Silvia W. Lestari1,4, Khairunnisa F. Ilato2, M. Iqbal A. Pratama2, Nurin N. Fitriyah3, Mulyoto Pangestu4, Gita Pratama5 and Ria Margiana6
1Department of Medical Biology, Faculty of Medicine Universitas Indonesia.
2Bachelor Program for Medical Sciences, Faculty of Medicine Universitas Indonesia.
3Master Program for Biomedical Sciences, Faculty of Medicine Universitas Indonesia.
4Department of Obstetric and Gynecology, Monash Clinical School, Monash University.
5Department of Obstetric and Gynecology, Faculty of Medicine Universitas Indonesia.
6Department of Anatomy, Faculty of Medicine Universitas Indonesia.
Corresponding Author E-mail: finallysilvia@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1351
Abstract
Numerous studies reported that vitrification, an ultra-rapid cooling technique, seems to be highly effective and could increase oocyte survival rate rather than slow freezing. The successful of oocyte vitrification depends on the proper combination of type and concentration of cryoprotectant. This study was addressed to determine the effects of the combination of type and concentration of cryoprotectants of vitrification media, notably in the embryo development. This experimental research was conducted by using oocyte obtained from thirty-two adult female Deutschland, Denken and Yoken (DDY) mice (7-8 weeks old). The MII mice oocytes were vitrified within 24 h after retrieval using the Cryotop method with cryoprotectants as follow : sucrose (16.5% EG, 16.5% DMSO, 0.5 mol/l sucrose), trehalose (16.5% EG, 16.5% DMSO, 0.5 mol/l trehalose) and Kitazato. The embryo development and morphological grading was observed at 2-cell and 8-cells under reverse phase light microscope and inverted microscope. This study demonstrated a good embryo development and morphological grading in sucrose and trehalose vitrification media. In embryo development, trehalose medium seems more superior compared to sucrose medium, even though Kitazato was the most superior compared to both. In the morphological grading, in 2-cells embryo, there were no significant differences between the three cryoprotectants, While, in 8-cells embryo, trehalose medium appeared to be superior compared to sucrose medium, even though seemed more inferior compared to Kitazato. The appropriate type and concentration of sugar as extracellular cryoprotectant was trehalose in oocyte vitrification based on embryo development, compared to sucrose.
Keywords
Extracellular Cryoprotectant; Embryo Development Oocyte Vitrification; Sucrose; Trehalose;
Download this article as:| Copy the following to cite this article: Lestari S. W, Ilato K. F, Pratama M. I. A, Fitriyah N. N, Pangestu M, Pratama G, Margiana R. Sucrose ‘Versus’ Trehalose Cryoprotectant Modification in Oocyte Vitrification : A Study of Embryo Development. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Lestari S. W, Ilato K. F, Pratama M. I. A, Fitriyah N. N, Pangestu M, Pratama G, Margiana R. Sucrose ‘Versus’ Trehalose Cryoprotectant Modification in Oocyte Vitrification : A Study of Embryo Development. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18950 |
Introduction
Recently, some in vitro fertilization (IVF) centers in worldwide undergo forbidden regulation, such as embryo cryopreservation and gamete donation. As a consequence, oocyte selection and cryopreservation could be introduced as a solution.1-7 In addition, other reasons such as cancer, endometriosis surgery and career are also convincing the oocyte selection and cryopreservation methods to ensure fertility preservation.8
Slow freezing as one of the oocyte cryopreservation method were performing some limitations such as low oocyte survival rate,7,9,10 increased risk of oocyte ageing7,11,12 and reduced embryo development compared to the fresh cycle.7,13 In contrast, vitrification as another method of oocyte cryopreservation has been suggested by other researchers.7,14 The efficacy of vitrification is different from slow freezing based on the oocyte physiology.7,15
Vitrification can be succeeded by performing a proper combination of type and concentration of cryoprotectants. Cryoprotectants are used to avoid cell damage, while in the same time, it could damage the cell too due to the osmotic effect and chemical toxicity. There are two types of cryoprotectants: intracellular and extracellular cryoprotectants. Intracellular cryoprotectants, such as dimethyl sulfoxide (DMSO), ethylene glycol (EG), and propanediol have been reported to provide good result, in contrast to the extracellular cryoprotectants (i.e. sucrose and trehalose).16 In addition, the certain concentration of intracellular cryoprotectans demonstrated a good survival rate (66% with 0.15 M of trehalose) and also a high cleavage rate (80% with 1.0 – 1.5 M of sucrose)17,18
Although vitrification had improved, there is not any of the most proper combination and concentration of cryoprotectants. Currently, studies that compare the effects of type and concentration of cryoprotectants of vitrification media on the embryo development are still lacking. Therefore, the purpose of this study was to determine the effects of the combination of type and concentration of cryoprotectants of vitrification media, on the embryo development.
