Manuscript accepted on :28 April 2018
Published online on: 15-05-2018
Plagiarism Check: Yes
Ahmed Mohamed Mohamed Youssef 1 and Zeinab Ahmed Said El-Swaify2
1Department of Pharmacology, Faculty of Pharmacy, Mutah University, Mutah, Karak, Jordan.
2Department of Botany, Faculty of Science, Al-Azhar, University, Girl Branch, Cairo, Egypt.
Corresponding Author E-mail: ammyouss@mutah.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/1416
Abstract
The active constituents present in Persicaria salicifolia and Persicaria senegalensis seeds may possess anti-tumour activity. Therefore, P. salicifolia and P. senegalensis seeds were extracted and analysed to identify their active constituents. Phytochemical compounds exist in 50 % methanol extracts of P. salicifolia and P. senegalensis seeds were identified through High-Performance Liquid Chromatography (HPLC), Liquid Chromatography/ Mass Spectrometry (LC/MS), and Gas Chromatography-Mass Spectrometry (GC/MS). MTT assay was utilized to analyse the anti-tumour activity of P. salicifolia and P. senegalensis seeds compared to their aerial parts against CaCo-2 and PC3 cell lines. The constituents of Persicaria species seeds have phenolic acids, flavonoid, and lipid compounds. The cytotoxicity of aerial parts of P. salicifolia showed half maximal inhibitory concentration (IC50) of 1.1 ± 0.15 µg/ml and 0.5 ± 0.0011 µg/ml and the seeds were 0.6 ± 0.0018 µg/ml and 1.0 ± 0.009 µg/ml against PC3 and CaCO-2 cell lines, respectively. While, the aerial parts of P. senegalensis showed IC50 of 2.3 ± 0.03 µg/ml and 2.0 ± 0.03 µg/ml, and the seeds were 3.5 ± 0.06 µg/ml and 1.5 ± 0.03 µg/ml against PC3 and Caco-2, respectively. The results showed that there was a potential cytotoxicity of two Persicaria species seeds against two human cancer cell lines comparing to their aerial parts that have antitumor activity as it is confirmed by the literature.
Keywords
P. Salicifolia Seed; P. Senegalensis Seed; Phenolic Acids; Flavonoid; Colon Cancer; Prostate Cancer
Download this article as:| Copy the following to cite this article: Youssef A. M. M, El-Swaify Z. A. S. Anti-Tumour Effect of two Persicaria Species Seeds on Colon and Prostate Cancers. Biomed Pharmacol J 2018;11(2). |
| Copy the following to cite this URL: Youssef A. M. M, El-Swaify Z. A. S. Anti-Tumour Effect of two Persicaria Species Seeds on Colon and Prostate Cancers. Biomed Pharmacol J 2018;11(2). Available from: http://biomedpharmajournal.org/?p=20453 |
Introduction
In Egypt, the Persicaria species (Polygonaceae) have been used traditionally since many years for the cure of various ailments. In Egypt, medicinal herbs P. salicifolia (Brouss ex Wild) Assenov and P. senegalensis (Meism) Sojak (Polygonaceae) are two of the seven species of the genus.1 They are perennial herbs commonly present along borders of watercourses of Nile Delta, shores of lakes, drains, and canals.2 The major groups of phytochemical compounds include; phenolics and flavonoids.1 Phenolics and flavonoids demonstrate pharmacological features in the form of antifungal, antibacterial, anti-inflammatory, and anti-tumour properties.
Persicaria species play a significant role in alternative medicines as they are used for treating various skin conditions (abscesses, boils, and scabies), colic pain, and inflammatory conditions (rheumatic pain, gout, knee pain, amenorrhea, and menstrual pain), since long. These species comprise of active biochemical ingredients, which possess anti-tumour, antioxidant, analgesic, antileukemic, antimicrobial, and tyrosinase inhibiting properties.3 Moreover, they are considered as traditional medicines to treat disorders; like dyspepsia, haemorrhoids, diarrhoea, and itchy skin.3 These species contain phenolic acids and flavonoid compounds and have shown potential therapeutic effects.4,6
Persicaria species are known to possess anti-tumour and antioxidant properties; however, the constituents and anti-tumour activity of seeds alone of two Persicaria species have not been studied before. Therefore, the phytochemical analysis and anti-tumour effects of both seeds have been investigated in the present study. The present study has mainly focused on the evaluation of the anti-tumour effects of active constituents, presented in P. salicifolia and P. senegalensis seeds against human cancer cell lines. The determination of anti-tumour effects of the two investigated Persicaria seeds has been performed for the first time in this study against two kinds of cancer cells.
