Manish Kumar1 and Nitin Bansal2
and Nitin Bansal2
1IKG Punjab Technical University, Kapurthala, Punjab, 144603 India.
2Department of Pharmacology, ASBASJSM College of Pharmacy, Bela, Ropar, 140111, India.
Corresponding Author E-mail: nitindsp@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/1347
Abstract
Administration of streptozotocin (STZ) through intracerebroventricular (ICV) route manifests AD symptoms in rats. STZ deregulates the control over GSK-3 and eNOS activities through disruption of phosphoinositides mediated signaling. We attempted to elucidate the functions of GSK-3 and eNOS in memory enhancing activity of ellagic acid (EGA) in STZ (ICV) triggered AD type dementia. A 3 mg/kg dose of STZ was injected gently in lateral cerebral ventricles of rats on day 1 and 3. The rats were given EGA (35 mg/kg b.w.) through oral route for four weeks daily. LiCl (GSK-3 inhibitor) and L-Arginine (NO precursor) were administered for four weeks to explore the modulation of GSK-3 and eNOS respectively by EGA in STZ (ICV) injected rats. MWM and EPM paradigms were utilized for evaluation of memory of rats. The rats were sacrificed on day 28 to determine markers of oxidative stress (TBARS, GSH, SOD, CAT), nitrite, AChE, LDH, TNF-α and eNOS in brain. STZ (ICV) initiated cognitive deficits through enhancement of brain oxidative stress, nitrite, TNF-α, AChE, LDH activity and reduction in eNOS levels. EGA attenuated the rise in oxidative stress, inflammation and LDH activity in STZ (ICV) treated rats. Decrease in nitrite content, AChE activity and resurrection of eNOS activity by EGA averted STZ (ICV) induced memory dysfunction in rats. Chronic inhibition of GSK-3 by LiCl (100 mg/kg, i.p.) enhanced these effects of EGA in STZ (ICV) injected rats which thereby exhibited marked cognitive improvement. L-Arginine group manifested inflation in brain oxidative stress, TNF-α content, AChE and LDH activities. L-Arginine (200 mg/kg, i.p.) surged the nitrite content even though eNOS expression was diminished in brain of EGA and STZ (ICV) administered rats resulting in profound loss of memory. It can be concluded that GSK-3 and eNOS are involved in memory enhancing activity of EGA in STZ (ICV) injected rats.
Keywords
Ellagic Acid; Endothelial NOS; GSK-3;Memory; Streptozotocin
Download this article as:| Copy the following to cite this article: Kumar M, Bansal N. Effects of Chronic Lithium Chloride and L-Arginine Treatment on Prevention of Streptozotocin Induced Cognitive Deficits by Ellagic Acid. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Kumar M, Bansal N. Effects of Chronic Lithium Chloride and L-Arginine Treatment on Prevention of Streptozotocin Induced Cognitive Deficits by Ellagic Acid. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19091 |
Introduction
Ellagic acid (EGA) constitutes natural polyphenol in nuts, pomegranates, berries and beverages.1 EGA enriched dietary supplements (e.g. PomActiv™) enjoy wide popularity owing to its potent antioxidative, anti-inflammatory, vasorelaxing, hypolipidemic, antidiabetic and antitumor properties.2 A clinical study revealed upper GIT as major site of absorption of EGA with extensive in vivo protein binding and intestinal transformation.3 Approximately 90% of orally administered EGA is detected unchanged in blood plasma, urine and faeces, however, a small fraction (10%) of EGA is metabolized to urolithins by gut microbiota in rodents. The broad chemoprotective properties of EGA are validated by its antiapoptotic, cardioprotective, genoprotective, hepatoprotective, neuroprotective, nephroprotective and ulceroprotective activities against a plethora of toxins such as arsenic trioxide, mercuric chloride, 6-hydroxidopamine, cisplatin and acetic acid.4 The neuroprotective activity of EGA has been evaluated in animal studies against Aβ,25-35 colchicine, scopolamine and diazepam signifying the memory enhancing potential of EGA.5
Earlier we demonstrated that EGA prevented cognitive deficits in rats treated with intracerebroventricular streptozotocin (STZ-ICV).1 STZ is a neurotoxic hexose linked N-methyl-N-nitrosourea analog which precipitates Alzhemier’s disease (AD) like dementia in rodents. Centrally administered STZ triggers microglia induced inflammation, redox imbalance, and depletion of cholinergic transmission and ATP/ADP ratio in brain.6 Disruption of insulin signaling and downstream phosphoinositide-3-kinase (PI3K) pathway is key feature of STZ-induced memory dysfunction.7,8 Phosphoinositides mediated signaling regulates miscellaneous cell functions including cell survival, brain development, neuronal plasticity, glucose metabolism, transcription, cellular transport and neurodegeneration through downstream targets glycogen synthase kinase (GSK-3) and endothelial nitric oxide synthase (eNOS). GSK-3 has basal serine-threonine kinase activity, ubiquitously expressed in brain and is implicated in formation of neurofibrillary tangles (NFTs), Aβ plaques, cholinergic hypoactivity and cognitive deficits.9 Involvement of GSK-3 in familial and sporadic forms of AD has prompted its pharmacological targeting (e.g. Tideglusib, LY2090314, Enzastaurin, lithium chloride) with substantial amelioration in AD manifestations.10 Nitric oxide is a retrograde messenger involved in synaptogenesis, neurogenesis and memory functions.11,12 Neuronal and endothelial isoforms of NOS fulfill the physiological NO demands with mutual cooperation rendering eNOS activity pivotal during traumatic or neurotoxic brain insult and is substantiated by a study that revealed intracranial inhibition of eNOS in chicks instigated AD like symptoms.13
ICV injection of STZ compromises the PI3K-Akt mediated control over GSK-3 and eNOS activities in brain.7,8 Aberrant overactivation of GSK-3, fading of eNOS expression and loss of cognitive abilities in response to STZ administration in brain of rats is reported by many studies.8,9 In a previous study chronic EGA administration prevented the loss of memory in STZ (ICV) treated rats.5 In vitro study has disclosed prevention of lipid peroxidation and endothelial apoptosis by EGA through modulation of eNOS activity.14 However, the molecular mechanism of neuroprotective activity of EGA is still eluded. We propose that suppression of GSK-3 overactivity and resurrection of eNOS activity in brain of rats is involved in prevention of memory loss by EGA in STZ (ICV) model.