Methods
Animal, Oocyte and Sperm Treatment
All procedures on the animals were approved by the Ethical Committee of Medical Faculty of Universitas Indonesia. Animals were maintained in accordance with the Animal Care and Use Committee Bogor Agricultural University on February 27th, 2017. Thirty-two adult females and sixteen adult male Deutschland, Denken and Yoken (DDY) mice (7-8 weeks old) were used as oocyte and sperm donors. Animals were kept in a room at 22.2°C, under a 12;12-hour light/dark cycle and were provided food pellet and water ad libitum. The female mice were superovulated by given 10 IU of gonadotropin (Gonal F, Serono Ferring, Germany), followed by ovulation trigger by given 10 IU human chorionic gonadotropin (HCG) (Pregnil, Hoddesdon Hertfortshire, United Kingdom) by intraperitoneal injection, 48 hours apart.
The female mice were euthanized at 15-16 hours after ovulation trigger, by cervical dislocation, for oocyte collection. Under dissecting microscope (Leica Zoom 2000, Japan), the fallopian tubes were expurgated and the follicles or cumulus-oocytes complex (COC) obtained were washed with IVF culture media (G IVF, Vitrolife, Swedia).
The oocytes removed from COC by denuding process using the hyase enzyme (Hyase, Vitrolife, Swedia) through repetitive pipetting, then being classified based on metaphase II (MII) phase and polar body (PB) extrusion as maturation criteria. Prior being transferred into vitrification medium, retrieved denuded and matured oocytes were placed into IVF culture media dishes (G IVF, Vitrolife, Swedia). Eighty four MII stage oocytes were divided into four groups and used for vitrification process.
The same with the female mice, the male were euthanized too immediately before the warming process, cervical dislocation, for sperm collection. The epididymis was excised and the sperm were prepared by performing density gradient centrifugation (Sperm Grade, Vitrolife, Sweden) continued with swim up (Sperm Preparation, Vitrolife, Sweden) sperm preparation methods, in order to get viable and motile sperms. Density gradient centrifugation method was done by performing centrifugation (1.500 rpm for 20 minutes) over a different gradient of two layers of Sperm Grade and sperm layer. The pellet was resuspended and centrifuged (1.500 rpm for 10 minutes) using Sperm Preparation. Lastly, the viable and motile sperm were obtained from the swim up at the top layer.
Vitrification and Warming Solution
To investigate a direct impact of vitrification solution on oocytes collected, one control and two experimental groups were formed. In group 1 they were vitrified by sucrose, in group 2 by trehalose, and in group 3 by Kitazato/commercial agent. Group 3 acted as the control in which contained vitrified by Kitazato oocytes. In group sucrose and trehalose, oocytes were vitrified in different vitrification solutions (VS): 1). sucrose contains 16.5% EG, 16.5% DMSO, 0.5 mol/l sucrose (Merck, Darmstadt, Germany); 2). trehalose contains 16.5% EG, 16.5% DMSO, 0.5 mol/l trehalose (Merck, Darmstadt, Germany). While its warming solution were formed, there were: 1). WS1a contains 0.3 mol/l sucrose; 2). WS1b contains 0.15 mol/l sucrose; 3). WS2a contains 0.3 mol/l trehalose; and 4). WS2b contains 0.15 mol/l trehalose.
Vitrification and Warming Procedures
For vitrification, the denuded MII stage oocytes were incubated in ES containing 7.5% EG (Sigma-Aldreich, Steinhem, Germany) and 7.5% DMSO (Sigma-Aldrich) in HM for 10 minutes at room temperature.19 After initial shrinkage and recovery, the MII oocytes were aspirated and placed into the sucrose, trehalose and Kitazato vitrification media for 50-60 seconds at room temperature. After being confirmed, under observation, that oocytes shrinkage had taken place, one until three oocytes were aspirated and placed on the tip of the Cryotop (Kitazato, Japan).20 Cooling of the oocytes was performed by plummeted gently cryotop into liquid nitrogen (LN2) within 45 seconds and moved to LN2 goblets and placed at -196ºC storage containers. Oocytes to be warmed was initiated by removing the shielding covering of Cryotops while the propylene strips were still in LN2 and followed by quick transfer of Cryotop from LN2 into warming solution (WS1a or WS1b) for 5 minutes at 37ºC and placed into a dilution solution (WS2a or WS2b) for 5 minutes at room temperature. The morphologically normal warmed oocytes were placed 4-5 times into PBS (Gibco, New York, USA) and continued for subsequent analysis.
