Gek Ayu Krismayogi1 , IGA Dewi Ratnayanti2
, IGA Dewi Ratnayanti2 , Ni Made Linawati2
, Ni Made Linawati2 , IGN Sri Wiryawan2
, IGN Sri Wiryawan2 , I Wayan Sugiritama2
, I Wayan Sugiritama2 , Ida Ayu Ika Wahyuniari2
, Ida Ayu Ika Wahyuniari2 and IGK Nyoman Arijana2
and IGK Nyoman Arijana2
1Medical Student of Udayana University, PB. Sudirman Street, Denpasar, Bali, Indonesia.
2Histology Department of Medical Faculty Udayana University, PB. Sudirman Street, Denpasar, Bali, Indonesia.
Corresponding Author E-mail: ratnayanti@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1378
Abstract
Early manifestation of skin damage from acute UV-B exposure is erythema. Previous studies showed that antioxidants are necessary for inhibiting the process of skin damage due to UV radiation through antioxidant properties and inflammation inhibition. Purple Cabbage which plants in Tabanan, Bali, Indonesia was known to have high concentration of antioxidant. This study aimed to assess the purple cabbage ethanol extract cream effect on UV-B exposed skin erythema of male Wistar rats. A post-test only control group design research was conducted on 30 male rats. Erythema was induced by 200 mJ/cm2 UV-B radiation to the rat’s back skin. Groups were divided into control, cream base, 5% cream, 10% cream, and 20% cream concentrations. The dosage was applied once to the erythema area. Erythema scores were then evaluated based on visual evaluation and erythema diameter as described by Ali. All statistical analyses were performed using SPSS v. 20.0. The control group's erythema score was significantly different from the cream treated groups but not with cream base group. The mean difference, 95% CI, and p value for each dose was 2.17, 1.83-2.50, 0.003; 2.00, 2.00-2.00, 0.007; and 1.67, 1.66-1.76, 0.007 for 5% cream, 10% cream and 20% cream respectively. This study showed that the purple cabbage ethanol extract cream with 5%, 10%, and 20% dose could inhibit the effects of acute UV-B exposure.
Keywords
UV-B; Erythema; Purple Cabbage
Download this article as:| Copy the following to cite this article: Krismayogi G. A, Ratnayanti I. G. A. D, Linawati N. M, Wiryawan I. G. N. S, Sugiritama I. W, Wahyuniari I. A. I, Arijana I. G. K. N. Purple Cabbage Extract Cream Effect on Erythema Score of Male Wistar Rats Back Skin Exposed to UV-B Radiation. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Krismayogi G. A, Ratnayanti I. G. A. D, Linawati N. M, Wiryawan I. G. N. S, Sugiritama I. W, Wahyuniari I. A. I, Arijana I. G. K. N. Purple Cabbage Extract Cream Effect on Erythema Score of Male Wistar Rats Back Skin Exposed to UV-B Radiation. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19651 |
Introduction
People in a tropical country with sun exposure 10-12 hours per day are susceptible to skin damage and premature aging of the skin.1,2,3 The UVR spectrum (Ultraviolet Radiation) consists of UV-A, UV-B, and UV-C. UV-A and UV-B can cause skin aging while UV-C is absorbed by the ozone layer, but it is known that UV-C can reach the earth by penetrating the thinning ozone layer.4,5 Clinical features of skin damage from acute UVR exposure are sunburn and erythema by both UV-A and UV-B.6,7,8
UV-B induced-reactive oxygen species activates Nuclear Factor kappa B (NFkB) signalling through Mitogen Activated Protein Kinase (MAPK) pathway.[9,10]This signalling process will stimulate numerous cytokines and inflammatory mediators production resulting in acute reaction to UVB.11,12 Prostaglandin E2 (PGE2) and histamine are the main mediators causing blood vessels dilatation that leads to erythema. Prostaglandin E2 causes vasodilatation by activating prostaglandin E (EP) receptors 2 and 4. Histamine increases further production of PGE2 or directly induces blood vessel alteration through nitrite oxide (NO) mediated pathway.13,14
Since oxidative stress is the major underlying process of UVB reaction, antioxidants are essential to inhibit skin damage.15 A number of antioxidant compounds are contained in many tropical and subtropical plants that grow in Indonesia.16 Purple Cabbage is a plant that began to be developed in Eka Karya Botanical Garden Bedugul, Tabanan, Bali, Indonesia. Based on research by Tendaj M., it is known that purple cabbage is a vegetable with an average anthocyanin content of 172.39 mg 100 g-1, a value higher than that of purple violet sweet potatoes 61.85 mg 100 g-1.17 Previous studies showed that anthocyanin is a potent free radical scavenging agent and an effective inflammation inhibitor by regulating inflammatory mediators.18,19,20 But, the protective effect of purple cabbage ethanol extract cream on UV-B induced skin erythema has not been investigated.
