Gehan A. Hegazy1,2 , Hesham N. Mustafa3
, Hesham N. Mustafa3 , Rawan M. Altalhi4 and Jehad M. Yousef4
, Rawan M. Altalhi4 and Jehad M. Yousef4
1Clinical Biochemistry Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
2Medical Biochemistry Department, National Research Centre, Cairo, Egypt.
3Anatomy Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
4Biochemistry Department, Faculty of Science-AlFaisaliah, King Abdulaziz University, Jeddah, Saudi Arabia.
Corresponding Author E-mail: hesham977@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1373
Abstract
Renal ischemia/reperfusion injury (IRI) represents the main reason for acute kidney injury (AKI). Dexmedetomidine (Dex) and Benincasa cerifera (BC) have wide benefits due to their anti-inflammatory and antioxidant properties. This study aims to illustrate the protective effects of BC and Dex on renal IRI in a diabetic model. Sixty adult male albino rats (Wistar strain), weighing 250–300 g, were included in the study. The rats were divided into four groups, as follows: sham group: (non-diabetic); diabetes mellitus (DM) + IRI group: streptozotocin (STZ)-induced diabetic rats exposed to renal IRI on day 30 after diagnosis of diabetes; DM + IRI + BC group: STZ-induced diabetic rats treated with BC (500 mg/kg) for 30 days after diagnosis of diabetes, then exposed to renal IRI; and DM + IRI + Dex group: STZ-induced diabetic rats treated with Dex (100 µg/kg intraperitoneally) 5 min before induction of ischemia on day 30 after diagnosis of diabetes, then exposed to renal IRI. Biochemical parameters, histopathological examination, and immunohistochemical markers were evaluated. A significant improvement in the biochemical, histopathological, and immunohistochemical parameters were observed in the DM + IRI + BC group, while the DM + IRI + Dex group showed improvements in renal IRI and dyslipidemia. The present study demonstrated that oxidative stress plays a chief role in renal IRI in the STZ-induced diabetic model. Treatment with BC achieved excellent ameliorative effects, while treatment with DEX improved renal IRI.
Keywords
Diabetes; Dexmedetomidine; Ischemia/Reperfusion; Oxidative Stress
Download this article as:| Copy the following to cite this article: Hegazy G. A, Mustafa H. N, Altalhi R. M, Yousef J. M. The Ameliorative Potential of Dexmedetomidine and Benincasa Cerifera Extract in Renal Ischemia/Reperfusion Injury in A Streptozotocin-Induced Diabetic Model. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Hegazy G. A, Mustafa H. N, Altalhi R. M, Yousef J. M. The Ameliorative Potential of Dexmedetomidine and Benincasa Cerifera Extract in Renal Ischemia/Reperfusion Injury in A Streptozotocin-Induced Diabetic Model. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19533 |
Introduction
Diabetes mellitus (DM) is one of the most common diseases, with a high prevalence and incidence worldwide. 1 The International Diabetes Federation (IDF) reported that the prevalence of DM was 285 million people in 2010; this is expected to increase by 50%, reaching up to 439 million, in 20302 Chronic kidney disease is a common consequence of DM that may lead to end-stage renal failure. Previous experimental trials have concluded that DM increases the kidneys’ vulnerability to acute kidney injury (AKI), including renal ischemia/reperfusion injury (IRI).3
Renal IRI represents the main factor accounting for AKI; other causes include ureteral obstruction and kidney ischemia.4 Renal IRI occurs spontaneously in many clinical situations, such as renal transplantation, partial nephrectomy, renal artery angioplasty, and ureteral obstruction. IRI is responsible for 30% of the total cases of delayed graft dysfunction after renal transplantation.5 Multiple theories have been developed to explain the tissue damage occurring due to IRI, including hypoxic injury, inflammatory processes, and oxidative stress (OS) injury. All these mechanisms result in the insufficiency of both the renal endothelial and tubular cells and mitochondrial dysfunction, ending in tissue damage and necrosis.6
Dexmedetomidine (Dex) is a vastly selective and effective α2-adrenergic agonist used as a sedative, anxiolytic, analgesic, antioxidant, and anti-inflammatory agent. Dex is used for alleviating IRI conditions in several organs, including the heart, brain, and kidney.7
Benincasa cerifera (BC) Savi is a member of Cucurbitaceae family that has high biological value and is used in herbal medicine. It is mainly cultivated in East Asia, and its fruit is nutritive, acting as an immunopotentiator, diuretic, vasoconstrictor, and anti-anthelmintic. Moreover, it has been used in the treatment of many diseases, as it has anti-inflammatory, antipyretic, and antioxidant properties.8 Phytochemically, BC contains lupeol and p-sitosterol and their acetates, as well as cucurbitin, cucurbitacin-B, isovitexin, rhamnose, alkali, fat, glucose, adenine, alnusenol, trigonelline, histidine, n-triaconatol, vitamin-B, pectic polysaccharide, mannitol, and amino acids.8
IRI is a common condition that has serious effects; thus, it is important to find components that can ameliorate these side effects. Thus, the present work aims to evaluate the beneficial effects of BC extract and Dex on renal IRI in STZ-induced diabetes.
Materials and Methods
This animal experimental study was conducted in King Fahd Research Center, King Abdulaziz University (KAU), Jeddah, Saudi Arabia. The study protocol was approved by the Hospital Biomedical and Research Ethics Committee, Faculty of Medicine, King Abdulaziz University, Saudi Arabia, and the procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
Drugs and Plants
BC was obtained from Taif City, Saudi Arabia. Dex was obtained from Hospira, Inc. (Lake Forest, USA). Furthermore, ketamine was obtained from King Abdulaziz University (Jeddah, Saudi Arabia). Finally, streptozotocin (STZ) was obtained from Sigma-Aldrich Chemicals (St. Louis, MO, USA).
Experimental Animals
Sixty adult male albino rats (Wistar strain), weighing 250–300 g, were obtained from the Experimental Animal Care Center of King Fahad Medical Research Center, King Abdulaziz University. Animals were kept in special cages at 20–22°C and humidity (60%) under 12-h dark and light cycles. Rats were supplied with standard pellet chow with free access to tap water for 2 days for acclimatization before the experiment.