Materials and Methods
Plant Material
Seeds of two selected plant species were collected from September 2016 to October 2016 from different localities of banks in Alexandria and Al- Mansoura, Egypt. The plants were identified according to the description reported by Boulos.1
Preparation of Seed Extract
The seeds were washed, dried, and powdered in shade. The dried powder of seeds of two investigated Persicaria were extracted as detailed by Moustafa et al.7 However, there was a slight alteration that was made in the seed extraction conducted in this study. The seeds under investigation (75g) were percolated in 450 ml methanol (50%), and then fully extracted by percolation at ambient temperature. Following this, the extracts underwent a filtration process using Whatman No.1 paper. A temperature of 40 °C was set out for the concentration process of these extracts. These extracts were dried using high vacuum. The extracts were kept in a refrigerator at 4°C until used for the experiment.
Extraction and Identification of Flavonoids
The total content of the flavonoids was ascertained via the utilization of the aluminium chloride colorimetric method.8 The results were then denoted in the form of milligram of catechin equivalents (CE). A spectrophotometer (Hewlett – Packarol, model 8452A, Rockville, USA) and the Folin-Ciocalteu method was used to establish total content of the polyphenols at 750 nm. The gallic acid equivalents (GAE) per gram DW was extracted from these results.9
Extraction and Identification of the phenolic acids
The phenolic acids were isolated from the seeds of two investigated Persicaria species10 and separated by reverse-phase HPLC instrument (Knauer, Germany). A model 7125 injection valve was used to supply the instrument (Rheodine, Cotati, CA, USA). The procedure of a 50 µl sample loop was handled using a computer (Knauer, HPLC version 211 a). It was seen that the flow rate was 1.0 ml / min. The detection of the phenolic acids was carried out via UV at 280 nm.
Separating and Identifying Lipid and Phenolic acids
The methanol extracts of the two investigated seeds (4g) were dissolved in water and defatted with n-hexane. The hexane extract was concentrated and analysed by Gas Chromatography-Mass Spectrometry (GC/MS) using Shimadzu GC/MS- QP 5050A software class 5000. [Column: DBI, 30 m, 0.53 mm ID, 1.5 µm film. Ionization mode: EL (70 eV). Carrier gas: Helium (flow rate 1 ml/min).]. The temperature program was set at 40 °C (static for 2 min), which was progressively increased (160°C at a rate of 2°C /min) to a level of 250°C (static for 7.5 min). Both the temperature of the injector and the temperature of the detector was set at 250 °C. The qualitative identification of hexane extract was achieved through library searched data Wiley 229 LIB.11
The defatted 50% methanol extract of two studied seeds was concentrated under reduced pressure till it dried completely. It was introduced to Liquid chromatography-mass spectrometry (LC/MS). An Agilent (Waldbronn, Germany) Model 1100 quaternary pump was used to conduct the LC analysis and to supply an autosampler and a diode-array detector (DAD). The analysis of the data obtained was carried out using a chemstation HP Rev.A.08.03. Furthermore, the analysis of the results obtained was carried out using a Luna C18 column (150 d 2.1 mm i.d., 5m) (Phenomenex, Torrance, CA, USA). The identification of the compounds’ structure was carried out via spectroscopic means. The UV spectral data of the separated compounds were measured through the inclusion of visible and ultraviolet absorption spectrometer (UV-VIS, Labomed Inc.). The ultraviolet and visible absorption spectrometer was in range of 200-500 nm.