ICSI and Embryo Culture
Nearly before ICSI, the obtained sperm were placed to a drop which consist of Polyvinylpyrrolidone (PVP) (Medicult, Origio) in a ICSI dish. Besides the PVP drops, there were also drops which consist of GMOPs medium (Vitrolife, Sweden) in ICSI dish, to put the fresh and the vitrified oocytes. All of these drops were overlaid by liquid paraffin (Medicult, Origio).
In the ICSI procedures, each oocyte was injected by a single spermatozoon. The viable and motile sperm with normal morphology were immobilized by stroking the injecting pipette onto the sperm. The oocyte was placed in the 6 or 12 o’clock position onto the holding pipette.
The sperm was aspirated by the injecting pipette, then penetrated into the ooplasm at the 3 o’clock position. The sperm was released into the ooplasm, then the injected pipette was revoked smoothly from the oocyte. The injected oocytes or embryos were placed into drops in the culture dish which consist of ISM1 culture medium (Medicult, Origio) and stored at 37°C in a triple gas (5% O2, 6% CO2 and 5% N2) incubator for 3 days.
Embryo Development
Morphological grading of embryo development was focused on 2-cell and 8-cell cleavage, based on scoring criteria of embryo cleavage by Veek (1998)21 and Rienzi (2002)22 (Table 1), which carried out by observation under the reverse phase light microscope (Leica IRB, Japan) and inverted microscope (Olympus IX81 SF-3, Japan). Fertilized oocytes were assessed at 16-18 hours after ICSI and defined as the appearance of two equally sized pro-nuclei, while cleaved embryos were evaluated on day 2 (44 – 48 hours after insemination) and day 3 (68 – 72 hours after insemination) after ICSI.
Table 1: Scoring criteria for cleaved embryo.21
| Measurement | Grade | Description |
| Grade 1 | Embryo with blastomeres of equal size, no cytoplasmic | |
| fragments | ||
| Grade 2 | Embryo with blastomeres of equal size, minor | |
| cytoplasmic fragments | ||
| Cleavage stage morphology | Grade 3 | Embryo with blastomeres of distinctly of unequal size, |
| none or few cytoplasmic fragments | ||
| Grade 4 | Embryo with blastomeres of equal or unequal size, | |
| significant cytoplasmic fragments | ||
| Grade 5 | Embryo with few blastomeres of any size, severe or | |
| complete fragments |
Statistical Analysis
A comparison among fresh and vitrified groups in terms of cleavage and embryo development was performed. The data was statistically analyzed using Statistical Package for Social Science (SPSS) version 22 with no parametric Mann-Whitney test. P-values less than 0.05 were regarded as significant.
Results and Discussion
Results of embryo development in cryopreservation media were shown in fig. 1. Embryo development was recorded at 2-cells (48 hours) and 8-cells (67 hours) of cleavage state, the result showed there were significant different at all of groups during observation. Fig. 1A showed that the number of developing embryos forming 2-cells and 8-cells were significantly higher in Kitazato than in sucrose medium (p=0.02). Embryo development forming 8-cells in Kitazato performed significant higher than in trehalose medium with p value less than 0.05 (p=0.03) (Fig. 1B). However, the number of embryos development in 2-cells state in Kitazato vs Trehalose was not significantly different. Embryo numbers during 2-cells and 8-cells cleavage in trehalose were not significantly higher than in sucrose medium (p=0.08) as presented in Fig. 1C.