Methods
Collection and Preparation of Benincasa cerifera
BC was collected from Taif City in the summer season. The freshly peeled rind was cut into small pieces and dried in a freeze-dryer device (IlShin Bio Base, Korea), then crushed using an electrical blender (Waring Commercial, USA) at King Fahd Center of Medical Research. The resulting powder was then extracted by dissolution with 70% v/v ethanol; the mixed solution was shaken for 24 h. The mixture was evaporated and dried using a gas-dryer device (Hahnvapor, Korea). Following this, the evaporated solution was placed in the first freeze-dryer device (IlShin Bio Base, korea) to evaporate the water and obtain a solid powder form of the extracted plant.9 The extracted powder was dissolved in water and administered orally in a dose of 500 mg/kg/day per group [3] for 30 days after diagnosis of STZ-induced DM via fasting blood glucose assessment.10
Dexmedetomidine
Dex was administered as single dose (100 µg/kg intraperitoneally [i.p.]) 5 min before renal IRI induction.11
Induction of Type 2 Diabetes Mellitus
Hyperglycemia was induced by a single i.p. injection of STZ at a dose of 55 mg/kg 12 into animals deprived of food for 18 h. STZ was dissolved in 0.1 M citrate buffer (pH 4.5) just prior to injection. Seventy-two hours later, hyperglycemia was confirmed by determining the blood glucose level using an Accu-check glucose meter (Roche Diagnostics, Basel, Switzerland). Blood samples were withdrawn from the retro-orbital venous plexus under light ether anesthesia. Rats with blood glucose levels less than 20 mmol/L were excluded from the study. The day on which hyperglycemia was confirmed was designated as day zero (72 h after STZ injection and confirmation of hyperglycemia).12
Induction of Renal Ischaemia Reperfusion Injury
The diabetic rats were anesthetized with ketamine (60 mg/kg, i.p.) 13 and then a midline laparotomy was performed, and the abdominal cavity was fully exposed. Body temperature was maintained at 37°C ± 0.5°C throughout the surgery. Bilateral renal pedicles were carefully isolated without damaging the ureter and clamped using nontraumatic microvascular clamps to result in complete cessation of the renal arterial blood flow. After 1 h, the clamps were removed to allow the return of blood flow to the kidneys, and the kidneys were observed while they underwent reperfusion for 1 h.14-16
Experimental Design
The rats were divided into four main groups, as follows:
Group 1: Sham
Sham non-diabetic rats were used as the negative control group, which was administered a single dose of saline daily for 30 days;
Group 2: DM + IRI
STZ-induced diabetic rats exposed to renal IRI on days 30 after diagnosis of diabetes were used as the positive control group;
Group 3: DM + IRI + BC
STZ-induced diabetic rats treated with BC for 30 days after diagnosis of diabetes, then exposed to renal IRI; and
Group 4: DM + IRI + Dex
STZ-induced diabetic rats treated with Dex 5 min before induction of ischemia on day 30 after diagnosis of diabetes, then exposed to renal IRI.
Physical Parameters
The body and kidney weights of each rat were measured.
Preparation of Blood Samples and Serum
After 30 days of experimentation, the animals were fasted overnight (12–14 h), and 1 h after reperfusion initiation, blood samples were collected by eye puncture of the anesthetized rats. The blood sample obtained from each rat was divided into three tubes, one containing EDTA and mixed well for estimation of hemoglobin Alc (HbAlc), the second for glucose estimation, and the third for other biochemical markers. Following this, the samples from the second and third tubes centrifuged at 3,000 rpm for 20 min to obtain the serum and stored in a clean Eppendorf tube for biochemical marker measurement.
Tissue Sampling
At the end of the experiment, the rats were sacrificed; the kidneys were obtained and perfused with phosphate-buffered saline (PBS) to remove all blood. The right kidney was kept in 10% neutral buffered formalin for histopathological studies, while the left kidney was stored at –80°C until the assay. Tissue homogenates were prepared in ice-cold phosphate-buffer (pH 7.5). Homogenates were centrifuged at 12,000 g for 10 min, and the supernatants were used for further biochemical analysis.
Biochemical Investigations
Estimation of Fasting Serum Glucose, Insulin, and HbAlc
Serum glucose was quantitated via the glucose oxidase peroxidase method using the rat glucose assay kit (Cat. No. 81693, Crystal Chem Inc., Spain). Serum insulin was determined using an ultra-sensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Cat. No. 90060, Crystal Chem Inc, Spain) following the manufacturer’s instructions. In addition, HbAlc was measured using a commercial kit (Cat. No. 80300, Crystal Chem Inc. Spain).
Assessment of Renal Function
The serum creatinine (Creat) level was measured via the enzymatic colorimetric method using kits purchased from Crystal Chem (Cat. No. 80340, Crystal Chem Inc. Spain). Serum blood urea nitrogen (BUN) levels were measured using a rat BUN ELISA kit (Cat. No. MBS752119, My BioSource, Inc., San Diego, CA, USA) following the manufacturer’s instructions.
Assessment of Lipid Profile
Total cholesterol (TC) serum levels were measured using the rat TC ELISA kit (Cat. No. MBS752119, My BioSource, Inc., San Diego, CA, USA) following the manufacturer’s instructions. Serum triglyceride levels were determined, using a commercially available kit (Cat. ab65336; Abcam, Cambridge, UK). High-density lipoprotein (HDL) and low-density lipoprotein (LDL)–very LDL (VLDL) serum levels were measured via the fluorometric method using a commercially available kit (Cat. ab65390; Abcam, Cambrige, UK) following the manufacturer’s instructions.
Assessment of Serum Lactate Dehydrogenase
Rat lactate dehydrogenase (LDH) was measured using an ELISA kit (Cat. No. MBS043166, My BioSource, Inc., San Diego, CA, USA) following the manufacturer’s instructions.
Assessment of Oxidative Stress Markers in Serum and Tissue Homogenate
Reduced glutathione (GSH) levels in serum and tissue homogenate were measured using the rat reduced GSH ELISA kit (Cat. No. MBS046356, My BioSource, Inc., San Diego, CA, USA) following the manufacturer’s instructions. Malondialdehyde levels in serum and tissue homogenate were estimated using the rat malondialdehyde ELISA kit (Cat. No. MBS738685, My BioSource, Inc., San Diego, CA, USA) following the manufacturer’s instructions.
Myeloperoxidase (MPO) levels were measured using an ELISA kit (Cat. No. CSB-E08722r, Cusabio Biotech Co. Ltd., Wuhan, Hubei Province, China) following the manufacturer’s instructions. Plasma DNA oxidation levels were determined using the rat 8-hydroxy-deoxyguanosine (8-OHdG) ELISA kit (Cat. No. CSBE10526r, Cusabio Biotech Co. Ltd.) in accordance with the manufacturer’s instructions.
Histopathological Examination
The kidneys were dehydrated with a graded series of alcohol, cleared in xylene, and embedded in paraffin wax. Tissues were then cut into sections of 3–5 µm in thickness using a microtome and stained with hematoxylin and eosin (H&E) for general observations, periodic acid-Schiff (PAS) for observation of glycogen, and Masson’s trichrome (MT) for distinguishing collagen. For each specimen, at least three to five slides were examined using an Olympus BX53 microscope equipped with a DP73 camera (Olympus, Tokyo, Japan).12
Immunohistochemical Detection
Using the streptavidin-biotin-peroxidase technique, the endogenous peroxidase activity was eliminated using 10% H2O2 for 15 min. Sections were then incubated for 1 h with the primary antibody against Bcl2-associated X (BAX) protein, a monoclonal antibody (Dako, Carpinteria, CA, USA; dilution: 1:200; cytoplasmic cellular site), as a marker for apoptotic death. They were similarly incubated with the primary antibody against tumor necrosis factor alpha (TNF-α), a mouse monoclonal antibody (Dako; 5–10 µg/ml; cytoplasmic cellular site), as a marker for inflammatory cytokines. Sections were incubated for 20 min in 3,30-diaminobenzidine (DAB) chromogen and counterstained with Mayer’s hematoxylin. Negative control sections were prepared by omitting the primary antibody. Positive control standard laboratory slides were used for all stains to demonstrate the technique’s success. All the slides were examined under light microscopy, and the presence of labeled cells was documented. Absence of staining was recognized as a negative result (−), while the presence of brown staining was recognized as a positive result (+).17
Semi-Quantitative Morphometric Measurements
A minimum of ten non-overlapping fields for each animal were selected indiscriminately and histopathologically analyzed by a pathologist who was blind to the treatment given. The measurements were performed using Image-Pro Plus v6.0 (Media Cybernetics, MD, USA) and NIH ImageJ (v1.50; http://rsb.info.nih.gov/ij/) with an Olympus BX53 microscope. The renal cortex and outer medullary regions from control and other groups were examined for apoptosis (pyknotic nuclei), Erythrocyte extravasation, dilatation of Bowman’s capsule, tubular swelling, tubular dilatation, congestion, tubular cell degeneration, tubular necrosis, tubular hyaline casts and lymphocytic infiltration. Changes were scored as follows: no damage (–), mild (+), moderate (++), and severe (+++) damage.18-23 Reaction to PAS was evaluated and scored semiquantitatively as follows: none (–), mild (+), moderate (++), and severe (+++). In addition, apoptotic cells (BAX-positive cells) were counted in an area of 20,000 µm2 and selected randomly in the Bax-protein-stained sections at ×400 magnification. The optical density (OD) of TNF-α immunopositive cells was evaluated.24
Statistical Analysis
Quantitative data were expressed as the mean ± standard deviation (SD) of different parameters for the treated groups. The data were analyzed using one-way analysis of variance (ANOVA), followed by the LSD post hoc test. The statistical analysis was performed using SPSS version 23. The values were considered significant when p < 0.05.25
Results
General Toxicity
No death or visible signs of toxicity were observed in any group during the experiment.