MS analysis was performed by mass spectrometer, Water Corporation, Milford, and MA01757 U.S.A. Electrospray ionization (ESI-MS Positive) was utilized for the ionization of the analytes. MS source parameters were optimised to: ionization potential, 70 eV, ion source temperature 290°C, scan speed 200 amu/s, solvent delay 4.0 min, EV voltage 300 volts and scan range 30-600 amu was used. The process of ion acquisition was then conducted via the use of a XEVO TQD triple quadruple instrument [Column: ACQUITY UPLC-BEH C18 1.7um – 2.1x 50 mm. Flow rate: 0.2 ml/min, solvent system: consisted of A- water containing 0.1% Trifluoroacetic acid (TFA) and B- acetonitrile containing 0.1 % TFA]. Mobile phase gradient methods were optimised to allow maximum separation of analyte (Table 1)
Table 1: LC/MS mobile phase gradient methods
| Time (min) | % Mobile phase A | % Mobile phase B |
| 0 | 70 | 30 |
| 18 | 0 | 100 |
| 23 | 0 | 100 |
| 25 | 70 | 30 |
| 30 | 70 | 30 |
Cell viability assay
Cell lines
Human prostate carcinoma (PC3) in the epithelial cells was procured from the site of metastasis on the bone and the disease was a grade IV level adenocarcinoma. Additionally, the human colon carcinoma (CaCO-2) in the epithelial cells was procured from the colon and the disease was colorectal adenocarcinoma. The American type culture collection (ATCC, Rockville, MD) was approached to procure both PC3 and CaCO-2 cells.
Cell culture and MTT assay
The sterility of the procedure was maintained by conducting it in a sterile area via the use of a laminar air flow cabinet that was at a biosafety level of class II. The culture was maintained in Roswell Park Memorial Institute medium (RPMI 1640) that contained a 1% antibiotic-antimycotic mixture (10,000µg/ml streptomycin sulphate, 25µg/ml amphotericin B and 10,000U/ml potassium penicillin), 1% L-glutamine, and was augmented with a fetal bovine serum which was 10% heat-inactivated.7 Culturing and sub-culturing were performed according to Thabrew et al.12
To assess the cell viability, the mitochondrial dependent reduction of yellow MTT was used (3-(4,5- dimethylthiazol-2-yl– 2,5- diphenyl tetrazolium bromide). This yellow MTT undergoes a mitochondrial reduction to form purple formazan.13 The 96 well tissue culture plate were inoculated with 1X105 cells /ml (100 µl / well) and incubated at 37°C for 24 hours to develop a complete monolayer sheet. Growth medium was decanted from 96 well micro titer plates after the formation of confluent sheet of cells. Later, the cell monolayer was washed twice with wash media. Serial dilutions of both extracts of seeds and aerial parts of Persicaria salicifolia and P. senegalensis were prepared by dissolving each extract in dimethyl sulfoxide (DMSO) followed by dilution with RPMI–1640 medium to give a final concentration, 78.12, 156.25, 312.5, 625, 1250, 2500, 5000 and 10.000 µgml-1. 0.1 ml of each concentration was assayed in triplicate in different wells leaving 3 wells as control, receiving only maintenance medium (RPMI-1640) with 2% serum.The treated cells was incubated at 37°C for 24 hour and examined. Cells were checked for any physical signs of toxicity, e.g. partial or complete loss of the monolayer, rounding, shrinkage, or cell granulation. MTT solution at 5 mg /ml was dissolved in PBS (Bio Basic Canada Inc.) and 20 µl of it was added to each of the 96 wells. The solutions were placed on a shaker at 150 rpm for 5 minutes to mix the MTT into the media thoroughly and incubated at 37 °C (5 % CO2) for 4 hours to allow the MTT to be metabolized. The media was dump off and dry plate on paper towels was used to remove residue if necessary. The resuspended formazan (MTT metabolic product) in 200 µl DMSO was place on a shaker at 150 rpm for 5 minutes to thoroughly mix the formazan into the solvent. The optical density was recorded using a micro plate reader at 560 nm.13
Determination of IC50 values
GraphPad prism version 5 software, Inc., California, U.S.A was used to calculate IC50 values of extracts of seeds and aerial parts of two Persicaria species against PC3 and CaCO- 2 cell lines. The log concentrations of extracts were plotted along the horizontal axis and the cells viability percentages were plotted up the vertical axis. Therefore, the decrease of cells viability percentages over the increased of concentrations of extracts can be expressed by the general equation of a straight line (1)
Y = m. X + C …(1)
Thus
Y (the value plotted up the vertical axis) will be the cells viability percentages
m the gradient of the line.
X (the value plotted along the horizontal axis) will be the log concentrations of the extracts.
