Hamid A. Saleh1 , Gamal S. Abd El-Aziz1
, Gamal S. Abd El-Aziz1 , Hehsam N. Mustafa1
, Hehsam N. Mustafa1 , Magdy El-Fark1, Jawad Mansour Tashkandi6, Anas Hassan Alzahrani1, Ahmed Mal3, Magda AboRass4 and Abdel Halim Deifalla5
, Magdy El-Fark1, Jawad Mansour Tashkandi6, Anas Hassan Alzahrani1, Ahmed Mal3, Magda AboRass4 and Abdel Halim Deifalla5
1Department of Anatomy, Faculty of Medicine, King Abdulaziz University, Jeddah, KSA.
2Department of Anatomy, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
3Department of Marine Biology, Faculty of Marine Sciences, King Abdulaziz University, Jeddah, KSA.
4Department of Biological Sciences, Faculty of Sciences, King Abdulaziz University, Jeddah, KSA.
5Department of Anatomy, Faculty of Medicine, Arabian Gulf University, Bahrain.
6Faculty of Medicine, Rabigh, KSA.
Corresponding Author E-mail: hesham977@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1444
Abstract
This study was planned to explore the protective role of curcumin (Cur) against maternal and fetal oxidative stress and cerebral damage induced by lead (Pb) during pregnancy. Positively pregnant female rats were divided into seven groups: control group, Cur group (300 mg/kg of Cur/b.wt.), DMSO group (50% DMSO), two Pb-treated groups (exposed to 160 and 320 mg/kg b.wt./day of Pb acetate, respectively), and two groups treated with both Pb and Cur (exposed to Pb as previous groups together with 300 mg/kg b.wt./day of Cur). Treatments through oral gavage once a day started from gestation day 1 (GD1) till day 20 (GD20), where the mother rats of different experimental groups were sacrificed to obtain the fetuses. Different chemical parameters were assessed. Brain specimens of mother and fetal groups were processed with examination. The results displayed that Pb administration to pregnant rats resulted in a dose-dependent toxicity for both mothers and fetuses. Also, there was a significant rise in lipid peroxidation and decreased antioxidant enzyme activities in the brains of the different Pb-treated groups. The histological examination of the brain of treated dams and fetuses showed marked alterations. Co-treatment of Cur along with Pb caused a significant decrease in Pb levels as compared with those treated with Pb alone, improving the oxidative condition with amelioration of the brain’s histopathological changes. Co-administration of Cur could have ameliorative effect against Pb-induced neurotoxicity through the reduction of oxidative stress and reversal of histopathological changes.
Keywords
Lead; Oxidative Stress; Brain; curcumin; Fetal toxicity
Download this article as:| Copy the following to cite this article: Saleh H. A, El-Aziz G. S. A, Mustafa H. N, El-Fark M, Tashkandi J. M, Alzahrani A. H, Mal A, AboRass M, Deifalla A. H. Beneficial Effects of Curcumin Inmaternal and Fetal Oxidativestress and Brain Damage Induced by Gestational Lead Administration. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Saleh H. A, El-Aziz G. S. A, Mustafa H. N, El-Fark M, Tashkandi J. M, Alzahrani A. H, Mal A, AboRass M, Deifalla A. H. Beneficial Effects of Curcumin Inmaternal and Fetal Oxidativestress and Brain Damage Induced by Gestational Lead Administration. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20704 |
Introduction
Throughout history and even now, Lead (Pb) toxicity is a chief environmental health issue, especially for pregnant women and young children. Pb has been detected in all biological systems, and, despite its usefulness in life as an ingredient in many compounds in multiple industries, it is the most toxic heavy metals, causing various negative effects on multiple systems of the body.1 Pb can reach the body via ingestion or inhalation and is absorbedmainly in the respiratory and gastrointestinal tractswhere it is transported to different tissues.2
AlthoughPb has a wide range of impacts on body system toxicities, its neurotoxic effect is the most prominent.It has beendescribed that cerebrum is a vulnerable target to Pb cause it contains low levels of enzymes that are responsiblefordefending it against oxidative stress and because of its high myelin-related content, which increases the vulnerability to peroxidation.3 Also, it has been stated that Pb can interrupt the structure of the blood-brain barrier by destructing endothelial and glial cells and disturbing the building of tight junctions between barrier cells.4
Recently,Pb developmental toxicity has occurred as a major health problem for pregnant cause the fetus is vulnerable to Pb’smany toxic agents.5 GestationalPb exposure produces toxic effects,that includeteratogenesis,risk of low birth weight andreduced mental development.6 Moreover, studies have proved that developmental Pbexposure exerts neurotoxicity during differentiation, synaptogenesis,and end-stages of brain development.7
Accumulating data supporting the role of oxidative stress in the pathophysiology of Pb toxicity has recently been reported. Pbcan induce oxidative damage to cellular components involved in the production of reactive oxygen species (ROS). The mechanisms for Pb-induced oxidative stress may be either direct or indirect byuprising the creation of lipid peroxidation to levels that can disturb cell membrane functions, lipid metabolism and the antioxidant defense systems of cells.8, 9 In addition, interruption of the prooxidant/antioxidant balance via excessive production of ROS have asignificant role in brain pathology.10 The effect of Pb on fetal growth has caused great concern as many studies have reported shortfalls in prenatal development in cases where mothers were exposed to high Pb levels during their pregnancies. Also, it is worth mentioning that Pb exposure during early childhood has been described to cause various toxic effects in both humans and animals.11 The effects of Pb on pregnancy and the fetus are complex and not completely understood in cases where both the mother and her fetus are victims of high Pb exposure. It has been proven that Pb can cross the placenta, and there is a positive correlation between Pb levels in both maternal and umbilical cord blood.12
In the last few years, greater attention has been given to the curative potentials of medicinal plants as natural antioxidants, due to their lack of side effects and low cost. Also, many clinical studies have suggested that the antioxidants in fruits and vegetables are key in minimzingmany chronic diseases incidence.13 One of these plants iscurcumin (Cur),14 which is a famous, biologically active phenolic compound that is a chiefelement in turmeric, a yellow spice that is removed from the rhizome of Curcuma longaL. (family Zingiberaceae).15,16 Cur exhibits limited solubility in water and good solubility in chloroform and dimethyl sulfoxide (DMSO), which may be account for its low bioavailability.17
Cur is reported to have many therapeutic features, including anti-inflammatory,18 hypoglycemic,19 hypocholestrolemic,20 chelating,21 and antioxidant.22-24 The antioxidant property of Cur is due its effective scavenging of ROS. It also decreases the level of lipid peroxides and augments the activity of antioxidant enzymes.25 Some studies have reported that Cur could protect against different brain disorders and neurodegeneration through suppression of oxidative stress.26-28
Cur has been used for a long time as a traditional medication during pregnancy, and no medical authority recommends against its use.29 It is beneficial in treatingseveral disorders that occur commonly in pregnant women, such as anorexia, coryza, cough, and sinusitis.26-28, 30 Cur is highly lipophilic and is capable of crossing the blood-brain barrier, as confirmed by its high concentration in the fetal brain after oral intake.31 The current study was intended to investigate the protective role of Cur against maternal and fetal developmental parameters, oxidative stress, and cerebral damage, which was produced by two doses of Pb during pregnancy.
Material and Methods
Chemicals
Lead acetate (PbAc) pure crystals with a molecular weight of 379.33 and analytical-grade were purchased from Sigma Chemical Company (USA), and other chemicals and staining reagents used in this study were purchased through local scientific agents in Jeddah, Saudi Arabia.