Subjects and Methods
Subjects
Thirty Wistar rats were used in this experimental study based on Federer formula calculation. Subjects were 3-4 months age, males and divided into five groups. The study protocol approved by Ethics Committee of Udayana University of Medical Faculty/ RSUP Sanglah Denpasar (No.1009/UN.14.2/KEP/2017). Subjects were excluded if having any skin disease and die.
Induction of Erythema
To induce erythema, UV-B light from Polish Ultraviolet broadband, Philips type UVB-311nm (pl-s 9w/01) lamp were applied on shaved rat’s back skin with an area of 4×4 cm. Before induction of erythema, a preliminary test was performed to determine an appropriate minimal dosage for erythema due to UV-B radiation on subjects. Each group was given different treatment as follows, the first rat was given UV-B with dose 25 mJ/cm2, the second rat was given 50 mJ/cm2, the third rat was given 100 mJ/cm2, and the fourth rat was given 200 mJ/cm2. Erythema was observed at the rat’s back skin after 24 hours. Based on the preliminary test, it was found that the minimal erythema dose was 200 mJ/cm2.
Application of Substances
Substances applied to 30 rats based on the groups (control as untreated subjects, cream base, 5% cream of purple cabbage ethanol extract, 10% cream of purple cabbage ethanol extract, and 20% cream of purple cabbage ethanol extract). All rats were exposed to 200 mJ/cm2 UVB radiation. Each of cream formulation was applied once after the exposure to the treatment area.
Design of experiment
This research was an experimental research using control group post-test only design. Subjects were placed randomly into five groups. Post-test values were then compared to determine the effectiveness of treatment.
Erythema assessment
Assessment was performed by the authors and two non author observers 24 hours after treatment application and the score was given as described by Ali (Table 1).21
Table 1: Score of Erythema
| Skin Reaction | Score |
| Without erythema | 0 |
| Very little erythema (barely visible. diameter <25 mm) | 1 |
| Erythema is clearly visible (diameter 25. 1-30 mm) | 2 |
| Moderate erythema (diameter 30. 1-35 mm) | 3 |
| Severe erythema (dark red with forming scar. diameter> 35 mm) | 4 |
Statistical analysis
All statistical analysis was performed using SPSS v. 20.0 (IBM Corp., Armonk, NY, USA). Statistical significance was considered when p < 0.05. Data were analysed using Kruskal Wallis test and Mann Whitney test to compare data between groups.
Result
Based on descriptive test result of rat’s back skin erythema score, the means were varied (Table 2) and could be seen in Figures 1-5. The Shapiro-Wilk test was used to test the normality of the erythema score data. It could be concluded that the data were not normally distributed at p<0.05 (Table 3). Data transformation with square root presented in Table 4 were also resulted in abnormal distribution (p < 0,05), therefore treatment effect analysis should use non parametric test.
Levene’s test was performed to investigate the homogeneity of erythema scores and it was found that all data were homogeneous with p > 0.05 (Table 5). Kruskal Wallis test was used to compare erythema score in each group. Data in Table 6 show that the erythema scores after treatment were significantly different (p < 0.05). Mann-Whitney test was performed to find out the difference between each group (Table 7). There was no difference between control and cream base group (p > 0.05). Groups treated with 5%, 10%, and 20% cream showed significant difference (p < 0.05) with the control group and cream base group, even though there was no difference between the three groups (p > 0.05).