Materials and Methods
Drugs
A suspension of ellagic acid (Himedia Labs, Mumbai, India) was prepared in 0.1% gum acacia maintaining the temperature 45±5°C and pH 6-81. Streptozotocin (SRL Pvt Ltd., Mumbai, India) was dissolved in 5% DMSO in aCSF vehicle (0.147 mM NaCl, 0.0029 mM KCl, 0.0016 mM MgCl2, 0.0017 mM CaCl2, 0.0022 mM dextrose, pH 7.3)1. L-Arginine and lithium chloride (Himedia Labs, Mumbai) were dissolved in normal saline. The drug solutions were freshly prepared right before administration.
Experimental Animals
The experimental design was approved by Institutional Animal Ethics Committee of the institute. Wistar rats (180-220 g, either sex) were acquired from Central Animal Facility (CAF), AIIMS, New Delhi and were reared at CAF of the institute as per guidelines of CPCSEA, Ministry of Forests and Environment, Government of India. Prior to surgery three rats per cage (44×29×16 cm3) were harbored (temperature, 21-25°C; humidity, 30-50%; 12:12 light-dark cycle) and nurtured with customary rodent diet (pellets from Ashirwad Industries, Mohali, India) and water ad lib. Post-surgery each rat was placed individually in separate cage (30×23×14 cm3) for 7 days allowing access to food and water gratis.
Stereotaxic Surgery of Rat Brain
The body of anesthetized rat (chloral hydrate, 350 mg/kg, i.p.) was placed on warm pad with head positioned in the stereotaxic frame (INCO, Ambala, India). The scalp was incised (mid-sagittal), skin was retracted and the skull was uncovered. A hole was drilled through the parietal bone to access a lateral cerebral ventricle (stereotaxic coordinates: antero-posterior from bregma = -0.8 mm, mediolateral from mid-sagittal suture = ±1.5 mm, dorso-ventral from the skull = ±3.6 mm).1,15 On day one STZ (3 mg/kg b.w.) was injected in randomly chosen lateral cerebral ventricle over 10 min duration (1 µl/min).8 After injection the Hamilton® microneedle was not displaced for 5 min to facilitate diffusivity of drug in CSF. Post drug treatment the hole was repaired with dental cement, skin was sutured and Neosporin® was applied pro re nata to prevent contamination. STZ administration was repeated in remaining lateral ventricle once after 48 h. Sham rats received same volume (10 µl) of ICV-vehicle only on day 1 and 3. Sepsis was avoided by injecting cephazolin sodium (Ranbaxy) (30 mg/kg, i.p.) once postoperatively. Postsurgical hypothermia was prevented by keeping the rats warm (37±0.5°C).
Experimental Design
The animals were acclimatized two weeks before experiments. AD type dementia was induced in rats by injecting STZ (ICV) on day 1 and day 3 one hour after EGA administration1. EGA (35 mg/kg) was administered through oral route for four weeks daily1. L-Arginine (NO precursor; 200 mg/kg, i.p.)16,17 and lithium chloride (GSK-3 inhibitor; 100 mg/kg, i.p.)18 were administered to EGA and STZ (ICV) treated rats for 28 consecutive days. The rats were subjected to Morris water maze (MWM) and elevated plus maze (EPM) tests from day 22 onwards for evaluation of learning and memory functions. After behavioral tests animals were partially anesthetized using diethyl ether and sacrificed by decapitation for biochemical estimations in whole rat brain (Fig. 1).
The rats were indiscriminately disseminated to five different groups having six animals in each group: (i) Sham group (Sham-treated) rats were administered ICV-vehicle (10 µl); (ii) STZ (ICV) group (STZ-ICV) rats were given streptozotocin (3 mg/kg in 10 µl vehicle) alone; (iii) EGA+STZ group (EGA+STZ) was administered EGA (35 mg/kg, p.o.) for 4 weeks daily and STZ (ICV) (3 mg/kg, 10 µl); (iv) LiCl group (EGA+STZ+LiCl) received EGA (35 mg/kg, p.o.), STZ (ICV) (3 mg/kg, 10 µl) and LiCl (100 mg/kg, i.p.); (v) L-Arginine group (EGA+STZ+L-Arginine) received EGA (35 mg/kg, p.o.), STZ (ICV) (3 mg/kg, 10 µl) and L-Arginine (200 mg/kg, i.p.).