C the intercept on the vertical axis
So, IC50, the half maximal inhibitory concentration can be calculated from equation (2)
X (IC50) = (C-50)/m …(2)
Results
Through the phytochemical examination, a variety of phenolic acids were found in the seeds of two Persicaria species. The seeds constituents were analysed by LC/MS and revealed several phenolic acids and flavonoid compounds between the two studied Persicaria species (Table 2). The total phenolics of P. salicifolia were 46.3 mg GAE/g DW and flavonoids was 5.04 mg CE/g DW; while, the total phenolics of P. senegalensis were 37.6 mg GAE/g DW and flavonoids were 18.2 mg CE/g DW. The results have shown that the seeds of both Persicaria species contain five free phenolic acids; ferulic, caffeic, fumaric, coumaric and cinnamic. While, Gallic acid, chlorogenic acid and 3,4,5 methoxy cinnamic were only detected in seeds of P. senegalensis (Table 2). The phenolic acid of 50 % methanolic extracts of P. salicifolia and P. senegalensis have revealed 13 and 12 different flavonoid compounds, respectively. The analysis showed the presence of five flavonoids in both the seeds including; Luteolin (Figure 1), apigenin, kaempferol, quercetin, and rutin. Moreover, P. salicifolia seeds were characterized by the presence of a high amount of 2’-O-methylcajanone, which is a new isoflavonone separated for the first time from P. salicifolia seeds (Figure 2).
Table 2: The phenolic acids; the chemical constituents that were found in P. salicifolia and P. senegalensis seeds. LC/MS was used for detection and identification
| Phenolic acids | P. salicifolia | P. senegalensis |
| Ferulic | + | + |
| Caffeic | + | + |
| Fumaric | + | + |
| Coumaric | + | + |
| Gallic | – | + |
| Chlorogenic | – | + |
| 3,4,5 methoxy cinnamic acid | ||
| Flavonoids: | ||
| Luteolin (Figure 1) | + | + |
| (-)- Epicatechin | + | + |
| Luteo 6-glucose 8-arabinose | – | + |
| Apigenin | + | + |
| Apigenin 6-glucose 8-rhamnose | – | + |
| kaempferol | + | + |
| Kaempferol 3,7 dirhamnoside | – | + |
| Dehydrokaempferol | + | – |
| Quercetin | + | + |
| Rutin | + | + |
| Pentamethoxy quercetin | + | – |
| 2`-o- methylcajanone (Figure 2) | + | – |
| Coumarin | – | + |
| 2“,5“,4`,5,6“,7methoxy isovitrixin | + | – |
| Sciadpitysin | + | – |
| Diosmetin 7-o- glucoside | + | – |
| Narigenin | – | + |
| Hespertin | – | + |
| Hesperidin | – | + |
(+): Present; (-): Absent.
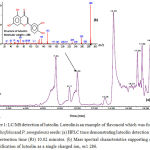 |
Figure 1: LC/MS detection of luteolin.
|
Luteolin is an example of flavonoid which was found in P. salicifolia and P. senegalensis seeds: (a) HPLC trace demonstrating luteolin detection at 280 nm, retention time (Rt) 10.02 minutes. (b) Mass spectral characteristics supporting correct identification of luteolin as a single charged ion, m/z 286.
 |
Figure 2: LC/MS detection of 2’-O-Methylcajanone.
|
2’-O-Methylcajanone is an example of flavonoid which was found in P. salicifolia Seeds: (a) HPLC trace demonstrating 2’-O-Methylcajanone detection at 280 nm, retention time (Rt) 14.25 minutes. (b) Mass spectral characteristics supporting correct identification of 2’-O-Methylcajanone as a single charged ion, m/z 436.
GC/MS was utilized to analyse polylipids of n-hexane extract of seeds that were obtained from the Persicaria species. As a result, P. salicifolia demonstrated 52 compounds; whereas, 29 compounds were found in P. senegalensis. Firstly, the major components of P. salicifolia were gamma-sitosterol (14.7 %), bis (2-ethylhexyl) phthalate (12.9 %), 9, 12-octadecadionoic acid, ethyl ester (9.7 %), hexadecanoic acid, ethyl ester (5.8 %), and ethyl oleate (1.9 %). Secondly, P. senegalensis contained hexadecanoic acid (33.3 %) (Figure 3), oleic acid (16.3 %), 1-[(2-aminoethoxy) hyroxyphosphinyl [oxy] methyl]-1, 2-ethanediylester (16.5 %), octadecanoic acid, 2, 3-dihydroxypropyl ester (6.25 %), and lucenin (3.6 %). While, other compounds recorded the lowest values in the two seeds of Persicaria species.