Animals and Experimental Work
Adult, sexually mature nulliparous female Sprague-Dawley albino rats (weighing 200-225 grams at the beginning of the experiment) were used in this study. They were obtained from the Animal House of King Fahd Medical Research Center. All experimental procedures were carried out in accordance with the international guidelines for the care and use of animals in the laboratory. This study was approved and registered by the Committee of Animal Investigations, Faculty of Medicine, King Abdulaziz University. During the study, the female rats were kept in separate metallic cages under standard temperature (24 ± 2ºC), humidity (55 ± 5%), and lighting (12h:12h light/dark) conditions. They were fed a standard chow diet ad libitum and had free access to water. After acclimatization for two weeks, mating procedure was conducted by placing the individual females overnight in the home cage of a singly-housed male of the same stock. Gestation was confirmed by positive identification of spermatozoa in a vaginal lavage smear;the day of confirmation is designated as gestation day 0 (GD0), with subsequent days of gestation numbered accordingly.32 Only positively pregnant females were chosen and randomly divided into the following seven groups (eight-rats each):
Group I: the female rats were treated with deionized water only from GD 1-20 of pregnancy (control group) through oral gavage.
Group II (Cur): the female rats were treated with Cur at doses of 300 mg/kg b.wt.through oral gavage once a day from GD 1-20 of pregnancy.
Group III (DMSO): the female rats were treated with 50% DMSO (solvent of Cur) through oral gavage once a day from GD 1-20.33-35
Group IV (L160): the female rats were given 160 ppm of Pb acetate (PbAc) through oral gavageonce a day from GD 1-20.
Group V (L160+Cur): the female rats were given160 ppm of PbAc plus Cur (300 mg/kg b.wt.) by oral gavage once a day from GD 1-20.
Group VI (L320): the female rats were given 320 ppm of PbAc by oral gavage from GD 1-20.
Group VII (L320+Cur): the female rats were given 320 ppm of PbAc plus garlic extract (300 mg/kg b.wt.) by oral gavage once a day from GD 1-20.
Pbsolution was prepared by dissolving PbAc in acidified,distilled water in concentrations of 0.1 and 0.2% (w/v), containing 160 and 320 ppm of Pbrespectively.36 Cur solution was prepared by dissolvingthe powder in 50% DMSO to give a dose of 300 mg/kg body weight and diluted further with distilled water in 1.0 ml volume; this was administered orally once a day.37
Evaluations of Pregnant Rats and Fetuses
The pregnant female rats of each group were observed dailythroughout gestation for mortality, morbidity, and body-weight gain following treatment. On day 20 of gestation and under ether anesthesia, blood samples were taken via cardiac puncture and centrifuged at 3,000 rpm for 15 minutes to separate the sera, which were stored at -80°C. Then, the abdominal cavity was opened, the gravid uterine horns were removed and weighed, and the uterine contents were examined to determine the number of corpora lutea, implantation sites, and resorptions (embryonic death); also, the number and position of viable and dead fetuses were counted. Additionally, pre-implantation losses were calculated as: (number of corpora lutea–number of implantations)×100/number of corpora lutea. Then, all viable fetuses and placentas were removed and weighed separately. Following this, fetal crown-rump length, head length, and biparietal diameter were measured using a Vernier caliper and recorded.The heads of the mother and fetal rats of each group were opened,and the cerebellum of mother and fetal brains were quickly extracted and weighed. All extracted specimens were divided sagittally into two halves; the right halves were fixed in 10% neutral-buffered formalin for histological and immunohis to chemical study, and the left halves were frozen and stored at -70°C.
Measurement of Pb Levels in the Brain Tissues
Left halves of the brains from rat mothers and fetuses of all groups were digested in concentrated HNO3Suprapur in a shaking water bath at 60°C for 30 minutes. After digestion, the solution was diluted (1:5 v/v) with deionized water. Pb levels were measured using a graphite furnace atomic absorption spectrophotometer (Perkin-Elmer Model 3030) at King Fahd Medical Research Center, King Abdulaziz University. Results were expressed as µg of Pb/dl blood, and Pb levels in brain and placenta were expressed as µg/g tissue weight.36, 38, 39
Biochemical Assays
Left halves of the brains from rat mothers and fetuses of all groupswere homogenized (10% w/v) in ice-cold 0.1 M sodium phosphate buffer (pH 7.4). The homogenate was centrifuged twice at 4,000 rpm for 15-20 minutes at 4°C. The resultant supernatant was used for estimation of various biochemical assays. The lipid peroxidation (LPO) was estimated by determining the amount of malondialdehyde (MDA), a product formed due to the peroxidation of membrane lipids using a thiobarbituric acid-reactive substances assay kit (BioAssay Systems, Hayward, CA).40 The antioxidant enzyme activity of the rat brains was evaluated by determining the superoxide dismutase (SOD) activity. Thiswas measured using a SOD assay kit (Cayman), in which a tetrazolium salt was used for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine.41 Catalase (CAT) activity was assayed according to the peroxidatic function of catalase using a catalase assay kit (Cayman).41 Glutathione peroxidase (GPx) activity was assayed by coupling the enzyme procedure with glutathione reductase using a GPx assay kit (Cayman).41
Histological and Immunohistochemical Methods
The fixed right halves of the maternal and fetal brains of all experimental groups were dehydrated, cleared, and embedded in paraffin, and then sections of 5 um thickness were cut. Some sections were stained with hematoxilin-eosin (H&E) for general examination. Other sections were immunohistochemically stained with a modified avidin-biotin peroxidase technique for anti-GFAP to examine the astrocytes. Deparaffinized and rehydrated sections were treated with 0.01 M citrate buffer (pH 6.0) for 10 minutes to unmask antigens, and then incubated with a monoclonal antibody against GFAP (1:100 monoclonal mouse anti-GFAP) for 18-20hours. Detection of the antibody was carried out using a biotin-streptavidin detection system with 0.05% diaminobenzidine as a chromogen and counterstained with hematoxylin. GFAP-positive cells appeared brown and their nuclei appeared blue. The positive results were indicated by brown coloration of the cell membrane and cytoplasm of the astrocytes. Finally, all slides were examined with an Olympus BX53 microscope equipped with a camera (Olympus, Tokyo, Japan) at different magnifications.42
Quantitative Morphometric Study
Ten slides of non-overlapping fields from each group (at least five animals/group), at least one slide from each animal, were analyzed with the use of Image-Pro Plus v6 (Media Cybernetics Inc., Bethesda,Maryland, USA) and Image J (National Institute of Health, USA) was used to quantify cells, measurearea% covered by cells and colour intensity (GFAP immunoreaction in astrocytes) within the cerebral cortex.All morphometric measurements were done at x100 magnification.42, 43 For each calculation, the background was determined by manually adjusting the density window of the system until only the GFAP-positive tissue was selected. i.e. Digital brightfield images were uploaded into the image Jsoftware and scale set using a digital micrcrometer gauge reading to convert measurements in pixels to microns, and this was applied to all images. Cells were counted using the cell-counter plug-in available on the image J software after a grid had been applied across the image; number of different cell types in the respective brain regions was then counted. Total number of cells, area% covered by cells and average cell size were calculated using the automated cell-counter after thresholding for colour. Then, immunoreactive blood vessels, nonspecific staining, and artifacts were omitted before quantitation. When a subfield was too large to be analyzed at one time, several measurements of non-overlapping areas were made and then averaged. For whole cortex,the GFAP-positive area was divided by the total area analyzed to obtain an estimate of the area% occupied by astrocytes and their processes.43-45
Statistical Analysis
All the data were presented as mean± standard deviation of studied parameters from each group. Data were analyzed using a one-way analysis of variance followed by Bonferroni’s post hoc test or Student’s t-test, wherever applicable. Four different maternal and fetal parameters were used. All statistical analyses were done using the Statistical Package for the Social Sciences (SPSS, version 23) (USA). The values of P < 0.05 were considered significant.