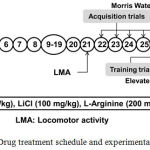 |
Figure 1: Drug treatment schedule and experimental protocol.
|
Closed Field Activity
The mean ambulatory score of different groups was determined on day 1,21 and 28 using actophotometer (INCO, Ambala, India) for a period of 10 min in a dark room and expressed as counts per 10 min1.
Morris Water Maze Test
Spatial navigation memory of rodents was assessed by using MWM in place-condition paradigm which involves learning using allocentric and egocentric cues to escape on a submerged platform placed in a fixed location with start position of each animal randomized with each trial.19 A black colored metallic (iron) circular tank (radius 100 cm, height 60 cm) was filled to a depth of 30 cm with water (temperature 25±1°C) and two threads were fixed at right angle to each other on the rim of the pool to divided the tank into four equal quadrants. A clockwise nomenclature was assigned to quadrants viz. North (N), East (E), South (S) and West (W).1 The standard procedure was adopted having three phases. On day 21 each rat was familiarized with MWM by allowing maze exploration for 120 s without platform. During acquisition phase a black painted metal (iron) platform (area 11 cm2, search area: target ratio of 314:1) was submerged 2 cm below surface of water in the centre of target quadrant (W) of this tank1. The platform was camouflaged by making the water opaque. In place-condition paradigm submerged platform was kept in a fixed location (W) throughout the training session with start position of each animal randomized with each trial. The rat was smoothly released in the water with head facing the wall of tank and allowed 120 s to locate submerged platform. The start location of each animal was randomized viz. from N to W on day 22, E to N on day 23, S to E on day 24, W to S on day 25, N to W on day 26 for each trial. Each rat received four training trials consecutively per day with inter-trial gap of 30 s on the platform. The animal unable to locate the hidden platform within 120 s was guided gently onto platform. The time taken by each rat to locate the hidden platform denotes mean escape latency (MEL). After 24 h of the last acquisition trial retention of memory of each rat was determined in probe trial. The platform was removed, each rat was placed 180° from original platform position to navigate the tank for 60 s to eliminate thigmotaxis20 and mean time spent in all four quadrants was noted1. The reference memory is denoted by percentage of mean time spent by the animal in target quadrant [TSTQ (%)] searching for the camouflaged platform. The distal visual cues and position of experimenter remained same during whole study1.
Elevated Plus Maze Test
EPM consisted of a wooden apparatus having a square central platform (area 100 cm2) connected to two open arms (50 cm×10 cm) and two laterally covered arms with open roof (50 cm×40 cm×10 cm), placed 60 cm above the floor. The duration of entry of rat from the open arm into one of the covered arms (transfer latency) with its entire four paws denoted memory of the animal. On day 27 each rat was placed at the distal-most end of an open arm with head opposite to the central platform and allowed to explore the maze for 90 s. The animal which failed to passage into a closed arm within 90 s was gently guided in one of the covered arms. The rat was further given exploration time of 20 s and then returned to its home cage. The reference memory of this EPM learning was evaluated 24 h after the last trial1.
Whole Brain Tissue Preparation
The whole brain was dissected out and rinsed with ice-cold sterile normal saline (0.9 g/L sodium chloride). Brain tissue homogenate (10% w/v) was prepared in 50 mM sodium potassium phosphate buffer (1% v/v Triton X-100, pH 7.4) at 4°C, centrifuged at 15×103 rpm for twenty minutes (4°C) in high speed refrigerated centrifuge (CPR-30 Remi Compufuge, India), and supernatant was separated from sediment for estimation of AChE, GSH, SOD, CAT, nitrite, LDH and TNF-α.
Determination of Oxidative Stress in Rat Brain
The markers of oxidative stress viz. thiobarbituric acid reactive substance (TBARS), glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) were measured. TBARS was measured by method provided by Ohkawa et al.21 Ellman method was used for GSH estimation.22 SOD activity was determined by method of Winterbourn et al.23 CAT activity is assessed following the method of Claiborne.24
Measurement of Nitrite Content in Rat Brain
Briefly, a mixture of 0.5 ml of Greiss reagent (equal volumes of 1% sulphanilamide in 3 M HCl and 0.1% N-1-Napthyl ethylene diamine dihydrochloride in water) and 0.1 ml supernatant was incubated at room temperature in the dark for 10 min, and the absorbance was measured at λmax= 548 nm (Shimadzu UV-1700, Pharmaspec).25 Total nitrite content (µM/mg of protein) was determined from standard curve of sodium nitrite (10-100 μM).