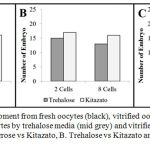 |
Figure 1: Embryo development from fresh oocytes (black), vitrified oocytes by sucrose media (dark grey), vitrified oocytes by trehalose media (mid grey) and vitrified oocytes by Kitazato media (light grey). A. Sucrose vs Kitazato, B. Trehalose vs Kitazato and C. Sucrose vs trehalose
|
Our findings about morphological grading of embryo in vitrification media demonstrated in Fig. 2 and 3. In Fig. 2, there were more abundant of Grade I embryos to other grades in all of groups significantly, even there were no significant differences in embryo morphology among groups.
 |
Figure 2: Morphological grading of cleavage embryo at 2-cells from fresh oocytes (black), vitrified oocytes by sucrose media (dark grey), vitrified oocytes by trehalose media (mid grey) and vitrified oocytes by Kitazato media (light grey). A. Sucrose vs Kitazato, B. Trehalose vs Kitazato and C. Sucrose vs trehalose
|
At 8-cells cleavage state, this study showed significant differences in the morphological grading of Grades 1 and 5. (Fig. 3) There were significantly more abundant of Grade 1 embryos observed in Kitazato than in sucrose media (p=0.01). (Fig. 3A) In contrast, there were also significantly more Grade 5 embryos observed in sucrose than in Kitazato media (p=0.03). (Fig. 3A) In addition, in comparison between Kitazato and trehalose media, there were not significant differences. Kitazato showed more abundant of Grade 1 embryos observed compared to trehalose, while trehalose showed more Grade 2 until Grade 5 embryos in which not significantly compared to Kitazato media. (p=0.07) (Fig. 3B) At last, in comparison between sucrose and trehalose, the trehalose showed more Grade 1 embryos significantly observed compared to sucrose, while sucrose showed more Grade 5 embryos not significantly compared to trehalose. (p=0.04, p=0.03, respectively) (Fig. 3C) Trehalose appeared to be superior compared to sucrose media.
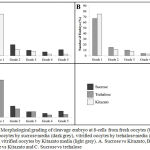 |
Figure 3: Morphological grading of cleavage embryo at 8-cells from fresh oocytes (black), vitrified oocytes by sucrose media (dark grey), vitrified oocytes by trehalose media (mid grey) and vitrified oocytes by Kitazato media (light grey). A. Sucrose vs Kitazato, B. Trehalose vs Kitazato and C. Sucrose vs trehalose
|
The appropriate/suitable combination of type and concentration of cryoprotectants is one of the approaches in the successful of oocyte vitrification.14 Cryoprotectants are high soluble and toxic solutions, which are associated with concentration and temperature and aim to prevent cells from damage during vitrification procedures.23 According to their ability to penetrate the oocyte membrane, there are two types of cryoprotectant, first is permeating or intracell cryoprotectant such as methanol, ethanol and dimethysulphoxide (DMSO) and second is non-permeating or extra cell cryoprotectant such as sucrose and trehalose.23 DMSO was reported to increase the risk for polyploidy due to spindle polymerization.24 Besides DMSO, ethylene glycol (EG) was reported to be used in oocyte vitrification which is related to high permeability and toxicity.25,26
In this study, we used three cryoprotectants, i.e. sucrose and trehalose as homemade vitrification media and Kitazato as commercial vitrification media. This present study used sucrose vitrification medium which is consisting of 16.5% EG, 16.5% DMSO, 0.5 mol/l sucrose, while trehalose vitrification medium consists of 16.5% EG, 16.5% DMSO, 0.5 mol/l trehalose and Kitazato vitrification medium consists of EG, DMSO, trehalose and hydroxypropyl cellulose. In the many publication of successful vitrification method, the vitrification medium includes EG and DMSO at a concentration of up to 15% per each.16,27 For homemade vitrification media, we also used 0.3 mol/l and 0.15 mol/l sucrose for sucrose thawing medium and 0.3 mol/l and 0.15 mol/l trehalose for trehalose thawing medium and in warming process. One of the factors that contribute in successful of oocyte vitrification is the embryo development.