Results
Maternal Findings
During the experiment, two pregnant rats from the high-dose Pb-treated group died. Post-mortem examination revealed that the fetuses aborted or died, and the treatment had no effect on the duration of the pregnancy.As represented in Table 1, there was a significant reduction in both maternal weight gain andplacental and brainweights in Pb-treated groups, which was more marked in the group that received the larger Pb dose, when compared to the control.Also,it was noticed that the co-administration of Curimproved theseeffects.
Table 1: Effect of gestational Pb and Cur co-administration on pregnant rats’parameters (Mean ± SD)
| Groups | Maternal Wt. Gain (gm) | Placental Wt. (gm) | Brain Wt. (gm) |
| Control (n=8) | 96.6 ± 11.9 | 0.73 ± 0.011 | 1.74 ± 0.09 |
| Cur (n=8) | 100.1 ± 9.6 | 0.74 ± 0.031 | 1.75 ± 0.11 |
| DMSO (n=8) | 98.35 ± 10.75 | 0.735 ± 0.021 | 1.745 ± 0.10 |
| L160 (n=8) | 80.6 ± 12.9
P1<0.05 P2<0.01 P3<0.05 |
0.63 ± 0.014
P1<0.001 P2<0.001 P3<0.001 |
1.59 ± 0.07
P1<0.05 P2<0.01 P3<0.05 |
| L160 + Cur (n=8) | 95.7 ± 7.83
P4<0.05 |
0.68 ± 0.019
P4<0.001 |
1.729 ± 0.04
P4<0.05 |
| L320 (n=6) | 75.0 ± 6.93
P1<0.001 P2<0.001 P3<0.001 |
0.58 ± 0.018
P1<0.001 P2<0.001 P3<0.001 |
1.56 ± 0.12
P1<0.01 P2<0.001 P3<0.01 |
| L320 + Cur (n=8) | 90.1 ± 7.12
P5<0.05 |
0.64 ± 0.028
P5<0.001 |
1.699 ± 0.05
P5<0.05 |
| F value | 7.668 | 67.733 | 6.545 |
Number of rat per treatment group = 8 except for the high lead dose. ANOVA test with Bonferroni post hoctest: P1: compared to control group. P2: compared to Cur group. P3: compared to DMSO group. P4: compared to L160 group. P5: compared to L320 group.
Fetal Findings
As seen in Table 2, the Pb-treated groups displayed a reduction of fetal weight and other fetal growth parameters (crown-rump length, head length, biparietal diameter, umbilical cord length), which was more marked in the group that received the larger Pb dose, when compared to the control; the co-administration of Cur along with Pb improved these effects.
Table 2: Effect of gestational Pb and Cur co-administration on fetal and brain weights and growth parameters(Mean ± SD)
| Groups (N of each) | Fetal Wt. (gm) | Crown-Rump
Length (cm) |
Head Length
(cm) |
Biparietal Diameter (cm) | Umbilical cord length (cm) | Fetal brain Wt. (gm) |
| Control (n=102) | 2.79 ± 0.037 | 2.96 ± 0.025 | 1.33 ± 0.003 | 0.75 ± 0.002 | 2.27 ± 0.055 | 0.142 ± 0.022 |
| Cur (n=98) | 2.83 ± 0.043 | 2.95 ± 0.015 | 1.34 ± 0.012 | 0.76 ± 0.011 | 2.25 ± 0.045 | 0.145 ± 0.027 |
| DMSO (n=102) | 2.81 ± 0.040 | 2.95 ± 0.02 | 1.35 ± 0.0075 | 0.741 ± 0.015 | 2.26 ± 0.050 | 0.1435±0.0245 |
| L160 (n=83) | 2.41 ± 0.055
P1<0.001 P2<0.001 P3<0.001 |
2.65 ± 0.026
P1<0.001 P2<0.001 P3<0.001 |
1.24 ± 0.004
P1<0.001 P2<0.001 P3<0.001 |
0.69 ± 0.009
P1<0.001 P2<0.001 P3<0.001 |
2.09 ± 0.051 P1<0.001
P2<0.001 P3<0.001 |
0.129 ± 0.017 P1<0.05
P2<0.001 P3<0.01 |
| L160 + Cur (n=92) | 2.69 ± 0.049
P4<0.001 |
2.79 ± 0.028
P4<0.001 |
1.29 ± 0.003
P4<0.001 |
0.73 ± 0.003
P4<0.001 |
2.21 ± 0.058 P4<0.001 | 0.141 ± 0.029
P4<0.05 |
| L320 (n=79) | 2.23 ± 0.041
P1<0.001 P2<0.001 P3<0.001 |
2.58 ± 0.044
P1<0.001 P2<0.001 P3<0.001 |
1.19 ± 0.005
P1<0.001 P2<0.001 P3<0.001 |
0.63 ± 0.002
P1<0.001 P2<0.001 P3<0.001 |
1.93 ± 0.026 P1<0.001
P2<0.001 P3<0.001 |
0.120 ± 0.032 P1<0.001
P2<0.001 P3<0.001 |
| L320 + Cur (n=88) | 2.62 ± 0.045
P5<0.001 |
2.69 ± 0.041
P5<0.001 |
1.24 ± 0.004
P5<0.001 |
0.71 ± 0.001
P5<0.001 |
2.05 ± 0.046 P5<0.001 | 0.135 ± 0.026 P5<0.01 |
| F value | 2262.7 | 2671.2 | 8116.4 | 2549.1 | 657.55 | 10.909 |
ANOVA test with Bonferroni post hoctest. P1: compared to control group. P2: compared to Curgroup. P3: compared to DMSO group. P4: compared to L160 group. P5: compared to L320 group.
Materno-Fetal Pb Analysis
The data in Table3show that there were parallel and significant increases in the mean values of Pb-level concentrations in the maternal blood and brains and fetal brainsin Pb-treated groups than inthe control group; this was higher in the group that received the larger Pb dose. Also, co-administration of Cur along with Pb resulted in the reduction of Pblevels versus the control group. Analysis showed that Pb content in the fetal brain was positively correlated to maternalPb levels,which indicatesPb transfer from the mother to the fetus.