Effects of BC and Dex on the Physical Parameters
Effect on Body and Kidney Weight
There was a significant decrease in total body weight in all the study groups compared with the sham group. Kidney weight exhibited a significant increase in the DM + IRI group compared with the sham group, while treatment with BC and Dex improved the kidney weight (Table 1).
Table 1: Effect of BC and Dexmedetomidine on body weight, Kidney
| Groups | Sham
N=15 |
DM+IRI
N=15 |
DM+IRI+BC
N=15 |
DM+IRI+Dex
N=15 |
| Body weight (g) | 417.00±21.93 | 264.66±4.16
1P= 0.000 |
318.66±1.52
1P= 0.000 2P= 0.000 |
268.66±1.52
1P= 0.000 2P= 0.674 |
| Kidney weight (g) | 1.34± 0.04 | 1.5±0.08
1P= 0.001 |
1.38±0.00
1P= 0.061 2P= 0.001 |
1.41±0.02
1P= 0.001 2P= 0.001 |
Values are Mean± SD: standard deviation. Comparison between groups done by ANOVA followed by LSD post hoc test. 1P: compared to Sham group. 2P: compared to DM+IRI groups.
Effect of BC and Dex on the Serum Levels of Different Biochemical Parameters
Diabetic Indices
This study indicated a significant increase in the serum levels of fasting glucose and glycosylated hemoglobin, as well as a significant decrease in the serum levels of insulin, in all studied groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex). The DM + IRI + BC group showed an improved diabetic condition, as indicated by the significant decrease in the serum levels of fasting blood glucose and glycosylated hemoglobin and increase in serum insulin levels (Table 2).
Table 2: Effect of BC and Dex on serum biochemical parameters:
| Groups | Sham
N=15 |
DM+IRI
N=15 |
DM+IRI+BC
N=15 |
DM+IRI+Dex
N=15 |
| Glucose (GLU)
(mmol L) |
5.80± 0.30 | 60.46±0.40
1P= 0.000 |
11.33±0.51
1P= 0.000 2P= 0.000 |
60.50±0.79
1P= 0.000 2P= 0.941 |
| Insulin (INS)
(mU/mL) |
18.00±0.100 | 12.9000±0.100
1P= 0.000 |
16.7000±0.100
1P= 0.000 2P= 0.000 |
13.033±0.15275
1P= 0.000 2P= 0.195 |
| Glycosylated Hemoglobin (HbA1c)
(%) |
5.33±0.83 | 12.00±9.07
1P= 0.000 |
7.3333±.0.90738
1P= .033 2P= 0.000 |
11.1667±1.04083
1P= 0.000 2P= 0.313 |
| Creatinine (Creat)
(mmole/L) |
30.66±1.52 | 120.00±1.00
1P= 0.000 |
59.00±1.00
1P= 0.000 2P= 0.000 |
100. 00±1.00
1P= 0.000 2P= 0.000 |
| Blood urea Nitrogen (BUN)
(mmole/L) |
5.86±0.25 | 25.56±0.92
1P= 0.000 |
9.03±0.152
1P= 0.000 2P= 0.000 |
23.23±0.65
1P= 0.000 2P= 0.001 |
| Lactate Dehydrogenase
(LDH) (U/L) |
217.00±1.00 | 1492.6±6.42
1P= 0.000 |
406.66±6.11
1P= 0.000 2P= 0.000 |
1286.6±5.85
1P= 0.000 2P= 0.000 |
| Triglycerides (TG)
(mmole/L)
|
1.35±0.05 | 7.50±0.26
1P= 0.000 |
2.03±0.05
1P= 0.001 2P= 0.000 |
6.23±0.15
1P= 0.000 2P= 0.000 |
| Cholesterol
(Chol) (mmole/L) |
1.93±0.15 | 8.06±0.15
1P= 0.000 |
3.20±0.10
1P= 0.000 2P= 0.000 |
7.00±0.20
1P= 0.000 2P= 0.000 |
| Low density lipoprotein (LDL) (mmole/L) | 0.90±0.10 | 5.10±0.10
1P= 0.000 |
1.40±0.10
1P= 0.000 2P= 0.000 |
4.40±0.10
1P= 0.000 2P= 0.000 |
| High Density Lipoprotein
(HDL) (mmole/L) |
1.50±0.10 | 0.43±0.05
1P= 0.000 |
1.36±0.05
1P= 0.045 2P= 0.000 |
0.43±0.05
1P= 0.000 2P= 1.000 |
Values are Mean± SD: standard deviation. Comparison between groups done by ANOVA followed by LSD post hoc test. 1 P: compared to sham group.2 P: compared to DM+IRI.
Renal Functions and Serum Lactate Dehydrogenase
The results of this study showed significant elevation in serum Creat, BUN, and LDH in all groups (DM + IRI, DM + IRI + BC and DM + IRI + Dex). DM + IRI + BC and DM + IRI + Dex showed significant reductions in serum Creat, BUN, and LDH compared with the untreated group (DM + IRI; Table 2).
Lipid Profile
There were significant elevations in the serum levels of triglycerides, cholesterol, and LDL in all the study groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex). Treatment with BC (DM + IRI + BC) and Dex (DM + IRI + Dex) resulted in significant reductions in the serum levels of triglycerides, cholesterol, and LDL when compared with the untreated group DM + IRI (Table 2). Moreover, there was a significant decrease in serum levels of HDL cholesterol (HDL-C) in all the study groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex). Treatment with BC (DM + IRI + BC) resulted in a significant increase in the serum levels of HDL-C when compared with the untreated group (DM + IRI; Table 2). No significant difference regarding serum HDL-C levels were detected between the untreated group (DM + IRI) and Dex (DM + IRI + Dex).
Effects of BC and Dex on Oxidative Stress Markers in Serum and Renal Tissue Homogenate
Lipid Peroxidation
There was a significant elevation in the serum levels of MDA and renal tissue homogenate MDA in all groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex). Treatment with BC (DM + IRI + BC) resulted in significant reductions in serum MDA levels, while treatment with Dex (DM + IRI + Dex) did not induce a significant difference (Table 3). Treatment with BC (DM + IRI + BC) and Dex (DM + IRI + Dex) resulted in significant reductions of MDA in renal tissue homogenate (Table 4).
8-Hydroxy-Deoxyguanosine
There were significant elevations of the serum 8-OHdG levels in all groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex). Treatment with BC (DM + IRI + BC) and Dex (DM + IRI + Dex) resulted in significant reductions in the serum levels of 8-OHdG in the untreated group (DM + IRI; Table 3). The renal tissue homogenate levels of 8-OHdG showed a significant increase compared with the untreated group (DM + IRI). Treatment with BC (DM +BC + IR) and Dex (DM+ Dex + IR) resulted in significant reductions in the renal tissue homogenate levels of 8-OHdG (Table 4).
