijel H, Zhu J, Kobayashi K. Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Tot. Environ. 2016;572:404–411.
CrossRef - Pellegrini E, Francini A, Lorenzini G, Nali C. Ecophysiological and antioxidant traits of Salvia officinalis under ozone stress. Sci. Pollut. Res. 2015;22:13083–13093.
CrossRef - Wang J, Zeng Q, Zhu J, Chen C, Liu G, Tang H. Apoplastic antioxidant enzyme responses to chronic free-air ozone exposure in two different ozone-sensitive wheat cultivars. Pollut. 2014;148(2):390–39.
CrossRef - Mishra K, Rai R, Agrawal SB. Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian J. Biochem. Biophys. 2013;50(2):139–49.
- Cho K, Tiwari S, Agrawal SB, Torres NL, Agrawal A, Sarkar J, et al. Tropospheric ozone and plants: absorption, responses, and consequences. Rev. Environ. Contam. Toxicol. 2011;212:111–116.
CrossRef