Table 3: Effect of gestational Pb and Cur co-administration on materno-fetal Pb concentrations (Mean ± SD)
| Groups (N of each) | Maternal blood Pb level (µg/dl) | Maternal brain Pb level (µg/gm) | Groups (N of each) | Fetal brain Pb level (µg/gm) |
| Control (N=8) | 5.3 ± 0.99 | 0.06 ± 0.004 | Control (N=102) | 0.010 ± 0.003 |
| Cur (N=8) | 5.1 ± 1.21 | 0.05 ± 0.011 | Cur (N=98) | 0.008 ± 0.002 |
| DMSO (N=8) | 5.2 ± 1.1 | 0.055 ± 0.0075 | DMSO (N=102) | 0.009 ± 0.0025 |
| L160 (N=8) | 23.7 ± 4.19
P1<0.001 P2<0.001 P3<0.001 |
1.05 ± 0.13
P1<0.001 P2<0.001 P3<0.001 |
L160 (N=83) | 0.43 ± 0.011
P1<0.001 P2<0.001 P3<0.001 |
| L160 + Cur (N=8) | 10.1 ± 4.45
P4<0.001 |
0.29 ± 0.17
P4<0.001 |
L160 + Cur (N=92) | 0.06 ± 0.009
P4<0.001 |
| L320 (N=6) | 42.5 ± 5.41
P1<0.001 P2<0.001 P3<0.001 |
1.60 ± 0.32
P1<0.001 P2<0.001 P3<0.001 |
L320 (N=79) | 0.85 ± 0.17
P1<0.001 P2<0.001 P3<0.001 |
| L320 + Cur (N=8) | 15.2 ± 3.24
P5<0.001 |
0.33 ± 0.12
P5<0.001 |
L320 + Cur (N=88) | 0.23 ± 0.07
P5<0.001 |
| F value | 132.12 | 124.93 | F value | 1992.1 |
Number of rat per treatment group = 8 except for the high lead dose. ANOVA test with Bonferroni post hoctest. P1: compared to control group. P2: compared to Cur group. P3: compared to DMSO group. P4: compared to L160 group. P5: compared to L320 group.
Biochemical Assays
As seen in Table 4, there was a significant increase of MDA levels inthe maternal brains of Pb-treated groups, which was significant when compared to the control group; this was higher in the group that receivedthe larger Pb dose. Moreover, there was a decrease of SOD, CAT, and GPxlevels in Pb-treated groups in a dose-dependent manner, which was significant when compared to the control group.Co-administration of Cur along with Pb resulted in the decrease of MDA level and the increase of antioxidant enzyme activities.
Regarding the fetal brains, Table 5 illustrates that there was a significant increase of MDA levels in the fetal brains of Pb-treated groups, which was significant when compared to the control group; this was higher in the group that received the larger Pb dose. Also, there was a decrease of SOD, CAT, and GPxlevels in Pb-treated groups, which was significant when compared to the control group; these levels werelower in the group given the higher Pb dose. In contrast, co-administration of Cur along with Pb resulted again in the decrease of MDA and the increase of antioxidant enzyme activities.
Table 4: Effect of gestational Pb and Cur administration on the lipid peroxidation and antioxidant enzyme activitiesof the maternal brains (Mean ± SD)
| Groups | MDA
(nmol/ gm tissue) |
SOD
(U/gm Tissue) |
CAT
(µmole/ gm Tissue) |
GPx
(µmol/ gm tissue) |
| Control (n=8) | 17.43± 0.10 | 69.11± 9.21 | 250.1 ± 4.1 | 22.3 ± 1.1 |
| Cur (n=8) | 15.88± 0.14 | 68.18± 6.22 | 243.1 ± 7.3 | 21.7 ± 2.7 |
| DMSO (n=8) | 16.66± 0.12 | 68.65± 7.72 | 246.6 ± 5.7 | 22 ± 1.9 |
| L160 (n=8) | 29.42 ± 0.86
P1<0.001 P2<0.001 P3<0.001 |
51.32 ± 3.41
P1<0.001 P2<0.001 P3<0.001 |
217.3 ± 6.3
P1<0.001 P2<0.001 P3<0.001 |
17.4 ± 2.4
P1<0.01 P2<0.01 P3<0.01 |
| L160 + Cur (n=8) | 19.94 ± 0.92
P4<0.001 |
63.27 ± 2.16
P4<0.001 |
236.4 ± 5.9
P4<0.001 |
21.1 ± 1.9
P4<0.001 |
| L320 (n=6) | 46.38 ± 1.27
P1<0.001 P2<0.001 P3<0.001 |
41.56 ± 4.01
P1<0.001 P2<0.001 P3<0.001 |
197.4 ± 7.1
P1<0.001 P2<0.001 P3<0.001 |
12.6 ± 1.8
P1<0.001 P2<0.001 P3<0.001 |
| L320 + Cur (n=8) | 23.74 ± 0.96
P5<0.001 |
59.42 ± 3.17
P5<0.001 |
218.1 ± 7.2
P5<0.001 |
19.8 ± 3.3
P5<0.001 |
| F value | 1590.0 | 26.933 | 75.433 | 19.284 |
Number of rat per treatment group = 8 except for the high lead dose. ANOVA test with Bonferroni post hoctest: P1: compared to control group. P2: compared to Cur group. P3: compared to DMSO group. P4: compared to L160 group. P5: compared to L320 group.
Table 5: Effect of gestational Pb and Cur administration on the lipid peroxidation and antioxidant enzyme activitiesof the fetal brains (Mean ± SD)
| Groups (N of each) | MDA
(nmol/ gm tissue) |
SOD
(U/gm Tissue) |
CAT
(µmole/ gm Tissue) |
GPx
(µmol/ gm tissue) |
| Control (n=102) | 6.33 ± 1.9 | 0.63 ± 0.18 | 16.6 ± 4.1 | 15.2 ± 2.8 |
| Cur (n=98) | 5.88 ± 1.6 | 0.62 ± 0.12 | 15.9 ± 2.3 | 17.1 ± 2.3 |
| DMSO (n=102) | 6.1 ± 1.75 | 0.625 ± 0.15 | 16.25 ± 3.2 | 16.15 ± 2.55 |
| L160 (n=83) | 11.12 ± 2.1
P1<0.001 P2<0.001 P3<0.001 |
0.51 ± 0.19
P1<0.001 P2<0.001 P3<0.001 |
23.1 ± 6.3
P1<0.001 P2<0.001 P3<0.001 |
11.4 ± 2.6
P1<0.001 P2<0.001 P3<0.001 |
| L160 + Cur (n=92) | 7.92 ± 1.8
P4<0.001 |
0.60 ± 0.16
P4<0.01 |
19.2 ± 6.1
P4<0.001 |
13.1 ± 2.9 P4<0.001 |
| L320 (n=79) | 22.47 ± 4.7
P1<0.001 P2<0.001 P3<0.001 |
0.38 ± 0.17
P1<0.001 P2<0.001 P3<0.001 |
35.4 ± 7.3
P1<0.001 P2<0.001 P3<0.001 |
8.8 ± 2.7
P1<0.001 P2<0.001 P3<0.001 |
| L320 + Cur (n=88) | 9.53 ± 1.7
P5<0.001 |
0.54 ± 0.15
P5<0.001 |
22.4 ± 6.8
P5<0.001 |
10.7 ± 3.1
P5<0.001 |
| F value | 514.68 | 27.228 | 141.05 | 115.44 |
ANOVA test with Bonferroni post hoc test: P1: compared to control group. P2: compared to Cur group. P3: compared to DMSO group. P4: compared to L160 group. P5: compared to L320 group.