Myeloperoxidase Activity
There was a significant elevation in the serum levels and renal tissue homogenate of MPO in all groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex; Tables 3 and 4). Treatment with BC (DM + IRI + BC) and Dex (DM + IRI + Dex) resulted in significant reductions of serum and renal tissue homogenate levels of MPO when compared with the untreated group (DM + IRI; Tables 3 and 4).
Table 3: Effect of BC and Dex on oxidative stress markers in serum.
| Groups | Sham
N=15 |
DM+IRI
N=15 |
DM+IRI+BC
N=15 |
DM+IRI+Dex
N=15 |
| Malondialdhyde (MDA)
(nmole /mL) |
1.70±0.01 | 4.14±0.15
1P= 0.000 |
2.99±0.01
1P= 0.000 2P= 0.000 |
4.09±0.17
1P= 0.000 2P= 0.585 |
| 8- Hydroxy-Desoxyguanosine (8-OHdG)
(ng/mL) |
0.19±0.01 | 0.88±0.01
1P= 0.000 |
0.31±0.01
1P= 0.000 2P= 0.000 |
0.83±0.01
1P= 0.000 2P= 0.000 |
| Myeloperoxidase (MPO)
(ng/mL) |
11.30±0.55 | 41.70±0.75
1P= 0.000 |
13.86±1.73
1P= 0.014 2P= 0.000 |
16.36±0.45
1P= 0.000 2P= 0.002 |
| Glutathione
(GSH) (micromole/L) |
19.20±0.20 | 4.73±0.25
1P= 0.000 |
14.96±0.20
1P= 0.000 2P= 0.000 |
5.76±0.25
1P= 0.000 2P= 0.001 |
Values are Mean± SD: standard deviation. Comparison between groups done by ANOVA followed by LSD post hoc test. 1 P: compared to Sham group. 2 P: compared to DM+IRI.
Table 4: Effect of BC and Dex on oxidative stress markers in renal tissue homogenate.
| Groups | Sham
N=15 |
DM+IRI
N=15 |
DM+IRI+BC
N=15 |
DM+IRI+Dex
N=15 |
| Malondialdhyde (MDA)
(Nano mole /gm renal tissue) |
3.5033±.05508 | 11.2420±0.17880
1P= 0.000 |
7.9471±0.32443
1P= 0.000 2P= 0.000 |
7.6667±0.34512
1P= 0.000 2P= 0.000 |
| 8- Hydroxy-Desoxyguanosine (8-OHdG)
(ng/mg renal tissue) |
8.1000±0.72111 | 20.5200±2.85517
1P= 0.000 |
10.3857±2.51690
1P= 0.200 2P= 0.000 |
8.9000±2.58147
1P= 0.655 2P= 0.000 |
| Myeloperoxidase (MPO)
(ng/mg renal tissue) |
3.5667±0.41633 | 19.0600±1.35757
1P= 0.000 |
9.1429±1.47970
1P= 0.000 2P= 0.000 |
5.8333±1.63666
1P= 0.037 2P= 0.000 |
| Glutathione
(GSH) (micromole/gm renal tissue) |
15.9667±1.30512 | 1.3000±0.59582
1P= 0.000 |
15.4143±1.57208
1P= 0.539 2P= 0.000 |
14.4667±1.57208
1P= 0.115 2P= 0.000 |
Values are Mean± SD: standard deviation. Comparison between groups done by ANOVA followed by LSD post hoc test. 1P: compared to Sham group. 2 P: compared to DM+IRI.
Reduced Glutathione
There were significant reductions in serum GSH in all groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex). Treatment with BC (DM + IRI + BC) and Dex (DM + IRI + Dex) resulted in significant increases in serum GSH when compared with the untreated group (DM + IRI; Table 3). Concerning renal tissue homogenate, GSH showed a significant decrease in the untreated group (DM + IRI). Treatment with BC (DM + IRI + BC) and Dex (DM + IRI + Dex) resulted in significant increase of renal tissue homogenate levels of GSH when compared with the untreated group (DM + IRI; Table 4).
Histopathological Examination
Histological Results
The normal histological architecture was demonstrated in the sham group (Fig. 1A). The DM + IRI group showed varying degrees of deterioration in the histological architecture, including degeneration and necrosis of the proximal tubule epithelial cells that poured into the lumen; severe erythrocyte extravasation surrounding the glomeruli and the tubules and accumulation of proteinaceous material at tubules; vacuolar degeneration of the tubules; and dilation of some tubules, with distorted cellular boundaries (Fig. 1B). The DM + IRI + BC group displayed minimal histological alterations of the glomeruli and tubules (Fig. 1C). The DM + IRI + Dex group revealed a slight degeneration of the tubules, mild erythrocyte extravasation, and reduced appearance of proteinaceous material at the tubules (Fig. 1D; Table 5).
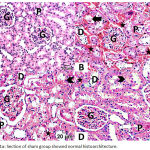 |
Figure 1a: Section of sham group showed normal histoarchitecture.
|
Note glomerulus (G), proximal tubule (P), distal tubule (D). Fig 1(B). Section of DM+IRI group showed degeneration and necrosis of proximal tubule epithelial cells (white-arrow) that poured into the lumen (black-arrow), severe erythrocyte extravasation surrounding the glomeruli and the tubules (star) and accumulation of proteinaceous material in the tubules (arrowhead) vacuolar degeneration of the tubules, and some tubules are dilated with distorted cellular boundaries. Fig 1(C). Sections of DM+IRI+BC showed reduced pathological findings with minimal degeneration of the tubules (white-arrow), minimal erythrocyte extravasation (star) and no apparent proteinaceus material (arrowhead). Fig 1(D). Sections of DM+IRI+Dex showed slight degeneration of the tubules (white-arrow) and mild erythrocyte extravasation (star) and reduced appearance of proteinaceus material at tubules (arrowhead) (H&E, Scale bar 20 µm).
Table 5: Histopathologic assessment of Renal Cortex and outer medullary regions of the different groups.
| Groups | Sham | DM+IRI | DM+IRI+BC | DM+IRI+Dex |
| Apoptosis (pyknotic nuclei) | — | ++ | + | + |
| Erythrocyte extravasation | — | +++ | + | + |
| dilatation of Bowman’s capsule | — | +++ | + | ++ |
| Tubular cell swelling | — | +++ | + | + |
| Tubular dilatation | — | +++ | ++ | ++ |
| Congestion | — | +++ | ++ | + |
| Tubular cell degeneration | ||||
| Tubular Necrosis | — | +++ | + | + |
| Tubular hyaline casts | — | +++ | + | ++ |
| Lymphocytic infiltration | — | ++ | + | + |
Note: None (–); mild (+), moderate (++), severe (+++). N= (15)
Histological Findings for Masson’s Trichrome
The sham group showed minimal collagen fiber deposition (Fig. 2A). The DM + IRI group revealed an apparently thickened parietal layer of the Bowman’s capsule; proximal and distal tubules with some collagen fiber condensation; obvious blood congestion; and a vacant area between some tubular cells and basal lamina (Fig. 2B). The DM + IRI + BC group displayed mild collagen deposition in the glomeruli surrounding the Bowman’s capsules and near the convoluted tubules (Fig. 2C). The DM + IRI + Dex group revealed minimal intraglomerular collagen fibers, with no remarkable changes in terms of glomerular basal lamina thickness or change in the basal lamina of convoluted tubules (Fig. 2D).