Table 2: Descriptive Test Result of Male Wistar Rat’s Back Skin Erythema Score
| Erythema | Sampels (n) | Mean | Standard Deviation |
| Control | |||
| Before | 6 | 2.5 | 0.548 |
| After | 2.5 | 0.837 | |
| Cream Base | |||
| Before | 6 | 2.5 | 1.049 |
| After | 2 | 0.894 | |
| 5% | |||
| Before | 6 | 2 | 0.632 |
| After | 0.33 | 0.516 | |
| 10% | |||
| Before | 6 | 2.17 | 0.983 |
| After | 0.5 | 0.837 | |
| 20% | |||
| Before | 6 | 2.33 | 0.816 |
| After | 0.83 | 0.753 | |
| Total | |||
| Before | 30 | 2.3 | 0.794 |
| After | 1.23 | 1.135 |
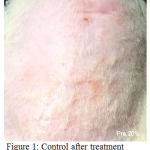 |
Figure 1: control after treatment
|
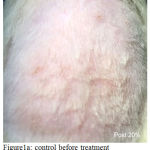 |
Figure 1a: Control before treatment
|
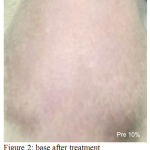 |
Figure 2: Base After Treatment
|
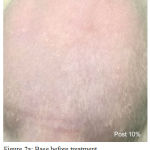 |
Figure 2a: Base Before Treatment
|
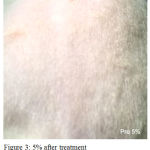 |
Figure 3: 5% after treatment
|
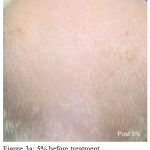 |
Figure 3a: 5% before treatment
|
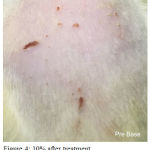 |
Figure 4: 10% after treatment
|
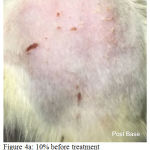 |
Figure 4a: 10% before treatment
|
 |
Figure 5: 20% after treatment
|
 |
Figure 5a: 20% before treatment
|
Table 3: Normality Test Result of Male Wistar Rat’s Back Skin Erythema Score
| Group | Sampels (n) | p |
| Control | ||
| Before | 6 | 0.004* |
| After | 0.006* | |
| Cream Base | ||
| Before | 6 | 0.82 |
| After | 0.167 | |
| 5% | ||
| Before | 6 | 0.101 |
| After | 0.001* | |
| 10% | ||
| Before | 6 | 0.035* |
| After | 0.006* | |
| 20% | ||
| Before | 6 | 0.091 |
| After | 0.212 |
Description: (p= p value) *= not normal at p<0.05
Table 4: Normality Test Result After Square Root Data Transform of Male Wistar Rat’s Back Skin Erythema Score
| Group | Sampels (n) | p |
| Control | ||
| Before | 6 | 0.004* |
| After | 0.007* | |
| Cream Base | ||
| Before | 6 | 0.771** |
| After | 0.158** | |
| 5% | ||
| Before | 6 | 0.090** |
| After | 0.001* | |
| 10% | ||
| Before | 6 | 0.032** |
| After | 0.007* | |
| 20% | ||
| Before | 6 | 0.079* |
| After | 0.168* |
Description: *= not normal at p<0.05;
**= not normal at p>0.05
Table 5: Homogeneity Test Result of Male Wistar Rat Back’s Skin Erythema Score
| Data Variation | Levene Statistic | p |
| Erythema Score | ||
| Before | 1.771 | 0.166* |
| After | 0.359 | 0.835* |
Description: (p= p value)* = homogeneous at p> 0.05
Table 6: Kruskal-Wallis Test Result of Male Wistar Rat’s Back Skin Erythema Score
| Group | Median | Mean±SD. | p |
| (min-max) | |||
| Before | |||
| Control | 2.50 (2-3) | 2.50±0.548 | |
| Base | 2.50 (1-4) | 2.50±1.049 | |
| 5% | 2.00 (1-3) | 2.00±0.632 | |
| 10% | 2.50 (1-3) | 2.17±0.983 | |
| 20% | 2.50 (1-3) | 2.33±0.816 | 0.748 |
| After | |||
| Control | 2.00 (2-4) | 2.50±0.837 | |
| Base | 2.00 (1-3) | 2.00±0.894 | |
| 5% | 0.00 (0-1) | 0.33±0.516 | |
| 10% | 0.00(0-2) | 0.50±0.837 | |
| 20% | 1.00 (0-2) | 0.83±0.753 | 0.001* |
Description: (Min= Minimum. Max= Maximum.