Histological Results
Examination of the H&E-stained sections of the cerebral cortex of the mother rats showed that there were nearly the same findings in the control, Cur, and DMSO-treated mother groups. In general, normal histoarchitecture was seen in the six layers of the cerebral cortex (Fig. 1 A, B & C). In the Pb-treated groups, the examination revealed variable degrees of histopathological changes, which were less evident in the lower dose groups (Fig. 2 A & B) than in the higher dose groups (Fig. 3 A & B).Disorganization and lack of characteristic, typical, layered structures were seen with some vacuoles of variable sizes between cells. Some pyramidal cells appeared irregular in shape and darkly stained with loss of their processes; others showed marked vacuolization with faintly stained cytoplasm. Most of granular cells were affected and became ill-defined and faintly stained with pericellular halos. Also, some apoptotic cells with dense nuclei were observed. Regarding the Pb and Cur treated groups (Fig. 4 A, B & C), the examination showed improvement of histological appearance of the cortical layers, which appeared similar to the control group with decreased cellular damage, especially with the low Pb-dose group. The pyramidal cells appeared almost normal with vesicular nuclei;a few were still vacuolated with faintly stained acidophilic cytoplasm. The immunohistochemical examination of GFAP-stained sections of the cerebral cortex from the above groups revealed a few GFAP-positive immuno-reactive astrocytes with their processes dispersed in between different cell layers of the cerebral cortex in the control, Cur, and DMSO-treated groups (Fig. 1 D). In the Pb-treated groups, an apparent increase in the number of GFAP immuno-reactive astrocytes was observed as compared to the control animals, which was more rigorous in the group that received the high dose of Pb (Fig. 3 C) than that which received the low dose (Fig. 2 C). In the Pb- and Cur-treated group (Fig. 4 D), a noticeable decrease in the GFAP immune-reactive astrocytes was seen as compared to the Pb-treated rats (Table 6).
Examination of the H&E-stained sections from the fetal brains belonging to the control, Cur, and DMSO-treated groups showed nearly the same, normal histological findings, which displayed normal appearance of the telencephalon (which appeared with distinct structure and was formed of five zones) with its lateral ventricle (Fig. 5 A & B). In the low-dose Pb-treated group, the picture was similar with no difference from that of the control group. In the high-dose Pb-treated group (Fig. 6 A & B), the examination of fetal brains revealed variable degrees of histopathological changes where there was a less degree of maturation than of the control fetuses in the form of dilation of the lateral ventricle as well as thinning and hardly distinguishable arrangement of the telencephalic wall layers. Also, thinned ventricular and subventricular zones, widening of the intermediate zone, hypoplasia, and reduction of the cortical zone were seen. While in the fetal brains from the Pb- and Cur-treated group (Fig. 7 A & B), the examination showed regressive changes, and the picture was similar to that of the control group regarding the thickness of the telencephalic wall and size of the lateral ventricle. The immunohistochemical examination of GFAP-stained sections of the fetal brain showed negative or very little GFAP-positive cellular reaction in the superficial layers of the telencephalic wall (Fig. 5 C). In the fetal brains from the high-dose Pb-treated group, there was apparent increase in the number of GFAP immuno-reactive astrocytes as compared to the control group (Fig. 6 C). However, in the fetal brain from Pb- and Cur-treated group, a reduction of GFAP immuno-reactive astrocytes was observed (Table 6, Fig. 7 C).
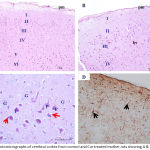 |
Figure 1: Photomicrographs of cerebral cortex from control and Cur treated mother rats showing A & B)
|
The normal layering pattern in the form of six layers; I: outer molecular layer, II: external granular layer, III: external pyramidal layer, IV: inner granular layer, V: inner pyramidal layer, VI: polymorphic layer. C) the pyramidal cells (P) with their multipolar shape, vesicular nuclei and basophilic cytoplasm. Granular cells (G) were seen with large nuclei, prominent nucleolus and little cytoplasm. The smaller neuroglia cells are scattered (↑). D) a few GFAP positive astrocytes with brown cytoplasmic granules (↑). pm= pia matter, bv= blood vessel. [A, B & C: H & E X 100, 200 & 400, D: GFAP X 200].
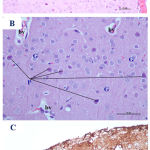 |
Figure 2: Photomicrographs of cerebral cortex from low dose Pb-treated mother rats showing A)
|
A little disorganization of cortical layers with some congested blood vessels (bv). B) some shrunken pyramidal cells (P) with loss of their processes, dark cytoplasm and small darkly stained nucleus. Some granular cells (G) are surrounded with halos. C) an increase in the GFAP immunoreactive astrocytes (↑) as compared to the control group. pm= pia matter [A&B: H&E X100 & 400, C: GFAP X 200].
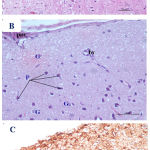 |
Figure 3: Photomicrographs of cerebral cortex from high dose Pb-treated mother rats showing A)
|
A distortion and disappearance of normal arranged cortical layers with dilated blood vessels. B) some pyramidal cells (P) were appeared darkly stained and irregular in shape with pyknotic nuclei; other cells showed marked vacuolization with faintly stained cytoplasm. Notice that some granular cells are shrunken and deeply stained (G). C) a marked increase in the number of GFAP immunoreactive astrocytes (↑) as compared to low dose Pb treated and control groups. pm= pia matter, bv= blood vessel. [A&B: H&E X100 & 400, C: GFAP X 200].
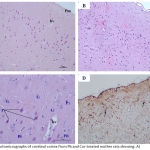 |
Figure 4: Photomicrographs of cerebral cortex from Pb and Cur-treated mother rats showing: A)
|
An improvement in the histoarchitecture with organized layers. B) more or less normal pyramidal cells (P) and granular cells (G). C) a decrease in the GFAP immunoreactive astrocytes as compared Pb-treated rats (↑). pm= pia matter, bv= blood vessel. [A: H&E X100 & B: 400, C: GFAP X 200].
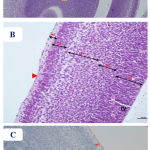 |
Figure 5: Photomicrographs of the fetal brain at GD20 from control and Cur treated groups showing: A)
|
Normal appearance of the telencephalic wall (TW) and lateral ventricle (LV). B) telencephalic wall (TW), which is formed of five basic zones: marginal zone (MZ), cortical zone (CZ), intermediate zone (IZ), subventricular zone (SZ) and ventricular zone (VZ). C) negative or very few and weak GFAP positive cellular reaction (↑), which are scattered in the superficial layers of the telencephlic wall. (Th= thalamus, Cp= choroid plexus). [A: H&E X100, B: H&E X 200, C: GFAP X 200].
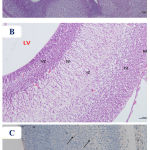 |
Figure 6: Photomicrographs of the fetal brain at GD20 from high dose Pb-treated group showing A)
|
Dilation of the lateral ventricle (LV) with thinning of the telencephalic wall (TW). B) a hardly distinguishable arrangement of the telencephalic wall layers, with thinned ventricular (VZ) and subventricular (SZ) zones and widening of the intermediate zone (IZ), hypoplasia and reduction of the cortical zone (CZ) and disruption of the marginal zone (MZ). C) a presence of GFAP positive reaction (↑), which was more scattered in the superficial layers of the telencephalic wall. (Th= thalamus). [A: H&E X100, B: H&E X 200, C: GFAP X 200].
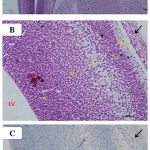 |
Figure 7: Photomicrographs of the fetal brain at GD20 from high dose Pb and Cur treated group showing: A)
|
Nearly normal appearance of the telencephalic wall (TW) and lateral ventricle (LV).B) a distinguishable arrangement of the telencephalic wall layers, which appeared similar to the control fetal brain. C) a reduction in the number of GFAP immunoreactive astrocytes (↑). (Th= thalamus, Cp= choroid plexus). [A: H&E X100, B: H&E X 200, C: GFAP X 200].