Overall, this study demonstrated a good embryo development and morphological grading in sucrose and trehalose vitrification media. (Fig. 1-3) This study is in agreement with Borini et al that a combination of cryoprotectants were more successful compared to a single of cryoprotectant.4 This present study showed that in embryo development, trehalose medium seems more superior compared to sucrose medium, even though Kitazato was the most superior compared to both, in embryo development. (Fig. 1) As explained in method section above, Kitazato contains of trehalose as well. In addition, in the morphological grading, this study demonstrated that in 2-cells embryo, there were no significant differences between the three cryoprotectants, even though Grade 1 embryos were significantly higher compared to other grades. (Fig. 2) Moreover, in morphological grading of 8-cells embryo, trehalose medium appeared to be superior compared to sucrose medium, even though seemed more inferior compared to Kitazato. (Fig. 3)
The newly highlight in order obtaining high glass-forming ability, less toxicity and low viscosity with a mixture of a cryoprotectant is the elusive goal of current vitrification research. High concentrations of cryoprotectants together with rapid cooling rates are necessary for a successful vitrification, yet there is still a possibility causing cryoprotectant toxicity. The introduction of cryoprotectants with higher membrane permeability, less toxicity, and possible use of combinations of non-permeable cryoprotectants, overcome cytotoxicity.30 Interestingly, it has been reported that oocyte vitrification may not require a high concentration of cryoprotectant in the vitrification solution.28,29 In this study, we were using 16.5% EG, 16.5% DMSO, 0.5 mol/l sucrose and 16.5% EG, 16.5% DMSO, 0.5 mol/l trehalose. Various substances including polymers with low toxicity have been suggested for this purpose. The traditionally utilization either sucrose or trehalose seems to be the most appropriate as well as another molecules by increasing viscosity such as ficoll. They act as an osmotic buffer, preventing ‘osmotic shock’ following dilution of the cryoprotectant after warming procedure. Nonetheless, non-penetrating cryoprotectants can assist vitrification, due to its nucleators are extracellular and dehydration allows for intracellular vitrification by bound water. Sugar involvement in extracellular vitrification may avoid cell membrane become touching and fusing to each other.31
This study was the sequence of our previous studies that conducted a comparison research between the sucrose and trehalose cryoprotectant media, based on apoptotic level and mitochondrial membrane potential. The result of this study was in agreement with our previously researches in which the vitrified oocytes using sucrose media were potential being injured than trehalose. This study confirmed those reported by other authors Crowe JH et al, thus indicating that, based on embryo development, trehalose was more effective and safe as cryoprotectant than sucrose. Numerous studies reported that trehalose was superior to another sugar such as in keeping membrane stabilization, maintaining liposomes stabilization during drying and preserving biologic materials.31
Conclusion
In conclusion, the results confirm that trehalose was proved as the more successful cryoprotectant in oocyte vitrification based on embryo development, compared to sucrose. A modification of cryoprotectant media as an update of oocyte vitrification consisting 0.5 mol/l trehalose concentration as extracellular cryoprotectant, combined with 16.5% EG and 16.5% DMSO as intracellular cryoprotectant, has designed.
Acknowledgement
The authors are grateful to Hibah PITTA DRPM 2017 for supporting this study and research assistant Meidika Dara Rizki and Debby Aditya for editorial assistance
Conflict of Interest
There is no conflict of interest
Funding Source
Funding support for this study was received from Hibah Publikasi Terindeks Internasional untuk Tugas Akhir Mahasiswa (PITTA), Direktorat Riset dan Pengabdian Masyarakat (DRPM) Universitas Indonesia, 2017.
References
- Chamayou S, Alecci C, Ragolia C, Storaci G, Maglia E, Russo E, et al. Comparison of in-vitro outcomes from cryopreserved oocytes and sibling fresh oocytes. Reprod Biomed Online. 2006;12(6):730-6.
CrossRef - Rienzi L, Ubaldi F.M, Iacobelli M, Minasi M.G, Romano S, Ferrero S, et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90(5):1692-700.
CrossRef - Borini A, Lagalla C, Bonu M.A, Bianchi V, Flamigni C, Coticchio G. Cumulative pregnancy rates resulting from the use of fresh and frozen oocytes: 7 years’ experience. Reprod Biomed Online. 2006;12(4):481-6.
CrossRef - Borini A, Sciajno R, Bianchi V, Sereni E, Flamigni C, Coticchio G. Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod. 2005;21(2):512-7.
CrossRef - Borini A, Bianchi V, Bonu M, Sciajno R, Sereni E, Cattoli M, et al. Evidence-based clinical outcome of oocyte slow cooling. Reprod Biomed Online. 2007;15(2):175-81.
CrossRef - La Sala G.B, Nicoli A, Villani M.T, Pescarini M, Gallinelli A, Blickstein I. Outcome of 518 salvage oocyte-cryopreservation cycles performed as a routine procedure in an in vitro fertilization program. Fertil Steril. 2006;86(5):1423-7.
CrossRef - Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25(1):66-73.
CrossRef - Nagy Z.P, Chang C-C, Shapiro D.B, Bernal D.P, Elsner C.W, Mitchell-Leef D, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92(2):520-6.