SD= Standard Deviation. p= p value)
* = significantly different at p <0.05
Table 7: Mann-Whitney Test Result of Male Wistar Rat’s Back Skin Erythema Score After Treatment
| Group | Mean Difference | p |
| (95% CI) | ||
| Control vs Base | 0.50 (0.44-0.56) | 0.388 |
| Control vs 5% | 2.17 (1.83-2.50) | 0.003* |
| Control vs 10% | 2.00 (2.00-2.00) | 0.007* |
| Control vs 20% | 1.67 (1.66-1.76) | 0.007* |
| Base vs 5% | 1.67 (0.88-1.27) | 0.008* |
| Base vs 10% | 1.50 (1.44-1.56) | 0.020* |
| Base vs 20% | 1.17 (1.10-1.32) | 0.044* |
| 5% vs 10% | -0.17(-0.50-0.17) | 0.847 |
| 5% vs 20% | -0.50(-0.74-(-0.1)) | 0.212 |
| 10% vs 20% | -0.33(-0.34-(-0.24)) | 0.382 |
Description: (CI= Confident Interval, p= p value)
* = significantly different at p <0.05
Discussion
This study showed that the cream extract of purple cabbage ethanol either cream 5%, 10%, or 20% were able to inhibit the effects of acute UV exposure. This is in accordance with several studies both in animals and humans that prove the benefit of antioxidants against UVR effect. The research by Rinawati showed that the presence of polyphenol compounds in the jatropha gum which has antioxidant activity is able to decrease the erythema of the treatment group more quickly than the control group.22
Kelagan water extract and waterleaf extract of pegagan as anti-inflammatory also showed the decrease of erythema treatment group faster than the control group.23 Similar studies had also been conducted by Wibawa, from the research the mimba leaf extract was able to reduce the area of erythema larger than the control group.24 Animal study about BCME anti-erythema activity explained that mice treated with Buddleja cordata extract after induced by UVB showed less redness than the control group.25 Application of 5% and 10% S-Methylmethionine sulfonium (SMMS) before and after UVB-irradiation significantly decreased the UVB-induced erythema index.26 In the human study done by Zhou concluded that topically applied Soy Oligopeptides in UVB-induced acute photo damage showed significant decrease of erythema index compare to control group and there was no significant difference in the administration of different doses.27
Erythema is induced by UV-B radiation, the absorbed UV-B light causes cellular immunologic changes leads to blood vessels dilatation. UV-B induces oxidative stress through direct photolysis of water, enzyme/substrates reactions, and cystein-containing protein. Increase reactive oxygen species is known to stimulate activation of NFkB through MAPK pathway that triggers complex cellular inflammation process in the skin.9,11 Following UVB exposure, IκB, negative regulator of NFkB, is directly degraded as early response, or phosphorylated by IkB kinase as late response.