Table 6: Area percentage of GFAP (Mean ± SD)
| Groups (N of each) | Maternal GFAP area percentage | Groups (N of each) | Fetal GFAP area percentage |
| Control (n=8) | 0.563 ± 0.188 | Control (n=102) | 0.285 ± 0.026 |
| Cur (n=8) | 0.55 ± 0.175 | Cur (n=98) | 0.275 ± 0.086 |
| DMSO (n=8) | 0.57 ± 0.19 | DMSO (n=102) | 0.280 ± 0.095 |
| L160 (n=8) | 1.1 ± 0.37
P1<0.01 P2<0.01 P3<0.01 |
L160 (n=83) | 0.55 ± 0.185
P1<0.001 P2<0.001 P3<0.001 |
| L160 + Cur (n=8) | 0.602 ± 0.209
P4<0.05 |
L160 + Cur (n=92) | 0.301 ± 0.105
P4<0.001 |
| L320 (n=6) | 1.42 ± 0.473
P1<0.001 P2<0.001 P3<0.001 |
L320 (n=79) | 0.71 ± 0.237
P1<0.001 P2<0.001 P3<0.001 |
| L320 + Cur (n=8) | 0.600 ± 0.2
P5<0.001 |
L320 + Cur (n=88) | 0.32 ± 0.11
P5<0.001 |
| F value | 12.289 | F value | 147.08 |
Number of rat per treatment group = 8 except for the high lead dose. Percent area/cell is the product of percent area/(100×density). ANOVA test with Bonferroni post hoc test: P1: compared to control group. P2: compared to Cur group. P3: compared to DMSO group. P4: compared to L160 group. P5: compared to L320 group.
Discussion
In the current study, Pb at a dose of 160 and 320 mg/kg b. wt. during pregnancy causeda significant reduction inbrain weight, body weight and fetal body weight when compared to the control rats, which wasobvious in those received the larger dose of Pb. In accordance with these findings, the authors reported that Pb causes several adverse health effects that are dose-dependent and somewhat irreversible.46 Also, it was found that Pb in ducesan important reduction in pups’ body and brain weightat postnatal days 0-21 when their dams consumed drinking-water containing 300 mg/L of Pb.12,47
The central nervous system is considered to be the primary target of Pb exposure, especially in the developing brain, leading to weakening of cognitive abilities and interruption of behavioral development and attentiveness in children.48 These results could be explained by the fact that infants absorb 50% of Pbthat are exposed to, whereas adults absorb 10%.11, 23, 49 Also, Pb traverses the blood-brain barrier simply while the brain is developing in a rapid growth and an intense cellular proliferation. This isdue toblood-brain barrier immaturity, which caused absorption of Pb.11, 23, 49 Studies haverevealed that the fetuses of pregnant mothers predisposed toPb level (0.01% and 0.05% w/v orally for 45 days) exhibited higher fetal brain Pb levels.50
The elevated maternal blood Pb levelin the current results that is obvious in the group with a higher dose of Pb, shows a correlation between maternal and umbilical cord blood Pb levels.Those findings support the concept that Pb mighteasily cross to the placentain a dose-responsive manner. It was described that the placenta can’t protect the fetus from exposure to Pb, because it is able to cross the placental barrier freely.51 Current results come in the same line with other studies that compared the Pb levels of umbilical cord blood and maternal blood and with studies that declared that the Pb in new-born infants’ blood was a reflection of that of the mother.52
Current results indicated that the dam’s Pb exposure caused an increase of Pb in fetus blood and placenta, in additon to elevation of the Pb in the brain of the developing fetus. Similarly, preceding studies showed that Pb was highly concentrated in the placenta,umbilical cord and brain,and it is supposed that the placenta and umbilical cord might be respectable biomarkers of fetal Pb exposure.53 Further more, studies have demonstrated that Pb in filtrate the immature blood-brain barrier and accumulate in the developing brain, which seemsto be vulnerable to metal infiltration; this results in a significant dose-dependent rise, confirming the trans placental metal passage from the mothers.6
In the current work, it was found that Pb causes a significant increase in LPO, as evidenced by the high MDA level in the maternal and fetal brains; this was accompanied by marked reduction of antioxidant enzymes (SOD, CAT, and GPx) in different Pb exposure groups. It was stated that Pb-induced disturbance of the prooxidant/antioxidant balance in the brain might encourage impairment to different cellular components, including nucleic acids, membrane lipids, and proteins.54 Also, it was shown that MDA level is strongly correlated with Pb concentration in the brains of exposed rats.55 Moreover, these results were consistent with earlier animal and human studies, which suggested that exposure to Pb was associated with increased oxidative stress and occurrence of neurotoxicity due to oxidative damages, as the brain consumes 20% of the body’s oxygen.56
The results also showed increased LPO in the fetal brains with pre-natal Pb exposure. It was declared that the toxic effects of Pb on the fetal brain are undoubtedly the main significant and meticulously studied consequence of intrauterine Pb exposure. This injurious effect of high Pb levels has been well-recognized.57,58 Such effects might be affecting the morphological developments and the sensory motor reflexes of the pups as well as the behavior of young adult offspring.59
Histolopathologically, the present study displayed a variable degree of structural impairment of the maternal and fetal brains in the Pb-treated groups, which were more understandable in those exposed to the higher dose of Pb. It was stated previously that Pb-induced damage occurred primarily in the cerebrum, hippocampus and cerebellum affecting various biological activities at the molecular, cellular, and intracellular levels, which cancause morphological modifications in the brain that may persist even after Pb levels have dropped.60 In this study, there were decreased pyramidal cells along with the presence of degenerated cells with pyknotic nuclei in Pb-treated groups in comparison to control. This agreed with studies that accredited these findings to the direct effect of Pb on brain cells.61 Correspondingly, Pb-induced cell death has been established in the cortex and cerebellum on neonatal rats in-vivo (2-4 weeks old). This indicates a sophisticated sensitivity in younger rats with apoptotic cells.49
The study showed that fetal brains from Pb-exposed groups displayed reduced differentiation and cell apoptosis degeneration. Accordingly, it was noticedthat the intrauterine exposure to Pb is harm fully disturbs cellular proliferation, differentiation and synaptic growth of the brain, which causes mental retardation or movement disorders.62 Accordingly, studies have described that Pb exposure during embryonic development causes cortical and cerebellar impairment in newborn and in the developing hippocampus. These effects happencause of Pb interferes with several proliferative and apoptotic pathways.63
In the current study, the effect of Pb on the astrocytes was observed using immunolabeling. Increased GFAP-positive astrocytes were found in the Pb treated maternal brains; this increase or gliosis is explained as hyper-reactivity of astrocytes that attempt to provide accommodation against the toxic effect of Pb, thus protecting neurons from its hazardous effects.61 The mechanism that explain the increased of GFAP-positive astrocytes and GFAP content in the neonatal brain after maternal Pb intake detected in the current study might be attributed to a potential role of cytokines, which play an important part in modulating astroglios is. Moreover, it was described that the observed glios is in the Pb-treated group might be caused by the formation of ROS and reduced antioxidants.42 Similarly, studies have observed an elevated GFAP in numerous regions of the brain after Pb exposure, mainly in the hippocampus and the cerebellar cortex. Consequently, the detection of GFAP expression migh tprobably be anappropriate marker to determine neurodegenerative impairments.64 Regarding the fetal brains at GD20, few GFAP-positive astrocytes were occasionally seen. GFAP-positive astrocytes were detected in the cortex of the brain in the examined groups. Focally accumulated GFAP-positive astrocytes in the cortex were apparent in brains from the Pb-treated group.