Estimation of AChE activity in brain of rats
Briefly, the reaction mixture consisted of 0.05 ml of the supernatant, 3 ml of phosphate buffer (100 mM, pH 8), 0.1 ml of 10 mM 5,5′-dithiobis-(2-nitrobenzoic acid), 0.1 ml of acetylthiocholine iodide (1585 mM). Acetylcholinesterase (AChE) activity is expressed as µM acetylthiocholine iodide hydrolysed/min/mg protein (ε=1.36 × 104 M-1cm-1, λmax=412 nm).26
Determination of LDH Activity in Rat Brain
The total reaction mixture (3 ml) contained 1 ml of 200 mM Tris-HCl buffer (pH 7.4), 0.15 ml of 100 mM KCl, 0.15 ml of 50 mM sodium pyruvate, 0.20 ml of 2.4 mM NADH and supernatant.27 A decrease in extinction (6220 M-1cm-1) at λmax=340 nm for 2 min at 25°C was measured and result was expressed in ‘n’ µM NADH oxidized/min/mg protein.
Determination of brain TNF-α and eNOS by ELISA
The rat brain TNF-α (Krishgen, Mumbai) and eNOS (KinesisDX, California) levels were determined by double antibody sandwich ELISA as per instructions provided in kits. The supernatant obtained by centrifuging the brain homogenate at 2500 rpm for 20 min was added to rat monoclonal antibody pre-coated wells (96 wells), treated with secondary antibodies labeled with biotin followed by Streptavidin-Horseradish Peroxidase and incubated at 37°C for 1 h after covering the plate. Afterwards, treatment with chromogenic solution A and B or TMB substrate produced bluish color, stop solution was added to stop the reaction and absorbance was noted at λmax=450 nm in ELISA microplate reader (iMARK, BIORAD) within 15 minutes of stopping reaction. Concentration of eNOS (ng/ml) and TNF-α (pg/ml) in unknown sample was calculated from standard curve.
Estimation of Total Protein in Rat Brain
The reaction mixture consisted of 0.25 ml of supernatant, 0.75 ml phosphate buffer, 5 ml of Lowry’s reagent and 0.5 ml of Folin-Ciocalteu reagent (1 N). After incubation the protein content was determined spectrophotometrically at λmax=650 nm with a standard curve (0.25-2.50 mg/ml of bovine serum albumin).28
Histopathology of Rat Brain
The rats (n=1) were injected with chloral hydrate (400 mg/kg, i.p.) and given transcardial perfusion with 10% neutral buffered formalin solution (10% NBF) by using gravity fed perfusion setup. The head was decapitated; whole brain was dissected out and fixed in 10:1 ratio of fixative (10% NBF) to tissue for 6 days at 4°C. Afterwards, the brain was stored in 70% ethanol at 4°C until sectioning. 5 μm sections were trimmed out by microtome and treated with haematoxylin-eosin (H&E) stain. Slides were then cover-slipped with permanent mounting medium (synthetic resin DPX) and examined in light microscopy at ×45 magnifications.
Statistical Analysis
One-way ANOVA followed by Tukey’s post-hoc test and two-way ANOVA followed by Bonferroni post-hoc test were utilized to interpret inter-group variation using software GraphPad Prism5 (GraphPad Software Inc., USA). All the values are denoted as mean±SEM and statistical significance was achieved at p < 0.05.
Results
Effect on Ambulatory Activity of Rats
The different groups exhibited no significant difference between mean locomotor activity on day 1, 21 and 28 (Fig. 2).
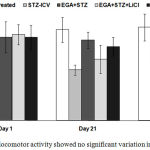 |
Figure 2: Mean locomotor activity showed no significant variation in actophotometer.
|
Two-way ANOVA followed by Bonferroni post-hoc test was applied. Values are denoted as mean±SEM (n=6). (STZ: Streptozotocin; EGA: Ellagic acid; LiCl: Lithium chloride).
LiCl and L-Arginine Modulate the Impact of EGA on Memory of STZ (ICV) Treated Rats in MWM
The different groups showed no significant difference in mean escape latency (MEL) on day 22, but major differences appeared on day 23 of acquisition trials in MWM. STZ alone group had significantly (p < 0.001) higher MEL with respect to sham-treated group and thereby exhibited decrease in spatial learning. EGA+STZ group manifested improvement in learning evident by declined (p < 0.001) MEL with respect to STZ (ICV) alone group. LiCl group exhibited decline of MEL (day 23, p < 0.001; day 24, p < 0.01; day 25 and 26, p < 0.05) in comparison to EGA+STZ group (Fig. 3A). L-Arginine group displayed considerable rise of MEL (day 23 and 24, p < 0.01; day 25, p < 0.05; day 26, p > 0.05) in comparison to EGA+STZ group.
On day 27 a reduction (p < 0.001) in percentage of time spent in target quadrant [TSTQ (%)] during probe trial in STZ (ICV) alone group denoted loss of spatial memory with respect to sham-treated group. EGA+STZ group showed significant (p < 0.001) rise in TSTQ (%) in comparison to STZ (ICV) alone group. LiCl group displayed increase of TSTQ (%) (p < 0.01) when compared to EGA+STZ group. L-Arginine group had low (p < 0.05) TSTQ (%) with respect to EGA+STZ group (Fig. 3B). These results revealed rise in memory functions of rats in response to chronic LiCl treatment while L-Arginine treatment enhanced the STZ (ICV) triggered cognitive deficits in EGA treated rats.