Phosphorylation of IkB leads to ubiquitination and degradation by the proteasome, resulting in the rapid translocation of NF-κB to the nucleus. NFkB binds to κB binding sites in the promoter region of target genes, and induces the transcription of pro-inflammatory mediators, including inducible nitric oxide synthase (iNOS), TNF-α, IL-1, IL-6, IL 8, VEGF, and others.10,28 These substances mediate subsequent complex immune reactions involving keratinocyte, fibroblast, mast cell, and multiple immune cells that result in acute reaction of UVB radiation.12,15,29,30
Interleukin-1 stimulates degranulation of mast cell, a major source of proinflammatory mediators, releasing more IL-1, TNF-α, PGE2, histamine etc. IL-1 and TNF-α can also stimulate PGE2 synthesis through phospholipase A2 and cyclooxigenase 2.9,12,30 Prostaglandin E2 induces vasodilatation due to increase cAMP through adenylyl cyclase activation by EP2 and EP4 prostaglandin receptors.13,31 Histamine increases further production of PGE2 or directly induces blood vessel alteration through nitrite oxide (NO) mediated pathway.14 TNF-α and histamine also induce activation of nitric oxide synthase (NOS) and xanthine oxidase (XO) result in increase release of Nitric Oxide (NO) and superoxide anions after UV-B exposure.29,32
No regulates vascular tone by stimulating the soluble guanylyl cyclase (sGC) in vascular smooth muscle cells to induce the formation of cyclic guanosine monophosphate (cGMP). Cyclic GMP activates protein kinase G (PKG) which prevents calcium influx, PKG also works on Sarco / endoplasmic reticulum calcium ATPase (SERCA) to recover cytosolic calcium into the sarcoplasmic reticulum. Intracellular calcium concentration decreases and calmodulin is inactivated, calcium depletion also increases myosin light chain phosphatase activity leading to the breakdown of myosin-actin bonding and vascular smooth muscle relaxation. [33,34] Proinflammatory cytokines also stimulate the inflammatory cells to secrete the NADPH oxidase (NOx) enzyme causing superoxide anion formation leading in further oxidative stress concentration in the dermis layer.15,29
Through which mechanism that the purple cabbage extract inhibit UV-B-induced erythema has not been elucidated in this study and are subject for further research. The purple cabbage contains anthocyanin, vitamin A, vitamin C, vitamin E, vitamin K, carotene, folate, sugar solution, and phenol compounds.16 A study of fresh purple cabbage ethanol extract cream by Senja et al found that high anthocyanin content was directly proportional to the high antioxidant potential.[35] Antioxidants can reduce the occurrence of acute oxidative damage or ROS through electron donor mechanism. Some studies using the extract of purple sweet potato showed that flavonoid content such as anthocyanin can act as free radical scavenging agent to protect the endothelial cell from oxidative stress, prevent the impaired renal function due to oxidative stress, and protect the oxidative stress of pancreatic β-cell.33,34,36 Moreover, anthocyanin inhibit NF-kB as the key transcription factor involved in inflammation. UVB-induced rat skin when given Cyanidin-3-Glucoside (C3G) prevent the nuclear translocation of NF-kB.28 Cross-sectional study in a population of US adults found higher intakes of anthocyanins and flavonols were associated with lower pro-inflammatory cytokine levels and oxidative stress biomarker concentrations.21 Previous research has also shown that polyphenolic compounds contained in green tea have anti-inflammatory properties.[22] Polyphenol compounds are able to act as photoprotective substances against skin inflammation due to UV exposure, oxidative stress, DNA damage, and all three.36 The study of interventions in carotene-rich dietary men suggests the micronutrients may protect against sun damage. Consumption of micronutrients such as vitamin E, Vitamin C, polyphenols contributes to defence against oxidants and endogenous photoprotection.37
The result of this study also showed that there was no significant difference of erythema score between groups treated with 5%, 10% and 20% creams, which means increasing cream dose did not correlate with the effectiveness of the cream. Lower effective dose of this substance needs to be evaluated further. On the other hand, the cream base did not have protective effect to UV-B radiation that suggested the protective effect was due to the purple cabbage extract rather than the cream base.
Conclusion
The purple cabbage ethanol extract cream is able to inhibit the effects of acute UV exposure indicated by reduce erythema score. All cream dose of purple cabbage ethanol extract has equal effectiveness. Further studies are needed to assess the minimum dosage, mechanism, side effects, clinical benefits, and human studies.
Acknowledgement
We would like to appreciate Department of Histology, Faculty of Medicine, Udayana University, all the faculties, all the doctor and students who helped us to carry out this study.