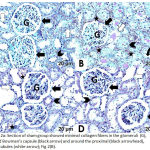 |
Figure 2a: Section of sham group showed minimal collagen fibers in the glomeruli (G), around Bowman’s capsule (black arrow) and around the proximal (black arrowhead), distal tubules (white arrow); Fig 2(B).
|
Section of DM+IRI group revealed some condensation of collagen fibers in the glomeruli (G), around the apparently thickened parietal layer of Bowman’s capsule (black arrow), the proximal (black arrowhead) and distal tubules (white arrow). Note congested blood (star) and vacant area between some tubular cells and basal lamina (curved arrow); Fig 2(C). Sections of DM+IRI+BC showed mild collagen in the glomeruli (G), surrounding Bowman’s capsule (black arrow) and the proximal (black arrowhead), distal tubules (white arrow). Note congested blood (star); Fig 2(D). Sections of DM+IRI+Dex revealed minimal intraglomerular (G) collagen fibers with no change in the basal lamina thickness (black arrow) and no notable changes in the proximal (black arrowhead), distal tubules (white arrow) (Masson’s trichrome, Scale bar 20 µm).
Histological Findings for PAS
The sections from the sham group showed a normal PAS reaction, with the presence of regular brush borders of the proximal convoluted tubules and basement membranes (Fig. 3A). The DM + IRI group sections showed an increased PAS reaction (Fig. 3B), while the DM + IRI + BC group showed an obvious reaction (Fig. 3C). Finally, DM + IRI + Dex showed an apparent PAS reaction (Fig. 3D; Table 6).
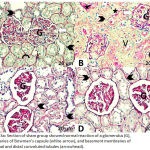 |
Figure 3a: Section of sham group showed normal reaction of a glomerulus (G), boundaries of Bowman’s capsule (white-arrow), and basement membranes of proximal and distal convoluted tubules (arrowhead).
|
Note regular curved brush border (curved arrow). Fig 3(B). Section of DM+IRI group showed increased reaction of the glomerulus (G), boundaries of Bowman’s capsule (white-arrow), and basement membranes around proximal and distal convoluted tubules (arrowhead) with the presence of vascular congestion (star). Note vacuoles inside and around the tubules (V). Fig 3(C). Sections of DM+IRI+BC showed a mild reaction in the glomerulus (G), boundaries of Bowman’s capsule (white-arrow), and basement membranes around proximal and distal convoluted tubules (arrowhead). Fig 3(D). Sections of DM+IRI+Dex showed a mild reaction in the glomerulus (G), boundaries of Bowman’s capsule (white-arrow), and basement membranes around proximal and distal convoluted tubules (arrowhead) (PAS, Scale bar 20 µm).
Table 6: Reaction to PAS of the different experimental groups.
| Histopathological findings | Sham | DM+IRI | DM+IRI+BC | DM+IRI+Dex |
| PAS (+) granules in tubules | – | ++ | + | + |
None (-); mild (+), moderate (++), severe (+++).
Immunohistochemical Detection of BAX Expression
An immunohistochemical study using the anti-BAX antibody showed an increase in the intensity of the glomerular and tubular epithelial cell staining in the DM + IRI group, with obvious apoptosis, in comparison with the sham group, which showed minimal BAX expression. The DM + IRI + BC group showed minimal cytoplasmic Bax expression, and the DM + IRI + Dex group showed mild cytoplasmic Bax expression (Figure 4).
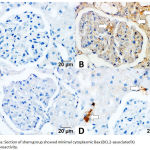 |
Figure 4a: Section of sham group showed minimal cytoplasmic Bax (BCL2-associated X) immunoreactivity.
|
Fig 4(B). Section of DM+IRI group showed strong positive cytoplasmic Bax immunoreactivity lining the glomeruli, cortical and in the tubular epithelial cells (white-arrow). Fig 4(C). Sections of DM+IRI+BC showed minimal cytoplasmic Bax immunoreactivity in the cytoplasm of some renal tubular cells (white-arrow). Fig 4(D). Sections of DM+IRI+Dex showed mild cytoplasmic Bax immunoreactivity (white-arrow) in most renal tubular cells (BAX, Scale bar 20 µm).
Apoptotic Cell Number
The mean number of apoptotic cells in the DM + IRI group was significantly increased compared with the sham group. In addition, the apoptotic cell number improved significantly in the DM + IRI + BC and DM + IRI + Dex groups compared with the DM + IRI group (Table 7).
Table 7: Mean ± SD of apoptotic cell number/20000 µm2 of the different groups
| Groups | Sham | DM+IRI | DM+IRI+BC | DM+IRI+Dex |
| Apoptotic cell number | 3.64 ± 0.76 | 9.57 ± 0.24
P1 < 0.001 |
4.36 ± 0.59
P1 < 0.01 P2 < 0.001 |
6.02±0.62
P1 < 0.001 P2 < 0.001 |
SD, standard deviation. P < 0.05 is significant. P1 Significance versus sham-operated. P2 Significance versus ischemia reperfusion.
Immunohistochemical Detection of TNF‑α Expression
The DM + IRI group showed strong inflammatory cytokine expression for TNF‑α in the glomeruli and renal tubular cells in comparison with the sham group, which showed weak cytoplasmic inflammatory cytokine expression. Furthermore, the DM + IRI + BC group showed minimal cytoplasmic inflammatory cytokine expression, and the DM + IRI + Dex group showed mild cytoplasmic inflammatory cytokine expression (Figure 5). The OD supported the immunohistochemical findings (Table 8).
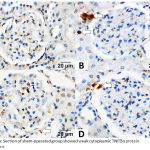 |
Figure 5a: Section of sham-operated group showed weak cytoplasmic TNF‑α protein expression.
|
Fig 5(B). Section of ischemia reperfusion group showed strong positive cytoplasmic immunoreaction for TNF‑α in the glomeruli and in the renal tubular cells (white-arrow). Fig 5(C). Sections of reperfusion treated with benincasa cerifera showed minimal cytoplasmic TNF‑α immunoreactivity in the cytoplasm of some renal tubular cells (white-arrow). Fig 5(D). Sections of reperfusion treated with dexmedetomidine showed mild cytoplasmic TNF‑α immunoreactivity (white-arrow) in renal tubular cells (TNF‑α, Scale bar 20 µm).
Table 8: OD of TNF‑α expression of the different groups, mean ± SD, (n=15).
| Groups | sham | DM+IRI | DM+IRI+BC | DM+IRI+Dex |
| OD of TNF‑α | 0.521±0.813 | 5.03±1.166
P1 < 0.001 |
3.85±0.124
P1 < 0.001 P2 < 0.001 |
4.23±0.089
P1 < 0.001 P2 < 0.05 |
TNF‑α, tumor necrosis factor‑α, SD, standard deviation. P < 0.05 is significant. P1 Significance versus sham-operated. P2 Significance versus ischemia reperfusion.