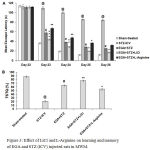 |
Figure 3: Effect of LiCl and L-Arginine on learning and memory of EGA and STZ (ICV) injected rats in MWM.
|
Comparison of (A) mean escape latency during five days acquisition trials (Analyzed by two-way ANOVA followed by Bonferroni post-hoc test), (B) percentage of time spent in target quadrant [TSTQ (%)] during probe trial (Analyzed by one-way ANOVA followed by Tukey’s post-hoc test). Values are denoted as mean±SEM (n=6). Significance at @ p < 0.001 vs. sham-treated group; # p < 0.001 vs. STZ-ICV; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. EGA+STZ group. (STZ: Streptozotocin; EGA: Ellagic acid; LiCl: Lithium chloride).
LiCl and L-Arginine Modulate the Impact of EGA on Memory of STZ (ICV) Injected Rats in EPM Task
During acquisition trial on day 27 of EPM task none of the groups showed significant difference in mean transfer latency (TL). During retrieval trial on day 28 STZ (ICV) group exhibited an increase (p < 0.001) in mean TL disclosed loss of memory in comparison to sham-treated group. EGA+STZ group showed decline (p < 0.001) of TL in comparison to STZ (ICV) group. LiCl group exhibited improvement in cognitive abilities evident by substantial decrease (p < 0.01) of TL with respect to EGA+STZ group. L-Arginine group displayed enhanced (p < 0.01) TL when compared with EGA+STZ group (Fig. 4).
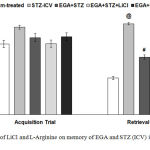 |
Figure 4: Effect of LiCl and L-Arginine on memory of EGA and STZ (ICV) injected rats in EPM.
|
Mean transfer latency of different groups during retrieval trial in EPM is compared. Analyzed by one-way ANOVA followed by Tukey’s post-hoc test. Values are denoted as mean±SEM (n=6). Significance at @ p < 0.001 vs. sham-treated group; # p < 0.001 vs. STZ-ICV; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. EGA+STZ group. (STZ: Streptozotocin; EGA: Ellagic acid; LiCl: Lithium chloride).
LiCl and L-Arginine Modulate the Effects of EGA on Brain Oxidative Stress of STZ (ICV) Administered Rats
Centrally administered STZ raised the brain TBARS content, and diminished the GSH level, SOD and CAT activity (p < 0.001) in comparison to sham-treated rats. EGA treatment prevented the STZ triggered surge in brain TBARS content, and decline of GSH level, SOD and CAT activity (p < 0.001). LiCl group showed attenuation of brain TBARS level (p < 0.01), and rise of GSH content (p < 0.05), SOD (p < 0.01) and CAT activity (p < 0.05) with respect to EGA+STZ group. L-Arginine group manifested higher brain TBARS content (p < 0.01), and lower GSH level (p < 0.05), SOD (p < 0.001) and CAT activity (p < 0.05) with respect to EGA+STZ group (Table 1).
LiCl and L-Arginine modulate the effects of EGA on brain nitrite level of STZ (ICV) injected rats
Total nitrite content in brain estimates the extent of NO transmission. STZ (ICV) group showed significant (p < 0.001) elevation in the brain nitrite content in comparison to sham-treated rats. EGA+STZ group had lower (p < 0.001) brain nitrite content as compared to STZ (ICV) group. LiCl or L-Arginine treatments enhanced (p < 0.05 and p < 0.001 respectively) the brain nitrite content in separate groups of EGA and STZ treated rats (Table 1). However, L-Arginine group showed exorbitant rise (p < 0.001) in brain nitrite content when compared with LiCl group.
Table 1: Effect of EGA and its modulation by LiCl and L-Arginine on brain thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and total nitrite in STZ (ICV) treated rats.
| Animal group | TBARS (nM/mg protein) | GSH (µM GSH/mg protein) | SOD (µM NBT reduced/min/mg protein) | CAT (µM H2O2 decomposed/min/mg protein) | Nitrite (µM/mg protein) |
| Sham-treated | 1.312±0.199 | 0.34±0.009 | 0.028±0.005 | 16.24±0.424 | 76.83±2.416 |
| STZ-ICV | 10.94±0.344@ | 0.013±0.0033@ | 0.177±0.005@ | 1.859±0.27@ | 174.3±2.013@ |
| EGA+STZ | 4.544±0.369# | 0.229±0.011# | 0.079±0.0047# | 11.7±0.504# | 120.5±3.374# |
| EGA+STZ+LiCl | 2.764±0.197** | 0.286±0.014* | 0.051±0.0048** | 13.96±0.615* | 133.8±2.93* |
| EGA+STZ+L-Arginine | 6.266±0.404** | 0.173±0.015* | 0.122±0.002*** | 9.279±0.518* | 152.9±2.944***$$$ |
Values are denoted as mean±SEM (n=5). Significance at @ p < 0.001 vs. sham-treated group; # p < 0.001 vs. STZ-ICV; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. EGA+STZ; $$$ p < 0.001 vs. EGA+STZ+LiCl group. (STZ: Streptozotocin; EGA: Ellagic acid; LiCl: Lithium chloride).