Conflicts of Interest
There is no conflicts of interest.
Financial Support
This Research Project was partially suppported by Research and Development Unit Medical Faculty of Udayana University (No SPK 3697/UN/14.2/PP/2017)
References
- Widodo D. A and Suryono T. A. Pemberdayaan Energi Matahari sebagai Energi Listrik Lampu Pengatur Lalu Lintas. Telementri. 2010;2(2):133–8.
- Rinnerthaler M., Bischof J., Streubel M. K., et al. Oxidative Stress in Aging Human Skin. Breitenbach M, Eckl P, eds. Biomolecules. 2015;5(2):545-89.
CrossRef - Cahoon E.K., Wheeler D. C., Kimlin M. G., et al. Individual, Environmental, and Meteorological Predictors of Daily Personal Ultraviolet Radiation Exposure Measurements in a United States Cohort Study. Chammas R., ed. PLoS ONE. 2013;8(2):e54983.
- Laga A. C and Murphy G. F. The Translational Basis of Human Cutaneous Photoaging : On Models, Methods, and Meaning. The American Journal of Pathology. 2009;174(2):357-60.
CrossRef - Crompton A., Gamage K., Bell S., et al. First Results of Using a UVTron Flame Sensor to Detect Alpha-Induced Air Fluorescence in the UVC Wavelength Range. 2017;17(12):2756.
- Gaddameedhi S., Selby C.P., Kemp M. G., et al. The Circadian Clock Controls Sunburn Apoptosis and Erythema in Mouse Skin. The Journal of investigative dermatology. 2015;135(4):1119-27.
CrossRef - Bosch R., Philips N., Suárez-Pérez J. A., et al. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants. 2015;4(2):248-68.
CrossRef - Marionnet C., Tricaud C., Bernerd F. Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. Piva T., ed. International Journal of Molecular Sciences. 2015;16(1):68-90.
CrossRef - Morgan M. J and Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research. 2011;21(1):103-115. doi:10.1038/cr.2010.178.
CrossRef - López-Camarillo C., Ocampo E. A., Casamichana M. L., et al. Protein Kinases and Transcription Factors Activation in Response to UV-Radiation of Skin: Implications for Carcinogenesis. International Journal of Molecular Sciences. 2012;13(1):142-72.
CrossRef - Radhiga T., Agilan B., Muzaffer U., et al. Phytochemicals as modulators of ultraviolet-b radiation induced cellular and molecular events: A review. J Radiat Cancer Res. 2016;7:2-12.
CrossRef - Ravi R and Piva T. The Role of Furin in the Development of Skin Cancer. Highlights in Skin Cancer Pierre Vereecken. Intech Open. 2013
- Kim G. H. Renal Effects of Prostaglandins and Cyclooxygenase-2 Inhibitors. Electrolytes & Blood Pressure E & BP. 2008;6(1):35-41.
CrossRef - Ashina K., Tsubosaka Y., Nakamura T., et al. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. Komarova Y., ed. PLoS ONE. 2015;10(7):e0132367.
- Kammeyer A and Luiten R. M. Oxidation events and skin aging. Ageing Res Rev. Elsevier B.V. 2015;21:16–29.
CrossRef - Sotelo T., Cartea M. E., Velasco P., et al.Identification of Antioxidant Capacity -Related QTLs in Brassica oleracea. PLoS One. 2014;9(9):e107290.
CrossRef - Tendaj M., Sawicki K., Mysiak B. The Content Of SomeChemical Compounds In Red Cabbage (Brassica Oleracea L.Var.Capitata F. Rubra) After Harvest And Long-Term Storag .2013;16(2):2.
- Amin F. U., Shah S. A., Badshah H., et al. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1–42-induced oxidative stress. Journal of Nanobiotechnology. 2017;15:12.
CrossRef - Rupasinghe H. P. V., Boehm M. M. A., Sekhon-Loodu S., et al.. Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent. Breitenbach M., Eckl P., eds. Biomolecules. 2015;5(2):1079-1098.