Co-administration of Cur together with Pb to the mother rats produced a significant improvement, as evidenced by the study parameters and the histopathological findings in maternal and fetal brains, which appeared like the control rats, especially in the group that received the smaller dose of Pb. Similarly, it has been reported that Cur has great potential for the prevention of multiple neurological conditions.65 Also, several studies have shown that Cur exhibits protective effects against oxidative damage by exerting a potent scavenging effect for ROS and increased intracellular glutathione concentration.66 Moreover, some animal studies have shown that administration of Cur after brain ischemia has protective effects and significantly diminishes lipid peroxidation, mitochondrial dysfunction, and glial activation, as well as infarct volume; moreover, it improves cognitive deficits, neurological scores, and locomotor activity.51
Furthermore, the protective role of the antioxidant Cur is proved by a reduced MDA concentration in the brain due toPb-caused oxidative stress.67 Suppression of SOD activity by Pb was shown in an in-vitro experiment that support the concept that Pb can causereduced scavenging of ROS, which results in oxidative impairment. SOD activity is a significant constituent of the cellular antioxidant system that defends cells from the destructive effects of oxidants.68 Cur showed a neuroprotective effect in the different regions of the brain 69. Cur is thought to exert its anti-apoptotic and neuroprotective actions in multiple ways, including as a strong anti-inflammatory and antioxidant, and through neuromodulating activities.70 Also, it was shown that Cur exhibits a great capacity to defend brain lipids from peroxidation, improve SOD activity and shows the important inhibitory action against H2O2-caused cell injury.68, 71
Conclusion
This study is important in providing evidence of the beneficial role of Cur as a natural antioxidant in the protection against maternal and fetal Pbneurotoxicity. Finally, efforts with active steps should be taken to wards the prevention of occupational exposure to this metal.
Disclosure of Interest
The authors declare that they have no conflicts of interest.
Referenc
- Mudipalli A. Lead hepatotoxicity & potential health effects. Indian J Med Res. 2007;126:518-527.
- Pizzol M, Thomsen M., Andersen MS. Long-term human exposure to lead from different media and intake pathways. The Science of the total environment. 2010;408:5478-5488
CrossRef - Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Reviews on Environmental Health. 2009:24:15.
- Liu J, Han D, Li Y, et al., Lead affects apoptosis and related gene XIAP and Smac expression in the hippocampus of developing rats. Neurochem Res. 2010;35:473-479.
CrossRef - Grandjean P, Bellinger D, Bergman A., et al., The faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic & clinical pharmacology & toxicology. 2008;102:73-75.
- Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdisciplinary toxicology. 2012;5:47-58.
CrossRef - Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic & clinical pharmacology & toxicology. 2008;102:218-227.
CrossRef - Bokara KK, Brown E, McCormick R, Yallapragada PR, Rajanna S, Bettaiya R. Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2008;21:9-16.
CrossRef - Bokara KK, Blaylock I, Denise SB, Bettaiya R, Rajanna S, Yallapragada PR. Influence of lead acetate on glutathione and its related enzymes in different regions of rat brain. Journal of applied toxicology: JAT. 2009;29:452-458.
CrossRef - Posser T, de Aguiar CBNM, Garcez RC, et al., Exposure of C6 glioma cells to Pb(II) increases the phosphorylation of p38MAPK and JNK1/2 but not of ERK1/2. Archives of toxicology. 2007;81:407-414.
CrossRef - Shallie P, Adefule A, Akpan H, Olubiyi O, Fakunle P, Adejumo E. Lead toxicity and some subsets of motor skill: Comparative evaluation of adult and prenatally exposed rats. Journal of Neuroscience and Behavioral Health. 2010;2:23-29.
- Ashafaq M, Tabassum H, Vishnoi S, Salman M, Raisuddin S, Parvez S. Tannic acid alleviates lead acetate-induced neurochemical perturbations in rat brain. Neurosci Lett. 2016;617:94-100.
CrossRef - Celiktas OY, Kocabas EEH, Bedir E, Sukan FV, Ozek T, Baser KHC. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chemistry. 2007;100:553-559.
CrossRef - Schmidt BM, Ribnicky DM, Lipsky PE, Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat Chem Biol. 2007;3:360-366.
CrossRef - Ellerkamp V, Bortel N, Schmid E, Kirchner B, Armeanu-Ebinger S, Fuchs J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer Res. 2016;36:3363-3372.
- Sahu BD, Kumar JM, Kuncha M, Borkar RM, Srinivas R, Sistla R. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life sciences. 2016;144:8-18.
CrossRef - Payton F, Sandusky P, Alworth WL. NMR study of the solution structure of curcumin. Journal of natural products. 2007;70:143-146.
CrossRef - Agarwal R, Goel SK, Behari JR. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. Journal of applied toxicology : JAT. 2010;30:457-468.
CrossRef - Soto-Urquieta MG, Lopez-Briones S, Perez-Vazquez V, Saavedra-Molina A, Gonzalez-Hernandez GA, Ramirez-Emiliano J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biological research. 2014;47:74.
CrossRef - Manogaran E, Ramanathan M, RamaRao T. Neuroprotective effect of curcumin against cholesterol induced neuroinflammation in-vitro and in-vivo Models. J Pharm Sci Res. 2015;7:189-196.
- Tandon SK, Singh S, Prasad S, Srivastava S, Siddiqui MKJ. Reversal of Lead-Induced Oxidative Stress by Chelating Agent, Antioxidant, or Their Combination In the Rat. Environmental research. 2002;90:61-66.
CrossRef - Agarwal NB, Jain S, Agarwal NK, Mediratta PK, Sharma KK. Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2011;18:756-759.
CrossRef - Yang J, Song S, Li J, Liang T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol Res Pract. 2014;210:357-362.
CrossRef - Tuzmen MN, Yucel NC, Kalburcu T, Demiryas N. Effects of curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol Mech Methods. 2015;25:120-127.
CrossRef - Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chemico-biological interactions. 2008;174:27-37.
CrossRef - Boyanapalli SS, Tony Kong AN. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr Pharmacol Rep. 2015;1:129-139.
CrossRef - Hu S, Maiti P, Ma Q, et al., Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother. 2015;15:629-637.
CrossRef - Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053-1064.
CrossRef - Chhunchha B, Fatma N, Kubo E, Rai P, Singh SP, Singh DP. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-κB regulation. American Journal of Physiology – Cell Physiology. 2013;304:C636-C655.
- Wu J, Li Q, Wang X, et al., Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PloS one. 2013;8:e59843.
- Sharifi AM, Mousavi SH, Jorjani M. Effect of chronic lead exposure on pro-apoptotic Bax and anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo. Cell Mol Neurobiol. 2010;30:769-774.
CrossRef - Adu EK, Yeboah S. The efficacy of the vaginal plug formation after mating for pregnancy diagnosis, and embyonic resorption in utero in the greater cane rat (Thryonomys swinderianus, Temminck). Trop Anim Health Prod. 2000;32:1-10.
CrossRef - Abu-Taweel GM. Effects of curcumin on the social behavior, blood composition, reproductive hormones in plasma and brain acetylcholinesterase in cadmium intoxicated mice. Saudi J Biol Sci. 2016;23:219-228.