Discussion
The transient cessation of renal blood flow occurs in numerous clinical conditions, such as renal transplantation and partial nephrectomy, as well as in major vascular and cardiothoracic surgeries.26 This cessation leads to renal IRI, which causes a decrease in the renal glomerular filtration and blood flow.5,23,27
In the current study, there was a disturbance in the diabetic profiles of the STZ-induced diabetic groups (DM + IRI, DM + IRI + BC, and DM + IRI + Dex); these results are in line with previous findings, which were explained in relation to multiple factors, such as improper insulin secretion or insulin resistance, along with the alteration in carbohydrate, lipid metabolism, and OS markers. 12,28-30 The present work demonstrated that BC ameliorated the diabetic condition; these results are in agreement with previous reports, which attributed the hypoglycemic effects of BC to insulin release from the remnant pancreatic ẞ-cells and enhancement of peripheral glucose metabolism.31 Researchers have analyzed the contents of BC extract and found that it is rich in catechins, carotenes, tannins, uronic acids, and polyphenol compounds, one of the most important of which is gallic acid.32 BC extract normalized the blood glucose level and restored the insulin level due to its enrichment of gallic acid.33 In contrast, Dex did not have any ameliorative effect on the diabetic condition, which may be explained by the inhibitory effects of Dex on insulin secretion.34
Renal IRI is more frequent in diabetics and considered a complex inflammatory process that is initiated with ischemia and aggravated during reperfusion of ischemic renal tissue. It results a complex series of cellular events, inflammatory processes, apoptosis, and OS.29
There was an improvement of serum Creat and serum BUN in this study that demonstrated the beneficial effects of both BC extract and Dex. These results agree with previous studies indicating that STZ-induced diabetes resulted in renal impairment, as evidenced by significant increases in the serum Creat and serum BUN.12,28-30
This study showed that treatment with BC resulted in a significant improvement in renal functions, in accordance with a previous study that attributed these results to the antihyperglycemic and antioxidant effects of the polyphenol content of BC.1 In addition, the renal functions improved due to treatment with Dex; these results agree with previous studies that attributed these results to reduced vasoconstriction, promotion of renal arterial vasodilation, decreased vascular resistance, and increased glomerular filtration rate and filtration fraction, 7,11,35 thereby improving renal IRI.36
In this study, there was a significant increase in serum LDH in the untreated group, which indicated tissue necrosis and tissue damage due to renal IRI; protection with BC and Dex resulted in significant decreases in the serum LDH levels, mainly due to their renoprotective effects, which resulted from their antioxidant properties.37 This study showed that untreated groups demonstrated dyslipidemia; these results are in accordance with previous studies stating that, due to disturbances in metabolism associated with STZ-induced diabetes as result of glucose intolerance, decreased insulin levels enhance lipolysis and increase LDL synthesis.38,39 Treatment with BC resulted in a significant improvement of all parameters of the lipid profile, in agreement with a previous study,40 which attributed this finding to the antihyperlipidemic properties of BC. The authors explained that the antihyperlipidemic effects of BC arise from multiple mechanisms, including its regenerative effects on pancreatic ẞ-cells, which result in increasing the serum insulin levels, inhibitory effects on PPAR signaling and HMG-CoA reductase, and antioxidant properties.41 In this study, Dex administration improved dyslipidemia, which may have been due to its antioxidant activity.36
OS plays a critical role in renal IRI, as with initiation of ischemia, renal tissues are exposed to hypoxia and the depletion of adenosine triphosphate (ATP); then, during the reperfusion phase, the OS continues via the release of reactive oxygen species (ROS) and free radicals [.36 ROS activate inflammation and vasoconstrictors, resulting in the damage of tubular cells.42 The results of the current work confirmed the role of OS in renal IRI, as evidenced by the significant elevation of MDA, 8-OHdG, and MPO and decrease of GSH in serum and renal ischemic tissue homogenate in the untreated group (DM + IRI) as indicators of OS.43,44
The results of this study agree with those of previous studies showing a significant increase in the MDA levels in renal IRI groups, which is used as an indicator of lipid peroxidation causing damage to the cellular and mitochondrial membranes, thereby leading to cellular disturbance and possibly ending with cell necrosis.3,42,45-48 The renal IRI group showed a significant increase in serum and renal tissue homogenate levels of 8-OHdG, which is considered a sensitive index for oxidative DNA damage resulting from OS produced due to renal IRI; these results agree with previous studies.49,50 Moreover, the MPO levels were increased in both serum and renal tissue homogenate; MPO is considered a marker of neutrophil infiltration in the renal tissue, leading to the release of multiple inflammatory cytokines; these results are in accordance with previous studies.51-53 Studies have demonstrated that neutrophil infiltration plays a critical role in initiating the inflammatory response as a consequence neutrophil degranulation, which exacerbates MPO, cytokine, and ROS release, leading to a cellular disturbance and loss of function in renal tissues.54
The results of the current work showed a significant decrease in the level of GSH in the untreated group, indicating the consumption of endogenous antioxidants like GSH. These findings agree with those of previous studies.55,56
The antihyperglycemic and antioxidants effects of BC and its power in stabilizing cellular membranes through the inhibition of lipid peroxidation, cellular infiltration, and restoration of reduced GSH levels mean that BC delays renal complications stemming from DM.10,41,57 The antioxidant effects of BC are due to its high content of polyphenols and flavonoids, which act as hydrogen donors for free radicals. They break the chain reaction and inhibit lipid peroxidation, promote the endogenous antioxidant defense system, and modulate the oxidant/antioxidant balance; hence, there is a positive correlation between phenolic content and antioxidant activity.57-59
In this study, the administration of Dex significantly ameliorated OS conditions, as found in other studies.60 Similar results documented that pretreatment with Dex results in the improvement of MDA and MPO levels in lung tissue homogenate after IRI. 61,62 These authors explained that the renoprotective action of Dex makes it an effective α2-adrenergic receptor agonist, producing effects on the kidney by decreasing the efferent sympathetic outflow of the renal nerve, resulting in increased diuresis and natriuresis.60 Moreover, by stabilizing cellular membranes to prevent lipid peroxidation, Dex antioxidant activity reduces malondialdehyde levels, restores total GSH levels, free radical scavenging activity, activates antioxidant enzymes, and maintains the integrity of DNA and cellular protein.36
In the current study, pretreatment with BC protected the kidney’s architectural and cytological structure from lipid peroxidation and OS, coinciding with previous findings.10 BC’s renoprotective effect on renal IRI can be explained by its antioxidant activity.63
A significant decrease was detected in BAX immunoreactivity in the groups treated with Dex and BC, suggesting that apoptosis, a major pathway of IRI, could be reduced or prevented by both substances. This coincided with other researchers’ findings.64
Kocoglu et al65 reported a significant decrease in the histopathological injury in a renal IRI model, supporting the current findings. Dex’s renoprotective effect of Dex may be attributed to the increase of renal blood flow and glomerular filtration by reducing the release of noradrenaline.66 Villela et al.67 reported that Dex decreased the urinary osmolality and plasma vasopressin level and produced free water diuresis. Previous researchers found that Dex improves renal function, urine flow, and glomerular filtration.68 In addition, it has been reported to increase renal arterial vasodilation via direct vascular effects.65 The renoprotective effect of Dex has been attributed to the increase in antiapoptotic Bcl-2 and (Mouse double minute 2 homolog) Mdm-2 expression; this increase has been associated with a decrease in the levels of proapoptotic Bax.69 This coincided with the current BAX immunohistochemistry findings. In the present study, the renoprotective role of Dex, was revealed by an improvement in post-ischemic attenuated histological damage, decreased number of apoptotic cells, and reduction of TNF-α.70
In conclusion, the present study demonstrated that oxidative stress plays a chief role in renal IRI in an STZ-induced diabetic model. Treatment with BC extract achieved excellent ameliorative effects on both diabetes and renal IRI, while treatment with Dex improved renal IRI, renal functions, and dyslipidemia, but it did not affect the diabetic condition.
Conflict of Interest
There is no conflict of interest.