LiCl and L-Arginine Modulate the Effects of EGA on Brain AChE Activity of STZ (ICV) Injected Rats
STZ (ICV) administration increased the brain AChE activity (p < 0.001) with respect to sham-treated group. EGA+STZ group displayed decrease (p < 0.001) in the AChE activity when compared with STZ (ICV) group. LiCl and L-Arginine groups showed significant attenuation of AChE activity (p < 0.001 and p < 0.05 respectively) with respect to EGA+STZ group. These results showed that facilitation of NO biosynthesis enhanced the AChE inhibitory activity of EGA and cholinergic activity in brain of rats (Table 2).
LiCl and L-Arginine modulate the effects of EGA on brain LDH activity of STZ (ICV) injected rats
Central administration of STZ compromised the neuronal coherence evident by enhanced (p < 0.001) brain LDH activity in comparison to sham-treated group. A decrease (p < 0.001) in brain LDH activity was noted in EGA+STZ group in comparison to STZ (ICV) group which manifested resurrection of neuron membrane integrity. LiCl group showed decline (p < 0.05) in LDH activity with respect to EGA+STZ group. L-Arginine treatment enhanced (p < 0.01) the STZ (ICV) induced surge in LDH activity in EGA treated rats (Table 2).
LiCl and L-Arginine modulate the effects of EGA on brain TNF-α content of STZ (ICV) injected rats
STZ (ICV) treatment enhanced (p < 0.001) the brain TNF-α content as compared to sham-treated group. Chronic administration of EGA attenuated (p < 0.001) the brain TNF-α levels in comparison to rats that received STZ (ICV) alone. Administration of LiCl reduced (p < 0.01) the TNF-α content in brain of EGA and STZ (ICV) treated rats. L-Arginine treatment increased (p < 0.05) the STZ (ICV) induced surge in brain TNF-α content in EGA treated rats when compared to rats administered with EGA and STZ only (Table 2).
Table 2: Effect of EGA and its modulation by LiCl and L-Arginine on brain acetylcholinesterase (AChE), lactate dehydrogenase (LDH) activity, TNF-α and eNOS levels in STZ (ICV) treated rats.
| Animal group | AChE (µM acetylthiocholine iodide hydrolysed/min/mg protein) | LDH (µM NADH oxidized/min/mg protein) | TNF-α (pg/ml) | eNOS (ng/ml) |
| Sham-treated | 0.0354±0.0017 | 0.0529± 0.0104 | 29.29±3.118 | 23.23±1.175 |
| STZ-ICV | 0.1463±0.0054@ | 0.3419±0.0103@ | 89.66±1.411@ | 1.415±0.4389@ |
| EGA+STZ | 0.0875±0.004690# | 0.1489±0.0052# | 51.6±1.416# | 15.08±0.858# |
| EGA+STZ+LiCl | 0.0553±0.004*** | 0.105±0.00612* | 40.37±1.795** | 13.11±0.687 |
| EGA+STZ+L-Arginine | 0.0679±0.0042* | 0.194±0.00884** | 61.66±1.72* | 9.323±0.891***$ |
Values are denoted as mean±SEM (n=5). Significance at @ p < 0.001 vs. sham-treated group; # p < 0.001 vs. STZ-ICV; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. EGA+STZ; $ p < 0.05 vs. EGA+STZ+LiCl group. (STZ: Streptozotocin; EGA: Ellagic acid; LiCl: Lithium chloride).
LiCl and L-Arginine modulate the effects of EGA on brain eNOS level of STZ (ICV) injected rats
STZ (ICV) group displayed lower (p < 0.001) brain eNOS levels with respect to sham-treated rats. Chronic EGA treatment revitalized (p < 0.001) the eNOS content in brain of STZ (ICV) injected rats with respect to STZ (ICV) alone group. LiCl group exhibited fall (p > 0.05) in brain eNOS level with respect to EGA+STZ. Chronic administration of L-Arginine downregulated (p < 0.001) the eNOS expression in brain of EGA and STZ (ICV) treated rats (Table 2). L-Arginine group portrayed decrease (p < 0.05) in brain eNOS content with respect to LiCl group.
Histology of rat Brain
STZ (ICV) administration catapulted neurodegeneration in cortical region of rat brain. EGA treatment for 28 days arrested the noxious effects of STZ (ICV) in rat brain. LiCl potentiated the neuroprotective activity of EGA in STZ (ICV) treated rats. L-Arginine group portrayed enhanced neurodegeneration in comparison to EGA+STZ group (Fig. 5).