CrossRef - Wang R, Tian S, Yang X, Liu J, Wang Y, Sun K. Celecoxib-induced inhibition of neurogenesis in fetal frontal cortex is attenuated by curcumin via Wnt/beta-catenin pathway. Life sciences. 2017;185:95-102.
CrossRef - Li X, Zhao L, Yue L, et al., Evidence for the protective effects of curcumin against oxyhemoglobin-induced injury in rat cortical neurons. Brain Res Bull. 2016;120:34-40.
CrossRef - Villeda-Hernandez J, Mendez Armenta M, Barroso-Moguel R, Trejo-Solis MC, Guevara J, Rios C. Morphometric analysis of brain lesions in rat fetuses prenatally exposed to low-level lead acetate: correlation with lipid peroxidation. Histology and histopathology. 2006;21:609-617.
- Abu-Taweel GM, Ajarem JS, Ahmad M. Protective Effect of Curcumin on Anxiety, Learning Behavior, Neuromuscular Activities, Brain Neurotransmitters and Oxidative Stress Enzymes in Cadmium Intoxicated Mice. Journal of Behavioral and Brain Science. 2013;03:74-84.
CrossRef - Miller DT, Paschal DC, Gunter EW, Stroud PE, D’Angelo J. Determination of lead in blood using electrothermal atomisation atomic absorption spectrometry with a L’vov platform and matrix modifier. The Analyst. 1987;112:1701-1704.
CrossRef - Sepehri H, Ganji F. The protective role of ascorbic acid on hippocampal CA1 pyramidal neurons in a rat model of maternal lead exposure. J Chem Neuroanat. 2016;74:5-10.
CrossRef - Mustafa HN, Hegazy GA, Awdan SAE, AbdelBaset M. Protective role of CoQ10 or L-carnitine on the integrity of the myocardium in doxorubicin induced toxicity. Tissue Cell. 2017;49:410-426.
CrossRef - Mustafa HN, El Awdan SA, Hegazy GA, Abdel Jaleel GA. Prophylactic role of coenzyme Q10 and Cynara scolymus L on doxorubicin-induced toxicity in rats: Biochemical and immunohistochemical study. Indian journal of pharmacology. 2015;47:649-656.
CrossRef - Mustafa HN, Hussein AM. Does allicin combined with vitamin B-complex have superior potentials than alpha-tocopherol alone in ameliorating lead acetate-induced Purkinje cell alterations in rats? An immunohistochemical and ultrastructural study. Folia Morphol (Warsz). 2016;75:76-86.
CrossRef - Onaolapo OJ, Onaolapo AY, Akanmu MA, Gbola O. Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology. 2016;23:147-156.
CrossRef - Sloane JA, Hollander W, Rosene DL, Moss MB, Kemper T, Abraham CR. Astrocytic hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain research. 2000;862:1-10.
CrossRef - Onaolapo AY, Onaolapo OJ, Nwoha PU. Alterations in behaviour, cerebral cortical morphology and cerebral oxidative stress markers following aspartame ingestion. Journal of Chemical Neuroanatomy. 2016;78:42-56.
CrossRef - Wang Y, Wang S. Effects of lead exposure on histological structure and antioxidant capacity in the cerebellum of 30-day-old mice. Neural regeneration research. 2011;6:1077-1081.
- Attia AM, Ibrahim FA, Nabil GM, Aziz SW. Antioxidant effects of ginger (Zingiber officinale Roscoe) against lead acetate-induced hepatotoxicity in rats. African Journal of Pharmacy and Pharmacology. 2013;7:1213-1219.
CrossRef - Balbuena P, Li W, Magnin-Bissel G, Meldrum JB, Ehrich M. Comparison of two blood-brain barrier in vitro systems: cytotoxicity and transfer assessments of malathion/oxon and lead acetate. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:260-271.
CrossRef - Chang BJ, Jang BJ, Son TG, et al. Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol. 2012;50:104-108.
CrossRef - Dribben WH, Creeley CE, Farber N. Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol. 2011;33:473-480.
CrossRef - Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav. 2013;110:238-244.
CrossRef - Guangwei X, Rongzhu L, Wenrong X, et al., Curcumin pretreatment protects against acute acrylonitrile-induced oxidative damage in rats. Toxicology. 2010;267:140-146.
CrossRef - Hamed EA, Meki AR, Abd El-Mottaleb NA. Protective effect of green tea on lead-induced oxidative damage in rat’s blood and brain tissue homogenates. J Physiol Biochem. 2010;66:143-151.
CrossRef - Dalia M. Effect of using pectin on lead toxicity. J Am Sci. 2010;6:541-554.
- Hassan A, Jassim H. Effect of treating lactating rats with lead acetate and its interaction with vitamin E or C on neurobehavior, development and some biochemical parameters in their pups. Iraqi Journal of Veterinary Sciences. 2010;24:45-52.
CrossRef - Abdel Moneim AE. Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res. 2012;148:363-370.
CrossRef - Sadek K. Barley phenolic compounds impedes oxidative stress in lead acetate intoxicated rabbits. Oxidants and Antioxidants in Medical Science. 2012;1:141-146.
CrossRef - Awasthi H, Tota S, Hanif K, Nath C, Shukla R. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life sciences. 2010;86:87-94.
CrossRef - Bagchi A, Mukherjee P, Bhowmick S, Raha A. Synthesis, characterization and antibacterial activity of a novel curcumin metal complex. Int J Drug Dev Res. 2015;7:011-014.
- Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci Rep. 2015;5:10278.
CrossRef - Chibowska K, Baranowska-Bosiacka I, Falkowska A, Gutowska I, Goschorska M, Chlubek D. Effect of Lead (Pb) on Inflammatory Processes in the Brain. Int J Mol Sci. 2016;17:2140.
CrossRef - Dkhil MA, Moneim AE, Al-Quraishy S. Indigofera oblongifolia Ameliorates Lead Acetate-Induced Testicular Oxidative Damage and Apoptosis in a Rat Model. Biol Trace Elem Res. 2016;173:354-361.
CrossRef - Gargouri M, Ghorbel-Koubaa F, Bonenfant-Magne M, et al., Spirulina or dandelion-enriched diet of mothers alleviates lead-induced damages in brain and cerebellum of newborn rats. Food Chem Toxicol. 2012;50:2303-2310.
CrossRef - Ciftci O, Tanyildizi S, Godekmerdan A. Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD). Immunopharmacol Immunotoxicol. 2010;32:99-104.
CrossRef - Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131:682-691.
- Ciftci O, Ozdemir I, Tanyildizi S, Yildiz S, Oguzturk H. Antioxidative effects of curcumin, beta-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicology and industrial health. 2011;27:447-453.
CrossRef - Huang HC, Lin CJ, Liu WJ, Jiang RR, Jiang ZF. Dual effects of curcumin on neuronal oxidative stress in the presence of Cu(II). Food Chem Toxicol. 2011;49:1578-1583.
CrossRef - Yu W, Wu J, Cai F, et al., Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PloS one. 2012;7:e52013.
- Liu Z, Yu Y, Li X, Ross CA, Smith WW. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol Res. 2011;63:439-444.
CrossRef - Rinwa P, Kumar A, Garg S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PloS one. 2013;8:e61052.
- Yu G, Liu L, Zhang P, Li Y. Protective effect of Curcumin on chronic cerebral ischemia by altering expression of α-synuclein in 2VO model. Molecular neurodegeneration. 2012:7: 33.