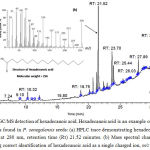 |
Figure 3: GC/MS detection of hexadecanoic acid.
|
Hexadecanoic acid is an example of polylipid which was found in P. senegalensis seeds: (a) HPLC trace demonstrating hexadecanoic acid detection at 280 nm, retention time (Rt) 21.52 minutes. (b) Mass spectral characteristics supporting correct identification of hexadecanoic acid as a single charged ion, m/z 256.
The use of the MTT assay analyse the anti-tumour activity of methanol extract of seeds and aerial parts of two persicaria species. Human colon carcinoma (Ca Co-2) and prostate carcinoma (PC 3) cell lines were used. IC50, the half maximal inhibitory concentration is a measure of a compounds effectivity used in hindering biochemical or biological activity and to estimate seeds extract cytotoxicity.
The results revealed that P. salicifolia seeds possessed anti-tumour activity against prostate carcinoma. In contrast, P. senegalensis seeds showed higher anti-tumour activity as compared to its aerial parts against CaCo-2 (Table 3) (Figure 4).
 |
Figure 4: Cytoxic effects of P. salicifolia and P. senegalensis seeds and aerial parts extracts against PC3 and CaCO-2 cell lines.
|
Table 3: Cytotoxicities of seeds and aerial parts of two persicaria species
| Species | Plant part used | IC50 (µg/ml) | |
| PC3 | CaCO-2 | ||
| P. salicifolia | Aerial parts | 1.1 ± 0.15 | 0.5 ± 0.011 |
| P. salicifolia | Seeds | 0.6 ± 0.018 | 1.0 ± 0.009 |
| P. senegalensis | Aerial parts | 2.3 ± 0.03 | 2.0 ± 0.03 |
| P. senegalensis | Seeds | 3.5 ± 0.06 | 1.5 ± 0.03 |
IC50: The half maximal inhibitory concentration; PC3: human prostate carcinoma; CaCO-2, human colon carcinoma
Discussion
Phytochemical analysis of both seeds of Persicaria species revealed several phenolic, flavonoid, and polylipid compounds that were present in their aerial parts. For example, phenolic acids found in the present study for seeds of the two Persicaria species were correlated to the aerial parts of four species of Polygonaceae (2 Periscaria + 2 Polygonum). These species included; apigenin, quercetin, and kaempferol.5 Luteolin, apigenin, kaempferol, quercetin, and rutin showed potential antioxidant, anti-tumour, and antihyperlipidemic effect.14,17 These five compounds were found in both seeds of Persicaria species in the present study (Table 1). Luteolin compound was present in high values that is 25.2% in seeds of p. senegalensis; while, 2’-O-methylcajanone (27.1 %) was present in P. salicifolia seeds. These two compounds showed anti-tumour activity18,19 The presence of anti-oxidative compounds in the form of gallic and caffeic acid was noted in the two Persicaria seeds. These compounds are noted to have potential anti-tumour activity20 Therefore, the identified compounds were confirmed by conducting a comparison of the data that was obtained and published in literature of aerial parts of Persicaria and Polygonum species.5,16,21,22
The cytotoxic activity against J82 (Bladder transitional carcinoma), HL60 (Human leukemia), P338 (Murine lymphocytic leukemia), LL2 (Lewis lung carcinoma) cancer cells, HepG2 (Hepatocellur carcinoma), and MCF7 (Human breast cancer) were analysed through the use of hexane fractions and chloroform with respect to Polygonum bistora sub-fractions. A moderate to very good activity against LL2 cancer cell lines, P338, and HL 60 were demonstrated through the hexane functions, sub-fractions and the chloroform.23 Through the methanol extract of Polygonum avicular, the apoptotic and cytotoxic influence on Hela-s cervical cell line was demonstrated.24 Reportedly, the n-butanol extract of Polygonum bellardii possesses the greatest cytotoxicity in HepG-2, Hela, and MCF-7 cells, with IC50 values of 30.09, 15.26, 50.66 µg/ml, respectively.25 In addition, myricetin –3-o–(5 acetyl – a – arabinofreranoside) demonstrated a notable cytotoxicity in HepG–2 (IC50 41.03 µg/ml) and Hela (IC50 75.04 µg/ml) cells.25 Another study conducted by Intisar et al. 26 demonstrated the anti-tumour activities of phenolic acids from Polygonum bistora L. that worked to resist the human hepatocellular carcinoma cell line (HCCLM3). They reported that eleven fractions demonstrated good to excellent levels of cytotoxicity, falling in the range of 200 µg/ml–800 µg/ml. However, the lack of any activity even at levels of 800 µg/ml were noted in two fractions and no anti-tumour component was detected in these. In the present study similar results were obtained for Persicaria species.