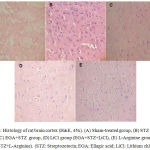 |
Figure 5: Histology of rat brain cortex (H&E, 45x). (A) Sham-treated group, (B) STZ (ICV) group, (C) EGA+STZ group, (D) LiCl group (EGA+STZ+LiCl), (E) L-Arginine group (EGA+STZ+L-Arginine). (STZ: Streptozotocin; EGA: Ellagic acid; LiCl: Lithium chloride).
|
Discussion
The functions of GSK-3 and eNOS in modulation of memory abilities by EGA in STZ (ICV) model of AD was evaluated in the current study. Administration of STZ in brain manifests symptoms akin to age associated progressive neurodegenerative disorders such as AD.6 The association of central cholinergic activity with memory has witnessed several pharmacological, electrophysiological and biochemical evidences. The cholinergic projection neurons of the basal forebrain and upper brainstem innervate brain regions (cortex, hippocampus) involved in memory.29 In present study STZ (ICV) injection compromised the cholinergic function evident by enhanced AChE activity in brain of rats. Chronic EGA administration attenuated the brain AChE activity in STZ (ICV) treated rats. LiCl (GSK-3 inhibitor) and L-Arginine (NO precursor) treatments enhanced the brain cholinergic function through inhibition of AChE activity in EGA and STZ (ICV) treated rats. Previous studies demonstrated improvement in brain cholinergic function through increase in central NO transmission and inhibition of GSK-3 activity by L-Arginine or LiCl.30,31
Commensurate to previous findings, administration of STZ in lateral cerebral ventricles of rats expedited the biogenesis of brain free radicals and diminished the antioxidant guard in the current study. TBARS is a widely acclaimed biomarker of lipid peroxidation conspicuously enhanced by STZ (ICV) treatment. GSH, SOD and CAT constitute endogenous brain antioxidants diminished in STZ (ICV) treated rats. Oxidative stress is an early event which hastens generation of reactive oxygen species, compromise antioxidative status and provides impetus to various age associated derangements in brain manifesting profound neurodegeneration and AD like cognitive deficits.6 Oral administration of EGA attenuated rise of brain TBARS and decline of GSH, SOD and CAT activity in STZ (ICV) treated rats. Uninhibited GSK-3 overactivity is known to hasten lipid peroxidation, protein oxidation and PARP activity.32 In the present study control of GSK-3 activity by chronic administration of LiCl (non-selective GSK-3 inhibitor) lowered the brain TBARS levels and enhanced the GSH, SOD, CAT activities in EGA and STZ (ICV) treated rats. However, L-Arginine (NO precursor) treatment spurred the STZ (ICV) induced oxidative stress in EGA administered rats.
Amyloid β aggregates and several neurotoxins (e.g. STZ, aluminum chloride, colchicine) trigger release of pro-inflammatory cytokines (e.g. IL-1, IL-6, TNF-α) by reactive microglia and astrocytes.6 Chronic elevation of TNF-α level in brain, CSF and plasma is associated with AD like neurodegenerative changes.33 Oral administration of EGA lowered the STZ (ICV) triggered increase of TNF-α in brain of rats. Inhibition of GSK-3 by LiCl facilitated the anti-inflammatory activity of EGA in STZ (ICV) injected rats. Chronic administration of L-Arginine abolished the decrease of brain TNF-α level by EGA in STZ (ICV) treated rats. Furthermore, STZ (ICV) treated rats showed high brain LDH activity which signified loss of cell viability. Lipid peroxidation and chronic inflammation infringe the plasma membrane and breach neuronal integrity that incites LDH activity.34 EGA averted STZ (ICV) induced neurodegeneration evident by abrogation of brain LDH activity. LiCl enhanced whereas L-Arginine declined the neuroprotective activity of EGA in STZ (ICV) injected rats. Furthermore, the histology of rat brain substantiated the present results.
The aforementioned biochemical results well supported the MWM and EPM findings. STZ (ICV) triggered brain oxidative stress, inflammation and cholinergic deficit reduced the spatial memory of rats. Chronic treatment with EGA abrogated the oxidative stress, inflammation, LDH, AChE activity and thereby prevented the loss of memory in STZ (ICV) treated rats. Administration of LiCl (GSK-3 inhibitor) enhanced the memory restorative activity of EGA in STZ (ICV) treated rats. However, contrary to our hypothesis facilitation of NO transmission by chronic L-Arginine (NO precursor) treatment decreased the memory of EGA and STZ (ICV) administered rats. Elevation in oxidative stress, inflammation and fall of antioxidative defense culminated in loss of memory of L-Arginine group rats. Furthermore, exploration of total brain nitrite content and eNOS levels by ELISA in brain of rats substantiated these results.
NO is ascribed a vital second messenger biosynthesized by constitutively expressed eNOS and is associated with fabrication of long-term memories. The eNOS mediated NO biosynthesis in cortex and hippocampus is associated with long term potentiation and synaptic plasticity.12 Nevertheless, excessive nitrite levels in brain leads to neurodegeneration through peroxynitrite mediated protein oxidation, lipid peroxidation, oxidative modification of DNA and mitochondria.35 STZ (ICV) causes excessive NO production through pathological iNOS activation that results in neurodegeneration and loss of memory. In the present study central administration of STZ heightened the NO (nitrite) levels in brain although decreased the expression and activity of eNOS. EGA revived the eNOS content and halted the exorbitant rise of brain nitrite levels in STZ (ICV) treated rats. LiCl and L-Arginine treatments increased the NO production in brain of EGA and STZ (ICV) treated rats. However, LiCl group showed no significant difference in brain eNOS content in comparison to EGA+STZ group. L-Arginine group exhibited profound downfall in eNOS expression (not activity of eNOS) in comparison to LiCl group and EGA+STZ group. NO regulated eNOS expression has been demonstrated in previous studies.36 These results imply that increase in nitrite content by L-Arginine downregulated the eNOS expression in brain of rats that resulted in loss of cognitive functions.