References
- Ozbilgin S., Ozkardesler S., Akan M., et al. Renal Ischemia/Reperfusion Injury in Diabetic Rats: The Role of Local Ischemic Preconditioning. BioMed research international. 2016;2016:8580475.
CrossRef - Pourghasem M., Shafi H., Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med. 2015;6:120-127.
- Hu B., Wu Y., Liu J., et al. GSK-3beta Inhibitor Induces Expression of Nrf2/TrxR2 Signaling Pathway to Protect against Renal Ischemia/Reperfusion Injury in Diabetic Rats. Kidney & blood pressure research. 2016;41:937-946.
- CrossRef
- Chang Y. K., Choi H., Jeong J. Y., et al. Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PloS one. 2016;11:e0158810.
CrossRef - Singh S. K., Cole E. H., Kim S. J. Delayed Graft Function and Kidney Transplantation. In: Weir RM, Lerma VE, eds. Kidney Transplantation: Practical Guide to Management. New York, NY: Springer New York. 2014:143-151.
CrossRef - Jain M., Rivera S., Monclus E. A., et al. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem. 2013;288:770-777.
CrossRef - Liu G., Song H., Qiu L., et al. Dexmedetomidine preconditioning inhibits the long term inflammation induced by renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2016;31:8-14.
CrossRef - Arora P., Kaushik D. Therapeutic potential of Benincasa cerifera: A review. Chinese Journal of Integrative Medicine. 2016:1-14.
CrossRef - Njoroge G. N., Bussmann R. W. Ethnotherapeautic management of skin diseases among the Kikuyus of Central Kenya. J Ethnopharmacol. 2007;111:303-307.
CrossRef - Bhalodia Y., Kanzariya N., Patel R., et al. Renoprotective activity of benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iran J Kidney Dis. 2009;3:80-85.
- Gonullu E., Ozkardesler S., Kume T., et al. Comparison of the effects of dexmedetomidine administered at two different times on renal ischemia/reperfusion injury in rats. Braz J Anesthesiol. 2014;64:152-158.
CrossRef - AbdEl-Moniem M., Mustafa H. N., Megahed H. A., Agaibyi M. H., Hegazy G. A., El-Dabaa M. A. The ameliorative potential of Hyphaene thebaica on streptozotocin-induced diabetic nephropathy. Folia Morphol (Warsz). 2015;74:447-457.
CrossRef - Moghbelinejad S., Nassiri-Asl M., Farivar T. N., et al. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol Lett. 2014;224:108-113.
CrossRef - Zhang N., Cheng G. Y., Liu X. Z., Zhang F. J. Expression of Bcl-2 and NF-κB in brain tissue after acute renal ischemia-reperfusion in rats. Asian Pacific Journal of Tropical Medicine. 2014;7:386-389.
CrossRef - Toth S., Jonecova Z., Curgali K., et al. Quercetin attenuates the ischemia reperfusion induced COX-2 and MPO expression in the small intestine mucosa. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;95:346-354.
CrossRef - Thokala S., Inapurapu S., Bodiga V. L., Vemuri P. K., Bodiga S. Loss of ErbB2-PI3K/Akt signaling prevents zinc pyrithione-induced cardioprotection during ischemia/reperfusion. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;88:309-324.
CrossRef - Hussein A. M., Mustafa H. N., Badawoud M. H. Ameliorative potentials of a combination of fenugreek and alpha-tocopherol on cadmium induced testicular toxicity: an ultrastructural study. Folia Morphol (Warsz). 2015;74:325-334.
CrossRef - Akbas A., Silan C., Gulpinar M. T., Sancak E. B., Ozkanli S. S., Cakir D. U. Renoprotective Effect of Humic Acid on Renal Ischemia-Reperfusion Injury: An Experimental Study in Rats. Inflammation. 2015;38:2042-2048.
CrossRef - Fouad A. A., Al-Mulhim A. S., Jresat I., Morsy M. A. Protective effects of captopril in diabetic rats exposed to ischemia reperfusion renal injury. The Journal of pharmacy and pharmacology. 2013;65:243-252.
CrossRef - Sayhan M. B., Kanter M., Oguz S., Erboga M. Protective effect of Urtica dioica L. on renal ischemia reperfusion injury in rat. Journal of molecular histology. 2012;43:691-698.
CrossRef - Altintas R., Parlakpinar H., Beytur A., et al. Protective effect of dexpanthenol on ischemia-reperfusion-induced renal injury in rats. Kidney & blood pressure research. 2012;36:220-230.
CrossRef - Mister M., Noris M., Szymczuk J., et al. Propionyl-L-carnitine prevents renal function deterioration due to ischemia reperfusion. Kidney Int. 2002;61:1064-1078.
CrossRef - Korkmaz A., Kolankaya D. Protective effect of rutin on the ischemia reperfusion induced damage in rat kidney. J Surg Res. 2010;164:309-315.
CrossRef - Hussein A. M., Saleh H. A. H. N. M. Effect of sodium selenite and vitamin E on the renal cortex in rats: an ultrastructure study. Tissue Cell. 2014;46:170-177.
CrossRef - Hussein A. M., Badawoud M. H., Noaman M. H. The effects of diethylstilbestrol administration on rat kidney. Ultrastructural study. Saudi Med J. 2013;34:1114-1124.
- Radhakrishnan J., Kiryluk K. Acute renal failure outcomes in children and adults. Kidney Int. 2006;69:17-19.
CrossRef - Murugan R., Kellum J. A. Acute kidney injury what’s the prognosis? Nat Rev Nephrol. 2011;7:209-217.
CrossRef - Fan J., Xu G., Jiang T., Qin Y. Pharmacologic Induction of Heme Oxygenase-1 Plays a Protective Role in Diabetic Retinopathy in RatsHeme Oxygenase-1 in Diabetic Retinopathy in Rats. Investigative ophthalmology & visual science. 2012;53:6541-6556.
CrossRef - Akan M., Ozbilgin S., Boztas N., et al. Effect of magnesium sulfate on renal ischemia-reperfusion injury in streptozotocin-induced diabetic rats. Eur Rev Med Pharmacol Sci. 2016;20:1642-1655.
- Mestry S. N., Dhodi J. B., Kumbhar S. B., Juvekar A. R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit. Complement Med. 2017;7:273-280.
CrossRef - Rupa L. M., Mohan K. Hypoglycaemic effect of aqueous extract of Benincasa hispida in rabbits. Inter Ayur Med J. 2013;1:1-5.
- Gill N., Dhiman K., Bajwa J., Sharma P., Sood S. Evaluation of free radical scavenging, anti-inflammatory and analgesic potential of Benincasa hispida seed extract. International Journal of Pharmacology. 2010;6:652-657.
- Fatariah Z., Zulkhairuazha T., Rosli W. W. Quantitative HPLC analysis of gallic acid in Benincasa hispida prepared with different extraction techniques. Sains Malaysiana. 2014;43:1181-1187.
- Zeng X., Wang H., Xing X., Wang Q., Li W. Dexmedetomidine Protects against Transient Global Cerebral Ischemia/Reperfusion Induced Oxidative Stress and Inflammation in Diabetic Rats. PloS one. 2016;11:e0151620.
CrossRef - Si Y., Bao H., Han L., et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med. 2013;11:141.
CrossRef - Cai Y., Xu H., Yan J., Zhang L., Lu Y. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol Med Rep. 2014;9:1542-1550.
CrossRef - Mahfoudh-Boussaid A., Ayed H. T. K., Zaouali M. A., Rosello-Catafau J., Abdennebi H. B . Effects of trimetazidine on the Akt/eNOS signaling pathway and oxidative stress in an in vivo rat model of renal ischemia-reperfusion. Ren Fail. 2014;36:1436-1442.