The crude methanol extract of P. glabrum demonstrated significant cytotoxic activity with highest lethality IC50 value 0.74 ± 0.045 µg/ml.27 The successive fraction of aqueous ethanol of P. salicifolia was conducted via the use of petroleum ether, methylene chloride, ethyl acetate, and n-butanol. The cell viability assays of these extractions were performed against MCF –7 and PC3 cell lines.21 As per the results, ethyl acetate and methylene chloride fractions were demonstrative of the greatest activity against breast carcinoma (IC50 6.01 µg/ml). Furthermore, the petroleum ether extract was demonstrative of the greatest activity against the prostate carcinoma (IC50 61.1 µg/ml) cell line.21
Conclusion
The results showed that there was a potential cytotoxicity of two Persicaria species seeds against two human cancer cell lines. Further investigations are recommended to identify the active principle responsible for anti-tumour activity of these plants. Therefore, seeds of P. salicifolia and P. senegalensis need to have a potential curative effect for prostate cancer and colon cancer, respectively.
Acknowledgement
Ahmed Mohamed Mohamed Youssef and Zeinab Ahmed Said El-Swaify designed the article, wrote this manuscript, participated in the literature search and conducted the data analysis.
Conflict of Interest
The research has no conflict of interest and is not funded through any source.
References
- Boulos L. Flora of Egypt, vol. 1. Cairo: Al Hadara Publishing. 1999:417.
- Shaltout KH, Galal TM, El-Komi TM. Biomass, nutrients and nutritive value of P. salicifolia Willd. in the watercourses of Nile Delta, Egypt. Rendiconti Lincei. 2014;1,25(2):167-79. doi: 10.1007/s12210-013-0269-6.
CrossRef - Khatun A, Imam MZ, Rana MS. Antinociceptive effect of methanol extract of leaves of Persicaria hydropiper in mice. BMC complementary and alternative medicine. 2015;13;15(1):63.
- Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, Kumar SS. Role of antioxidants and natural products in inflammation. Oxidative medicine and cellular longevity. 2016.
CrossRef - Hussein S, Usama EM, Tantawy M, Kawashty S, Saleh N. Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt. Arabian Journal of Chemistry. 2017;1;10(1):76-81. Doi: 10.1016/j.arabjc.2012.06.002.
CrossRef - Uddin MS, Nasrullah M, Hossain MS, Rahman MM, Sarwar MS, Amran MS, Sadik MG, Rashid M, Asaduzzaman M. Evaluation of nootropic activity of Persicaria flaccida on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: Implication for the management of Alzheimer’s disease. American Journal of Psychiatry and Neuroscience. 2016;4:26-37.
CrossRef - Moustafa SM, Menshawi BM, Wassel GM, Mahmoud K, Mounier MM. Screening of some plants in Egypt for their cytotoxicity against four human cancer cell lines. Int J Pharm Tech Res. 2014;6(3):1074-84.
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. Journal of agricultural and food chemistry. 2002;8;50(10):2926-30. Doi: 10.1021/jf0111209.
CrossRef - Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in enzymology. 1999;31;299:152-78. Doi: 10.1016/s0076-6879(99)99017-1.
CrossRef - Smolarz HD. Chromatographical analysis of phenolic acids in some species of Polygonum L. genus. Quantitative determination of the major components by high performance liquid chromatography (HPLC). Acta Societatis Botanicorum Poloniae. 2000;69(1):21-3. Doi: 10.5586/asbp.2000.003.