CrossRef - Chandrasenan P., Anjumol V. M., Neethu M. V., et al. Cytoprotective and antiinflammatory effect of polyphenolic fraction from Red cabbage (Brassica oleracea Linn var. capitata f rubra) in experimentally induced ulcerative colitis. J Appl Pharm Sci. 2016;6(1):137–46.
CrossRef - Ali A. In Vivo Skin Irritation Potential of a Cream Containing Moringa Oleifera Leaf Extract. African J Pharm Pharmacol. 2013;7(6):289–93.
CrossRef - Agustina R and Suhartono E. Penyembuhan Luka Dengan Penurunan Eritema Pada Tikus Putih (Rattus Norvegicus) yang Diberikan Getah Batang Jarak Cina (Jatropha Multifida L.). 2015;3(1):1–11.
- Mustahdiati N., Rizany I., Suhartono E., et al. Peran Ekstrak Air Kelakai dan Ekstrak Air Daun Pegagan dalam Mempercepat Penurunan Eritema pada Model Tikus Putih yang Mengalami Luka Terkontaminasi. Jurnal Dunia Keperawatan. 2014;2:93–100.
CrossRef - Wibawa K., Ekstrak E., Terhadap D.,pada E .,Putih T., oleh B., Alat R., Modifikasi U. V. 04-08. University of Surabaya-Fakultas Farmasi. Pharmacy and material medica. 2014.
- Acevedo J.G. A.,González A. M. E., Campos D. M. y., et al. Photoprotection of Buddleja cordataextract against UVB-induced skin damage in SKH-1 hairless mice. BMC Complementary and Alternative Medicine. 2014;14:281.
CrossRef - Kim W. S., Seo H. M., Kim W. K., et al. The Photoprotective Effect of S-Methylmethionine Sulfonium in Skin. Piva T, ed. International Journal of Molecular Sciences. 2015;16(8):17088-100.
CrossRef - Zhou B., Ma L., Liu J., et al. Protective Effects of Soy Oligopeptides in Ultraviolet B-Induced Acute Photodamage of Human Skin. Oxidative Medicine and Cellular Longevity. 2016;2016:5846.
CrossRef - Pratheeshkumar P., Son Y. O., Wang X., et al. Cyanidin-3-Glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signalling pathways in SKH-1 hairless mice skin. Toxicology and applied pharmacology. 2014;280(1):127-37.
CrossRef - Jeon S. Y., Lee C. Y., Song K. H., et al. Spectrophotometric Measurement of Minimal Erythema Dose Sites after Narrowband Ultraviolet B Phototesting: Clinical Implication of Spetrophotometric Values in Phototherapy. Annals of Dermatology. 2014;26(1):17-25.
CrossRef - Lee C., Lin C., Lee I., et al. Activation and induction of cytosolic phospholipase A2by TNF‐α mediated through Nox2, MAPKs, NF‐κB, and p300 in human tracheal smooth muscle cells. Journal of Cellular Physiology. 2011;226(8):2103-14.
CrossRef - Ricciotti E and FitzGerald G. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986-1000.
CrossRef - Deliconstantinos G and Villiotou V. NO Synthase and Xanthine Oxidase Activities of Rabbit Brain. NeurochemRes. 1996;21(1):51–61.
CrossRef - Utami A. N. 2009. Perbandingan Efek Antiinflamasi Kurkumin 1% dalamVehikulum Krim dan Salep Pada Kulit Mencit Yang Telah Disinari Ultraviolet. Jakarta. FK UI.
- Zhao Y., Vanhoutte P. M., Leung S. W. S. Vascular Nitric Oxide : Beyond eNOS. J Pharmacol Sci. 2015;129(2):83–94.
CrossRef - Senja R. Y., Issusilaningtyas E., Nugroho A. K., et al. The Comparison of Extraction Method and Solvent Variation on Yield and Antioxidant Activity of Brassica oleracea L . var . capitata f . rubra Extract. 2014;19:2–3.
- Pandel R., Poljšak B., Godic A., et al. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013;2013:1–11.
CrossRef - García E. F. Skin protection against UV light by dietary antioxidants. Food Funct. 2014;(9).