CrossRef - Ford P, Merot J, Jawerbaum A, Gimeno M, Capurro C, Parisi M. Water permeability in rat oocytes at different maturity stages: aquaporin-9 expression. J Membr Biol. 2000;176(2):151-8.
CrossRef - denAbbeel V.E, Schneider U, Liu J, Agca Y, Critser J.K, Van Steirteghem A. Osmotic responses and tolerance limits to changes in external osmolalities, and oolemma permeability characteristics, of human in vitro matured MII oocytes. Hum Reprod. 2007;22(7):1959-72.
CrossRef - Parmegiani L, Cognigni G, Bernardi S, Ciampaglia W, Infante F, Pocognoli P, et al. Freezing within 2 h from oocyte retrieval increases the efficiency of human oocyte cryopreservation when using a slow freezing/rapid thawing protocol with high sucrose concentration. Hum Reprod. 2008;23(8):1771-7.
CrossRef - Parmegiani L, Accorsi A, Cognigni G.E, Bernardi S, Troilo E, Filicori M. Sterilization of liquid nitrogen with ultraviolet irradiation for safe vitrification of human oocytes or embryos. Fertil Steril. 2010;94(4):1525-8.
CrossRef - Magli M.C, Lappi M, Ferraretti A.P, Capoti A, Ruberti A, Gianaroli L. Impact of oocyte cryopreservation on embryo development. Fertil Steril. 2010;93(2):510-6.
CrossRef - Vajta G, Nagy Z.P. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12(6):779-96.
CrossRef - Gardner D.K, Sheehan C.B, Rienzi L, Katz-Jaffe M, Larman M.G. Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology. 2007;67(1):64-72.
CrossRef - Kuwayama M, Vajta G, Kato O, Leibo S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod .Biomed .Online. 2005;11(3):300-8.
CrossRef - Emeka C. Sucrose and propylene glycol effect on the vitrification of oocytes in sheep. Glob J Anim Environ Biol. 2015;3(1):26-31.
- Eroglu A, Toner M, Toth TL. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil Steril. 2002;77(1):152-8.
CrossRef - Kasai M, Ito K, Edashige K. Morphological appearance of the cryopreserved mouse blastocyst as a tool to identify the type of cryoinjury. Hum Reprod. 2002;17(7):1863-74.
CrossRef - Mukaida T, Nakamura S, Tomiyama T, Wada S, Kasai M, Takahashi K. Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless technique. Fertil Steril. 2001;76(3):618-20.
CrossRef - Veeck L.L. Oocyte assessment and biological performance. Ann. NY. Acad. 1988;541(1):259-74.
CrossRef - Rienzi L, Ubaldi F, Iacobelli M, Ferrero S, Minasi M.G, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod. 2002;17(7):1852-5.
CrossRef - Elnahas A, Alcolak E, Marar E.A, Elnahas T, Elnahas K, Palapelas V, et al. Vitrification of human oocytes and different development stages of embryos: An overview. Middle East Fertil Soc J. 2010;15(1):2-9.
CrossRef - Glenister P, Wood M.J, Kirby C, Whittingham D. Incidence of chromosome anomalies in first‐cleavage mouse embryos obtained from frozen‐thawed oocytes fertilized in vitro. Gamete Res. 1987;16(3):205-16.
CrossRef - Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A. Birth following vitrification of a small number of human oocytes: case report. Hum Reprod. 1999;14(12):3077-9.
CrossRef - Yoon T.K, Kim T.J, Park S.E, Hong S.W, Ko J.J, Chung H.M, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization–embryo transfer program. Fertil Steril. 2003;79(6):1323-6.
CrossRef - Alcolak E, Marar E.A, Mytas S.C, Chalvatzas N, Palapelas V, Schöpper B, et al. Comparison of two different media for vitrification and rewarming of human zygotes: Prospective randomized study. Middle East Fertil Soc J. 2011;16(3):189-93.
CrossRef - Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to−196 C in dilutions of a standard vitrification solution. PloS one. 2012;7(4):e36058.
CrossRef - Chian R-C, Wang Y, Li Y-R. Oocyte vitrification: advances, progress and future goals. J Assist.Reprod .Genet. 2014;31(4):411-20.
CrossRef - Katayama K.P, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil Steril. 2003;80(1):223-4.
CrossRef - Crowe J.H, Carpenter J.F, Crowe L.M. The role of vitrification in anhydrobiosis. Annu Rev.Physiol. 1998;60(1):73-103.
CrossRef