CrossRef - Asad M., Aslam M., Munir T. A., Nadeem A. Effect of Acacia nilotica leaves extract on hyperglycaemia, lipid profile and platelet aggregation in streptozotocin induced diabetic rats. J Ayub Med Coll Abbottabad. 2011;23:3-7.
- Hegazy G. A., Alnoury A. M., Gad H. G. The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and an tioxidant in streptozotocin-induced diabetic rats. Saudi medical journal. 2013;34:727-733.
CrossRef - Yasir M., Shrivastava R., Jain P., Das D. Hypoglycemic and Antihyperglycemic Effects of Different Extracts and Combinations of Withania coagulans Dunal and Acacia arabica Lamk in Normal and Alloxan Induced Diabetic Rats. Pharmacognosy Communications. 2012;2:61-66.
CrossRef - Mahatma A., Kumar M. S., Sonowal A. Evaluation of Antidiabetic Potential of Methanolic Extract of Benincasahispida in Dexamethasone Induced Diabetic Rats. International Journal of Medical and Dental Science Invention. 2014;1:07-17.
- Qiao X., Li R. S., Li H., et al. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am J Physiol Renal Physiol. 2013;304:F112-119.
CrossRef - Shokeir A. A., Barakat N., Hussein A. M., et al. Activation of Nrf2 by ischemic preconditioning and sulforaphane in renal ischemia reperfusion injury: a comparative experimental study. Physiol Res. 2015;64:313-323.
- Fu Y., Lin Q., Gong T., Sun X., Zhang Z. R. Renal-targeting triptolide-glucosamine conjugate exhibits lower toxicity and superior efficacy in attenuation of ischemia/reperfusion renal injury in rats. Acta Pharmacol Sin. 2016;37:1467-1480.
CrossRef - Lin M., Li L., Li L., et al. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC complementary and alternative medicine. 2014;14:19.
CrossRef - Han S. J., Kim J. I., Park J. W., Park K. M. Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia reperfusion injury. Nephrol Dial Transplant. 2015;30:1497-1506.
CrossRef - Yu Y., Li M., Su N., et al. Honokiol protects against renal ischemia reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol Med Rep. 2016;13:1353-1360.
CrossRef - Xiao Y.D., Huang Y. Y., Wang H. X., et al. Thioredoxin-Interacting Protein Mediates NLRP3 Inflammasome Activation Involved in the Susceptibility to Ischemic Acute Kidney Injury in Diabetes. Oxid Med Cell Longev. 2016;2016:2386068.
CrossRef - Sun Z., Zhang X., Ito K., et al. Amelioration of oxidative mitochondrial DNA damage and deletion after renal ischemic injury by the K ATP channel opener diazoxide. American Journal of Physiology-Renal Physiology. 2008;294:F491-F498.
CrossRef - Tan X., Zhang L., Jiang Y., et al. Postconditioning ameliorates mitochondrial DNA damage and deletion after renal ischemic injury. Nephrol Dial Transplant. 2013;28:2754-2765.
CrossRef - Ozturk H., Ozturk H., Terzi E. H., Ozgen U., Duran A., Uygun I. Protective effects of rosmarinic acid against renal ischaemia/reperfusion injury in rats. J Pak Med Assoc. 2014;64:260-265.
- Yuan D., Collage R. D., Huang H., et al. Blue light reduces organ injury from ischemia and reperfusion. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:5239-5244.
CrossRef - Xiong C., Zang X., Zhou X., et al. Pharmacological inhibition of Src kinase protects against acute kidney injury in a murine model of renal ischemia reperfusion. Oncotarget. 2017;8:31238-31253.
CrossRef - Schofield Z. V., Woodruff T. M., Halai R., Wu M. C., Cooper M. A. Neutrophils–a key component of ischemia-reperfusion injury. Shock. 2013;40:463-470.
CrossRef - Liu H. B., Meng Q. H., Huang C., Wang J. B., Liu X. W. Nephroprotective Effects of Polydatin against Ischemia/Reperfusion Injury: A Role for the PI3K/Akt Signal Pathway. Oxid Med Cell Longev. 2015;2015:362158.
CrossRef - Shati A. A. Xanthohumol protects against renal ischaemia reperfusion (I/R) injury by scavenging ROS and inhibition of JAK-2/STAT-3 inflammatory pathway. Journal of Taibah University for Science. 2017;11:458-470.
CrossRef - Ojha S., Alkaabi J., Amir N., et al. Withania coagulans fruit extract reduces oxidative stress and inflammation in kidneys of streptozotocin-induced diabetic rats. Oxid Med Cell Longev. 2014;2014:201436.
CrossRef - Li J. L., Gao R., Li S., Wang J. T., Tang Z. X., Xu S. W. Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals an international journal on the role of metal ions in biology, biochemistry, and medicine. 2010;23:695-705.
CrossRef - Abd E. l Samad A. A., Raafat M. H. Comparative study on the effects of grape seed extract and telmisartan on doxorubicin-induced cardiotoxicity in adult male rats: light and electron microscopic study. Egyptian Journal of Histology. 2012;35:340-352.
CrossRef - Cakir M., Polat A., Tekin S., et al. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren Fail. 2015;37:704-708.
CrossRef - Zhang W., Zhang J. Dexmedetomidine preconditioning protects against lung injury induced by ischemia-reperfusion through inhibition of autophagy. Exp Ther Med. 2017;14:973-980.
CrossRef - İnci F., Doğan İ. V., Eti Z., Deniz M., Göğüş F. Y. The effects of dexmedetomidine infusion on the formation of reactive oxygen species during mesenteric ischemia-reperfusion injury in rats. Marmara Medical Journal. 2007;20:154-160.
- Lim S. J. Effects of fractions of Benincasa hispida on antioxidative status in streptozotocin induced diabetic rats. Korean Journal of Nutrition. 2007;40:295-302.
- Bagcik E., Ozkardesler S., Boztas N., et al. [Effects of dexmedetomidine in conjunction with remote ischemic preconditioning on renal ischemia-reperfusion injury in rats]. Rev Bras Anestesiol. 2014;64:382-390.
CrossRef - Kocoglu H., Ozturk H., Ozturk H., Yilmaz F., Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70-74.
CrossRef - Taoda M., Adachi Y. U., Uchihashi Y., Watanabe K., Satoh T., Vizi E. S. Effect of dexmedetomidine on the release of [3H]-noradrenaline from rat kidney cortex slices: characterization of α2-adrenoceptor. Neurochemistry international. 2001;38:317-322.
CrossRef - Villela N. R., Júnior P. d. N, Carvalho L. R. d., Teixeira A. Efeitos da dexmedetomidina sobre o sistema renal e sobre a concentração plasmática do hormônio antidiurético: estudo experimental em cães. Revista Brasileira de Anestesiologia. 2005;55:429-440.
CrossRef - Sugita S., Okabe T., Sakamoto A. Continuous infusion of dexmedetomidine improves renal ischemia-reperfusion injury in rat kidney. J Nippon Med Sch. 2013;80:131-139.
CrossRef - Engelhard K., Werner C., Eberspacher E., et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524-531. table of contents.
CrossRef - Chen Z., Chen Z., Chen H., Chen H., Zhou T., Lu H. Schwann cell apoptosis in Wallerian-degenerated sciatic nerve of the rat. Chinese journal of traumatology = Zhonghua chuang shang za zhi / Chinese Medical Association. 2004;7:220.