CrossRef - Adams RP. Identification of essential oils by ion trap mass spectroscopy. Academic Press. 2012.
- Thabrew M, Hughes RD, Mcfarlane IG. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. Journal of pharmacy and pharmacology. 1997;1;49(11):1132-5. Doi: 1111/j.2042-7158.1997.tb06055.x.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;16;65(1-2):55-63. Doi: 1016/0022-1759(83)90303-4.
- Bojic M, Debeljak Z, Tomicic M, Medic-Saric M, Tomic S. Evaluation of antiaggregatory activity of flavonoid aglycone series. Nutrition journal. 2011;11;10(1):73. Doi: 1186/1475-2891-10-73.
- Kim HJ, Woo ER, Park H. A novel lignan and flavonoids from Polygonum aviculare. Journal of natural products. 1994;57(5):581-6. Doi: 10.1021/np50107a003.
CrossRef - Shuli S, Wenli H, Bo L. Recent Advance on Anti-tumour Role of Polygonaceae. Progress in Modern Biomedicin. 2008;8:2191-2200.
- Urones JG, Marcos IS, Perez BG, Barcala PB. Flavonoids from Polygonum minus. Phytochemistry. 1990;1;29(11):3687-9. Doi: 10.1016/0031-9422(90)85309-4.
CrossRef - Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Current cancer drug targets. 2008;1;8(7):634-46. Doi: 10.2174/156800908786241050.
CrossRef - Nix A, Paull CA, Colgrave M. The flavonoid profile of pigeonpea, Cajanus cajan: a review. SpringerPlus. 2015;13;4(1):125. Doi: 1186/s40064-015-0906-x.
- Jang HG, Heo BG, Park YS, Namiesnik J, Barasch D, Katrich E, Vearasilp K, Trakhtenberg S, Gorinstein S. Chemical composition, antioxidant and anti-tumour effects of the seeds and leaves of indigo (Polygonum tinctorium Ait.) plant. Applied biochemistry and biotechnology. 2012;1;167(7):1986-2004. Doi: /10.1007/s12010-012-9723-7.
- El-Anwar R.M, Ibrahim A.R.S, Abo El-Seoud K.A, Kabbash A.M. Phytochemical and biological studies on salicifolia brouss. Ex willd growing in Egypt. International research journal of pharmacy. 2016;7:4-12. Doi: 10.7897/2230-8407.07889.
CrossRef - Hussein SR, Mohamed AA. Antioxidant activity and phenolic profiling of two Egyptian medicinal herbs Polygonum salicifolium Brouss ex Wild and Polygonum senegalense Meisn. Analele Universităţii din Oradea. Fascicula Biologie Tom. 2013;1;20(2):59-63.
- Pillai Manoharan K, Yang D, Hsu A, Tan Kwong Huat B. Evaluation of Polygonum bistorta for anti-tumour potential using selected cancer cell lines. Medicinal chemistry. 2007;1;3(2):121-6.
- Mohammad R, Hossein B, Davood F, Farnaz T, Ali F, Yusef R. The apoptotic and cytotoxic effects of Polygonum avicular extract on Hela-S cervical cancer cell line. African Journal of Biochemistry Research. 2011;31;5(14):373-8. Doi: 10.5897/ajbr11.095.
CrossRef - Abd El-kader AM, El-Readi MZ, Ahmed AS, Nafady AM, Wink M, Ibraheim ZZ. Polyphenols from aerial parts of Polygonum bellardii and their biological activities. Pharmaceutical biology. 2013;1;51(8):1026-34.
- Intisar A, Zhang L, Luo H, Kiazolu JB, Zhang R, Zhang W. Anti-tumour constituents and cytotoxic activity of methanol-water extract of Polygonum bistorta L. African Journal of Traditional, Complementary and Alternative Medicines. 2013;10(1):53-9.
- Khan MF, Rabbi SN, Aktar F, Kawsar MH. In vitro Cytotoxic, membrane stabilizing and thrombolytic activities of Polygonum glabrum Willd. Bangladesh Pharmaceutical Journal. 2015;21;17(2):202-4. Doi: 10.3329/bpj.v17i2.22341.
CrossRef