Conclusion
Suppression of GSK-3 activity by LiCl potentiated the antioxidative, anti-inflammatory, anti-AChE and cytoprotective activity of EGA in STZ (ICV) treated rats. This implies that EGA attenuated the STZ (ICV) incited aberrant overactivity of GSK-3 in brain of rats and revived their diminishing memory functions. L-Arginine treatment enhanced STZ (ICV) initiated brain oxidative stress, inflammation, cytotoxicity, nitrite content and AChE activity in EGA administered rats. Furthermore, ELISA results showed that L-Arginine aided the STZ (ICV) induced decrease of brain eNOS expression (not activity of eNOS) and thereby reduced the memory of EGA treated rats. These results highlighted that EGA prevented loss of memory in STZ (ICV) rats through upregulation of brain eNOS.
Acknowledgement
The authors are thankful to AICTE, New Delhi for providing financial assistance under Research Promotion Scheme, ASBASJSM college of Pharmacy, Bela for providing necessary research facilities and IKG Punjab Technical University, Kapurthala (Punjab).
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Bansal N, Yadav P, Kumar M. Ellagic acid administration negated the development of streptozotocin-induced memory deficit in rats. Drug Res. (Stuttg). 2017;67:425-431. doi: 10.1055/s-0043-108552.
CrossRef - Landete J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011;44:1150-1160.
CrossRef - Seeram N.P, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta. 2004;348:63-68.
CrossRef - Larrosa M, Garcia-Conesa M.T, Espin J.C, Tomas-Barberan F.A. Ellagitannins, ellagic acid and vascular health. Mol. Aspects. Med. 2010; 31:513-539. doi: 10.1016/j.mam.2010.09.005.
CrossRef - Oliveira D.M.R. The effects of ellagic acid on brain cells: A mechanistic view and future directions. Neurochem. Res. 2016;41:1219-1228. doi: 10.1007/s11064-016-1853-9.
CrossRef - Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol. Neurobiol. 2016;53:1741-1752. doi: 10.1007/s12035-015-9132-3.
CrossRef - Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J. Neurochem. 2007;101:757-770.
CrossRef - Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci. 2017;173:1-10. doi: 10.1016/j.lfs.2016.09.020.
CrossRef - Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008;104:1433-1439. doi: 10.1111/j.1471-4159.2007.05194.x.
CrossRef - Martinez A, Perez D.I. GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer’s disease? J. Alzheimers Dis. 2008;15:181-191.
CrossRef - Santos A.I, Martinez-Ruiz A, Araujo I.M. S-nitrosation and neuronal plasticity. Br. J. Pharmacol. 2015;172:1468-1478. doi: 10.1111/bph.12827.
CrossRef - Doreulee N, Sergeeva O.A, Yanovsky Y, Chepkova A.N, Selbach O, Godecke A, Schrader J, Haas H.L. Cortico-striatal synaptic plasticity in endothelial nitric oxide synthase deficient mice. Brain Res. 2003; 964:159-163.
CrossRef - Rickard N.S, Gibbs M.E, Ng K.T. Inhibition of the endothelial isoform of nitric oxide synthase impairs long-term memory formation in the chick. Learn. Mem. 1999;6:458-466.
CrossRef - Ou H.C, Lee W.J, Lee S.D, Huang C.Y, Chiu T.H, Tsai K.L, Hsu W.C, Sheu W.H. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2010;248:134-143. doi: 10.1016/j.taap.2010.07.025.
CrossRef - Paxinos G, Watson C.R, Emson P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Methods. 1980;3: 129-149.
CrossRef - Akar Y.F, Celikyurt I.K, Ulak G, Mutlu O. Effects of L-arginine on 7-nitroindazole-induced reference and working memory performance of rats. Pharmacology. 2009;84:211-218. doi: 10.1159/000235997.
CrossRef - Gocmez S.S, Yazir Y, Sahin D, Karadenizli S, Utkan T. The effect of a selective neuronal nitric oxide synthase inhibitor 3-bromo 7-nitroindazole on spatial learning and memory in rats. Pharmacol. Biochem. Behav. 2015;131:19-25. doi: 10.1016/j.pbb.2015.01.013.
CrossRef - Ponce-Lopez T, Liy-Salmeron G, Hong E, Meneses A. Lithium, phenserine, memantine and pioglitazone reverse memory deficit and restore phospho-GSK3β decreased in hippocampus in intracerebroventricular streptozotocin induced memory deficit model. Brain Res. 2011;1426:73-85.
CrossRef - Morris R.G.M. Development of a water-maze procedure for studying spatial learning in the rats. J. Neurosci. Methods. 1984;11:47-60.
CrossRef - Vorhees C.V, Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848-858.
CrossRef - Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351-358.
CrossRef - Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70-77.
CrossRef - Winterbourn C.C, Hawkins R.E, Brian M, Carrell R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975;85:337-341.
- Claiborne A. Catalase activity, in: Greenwald R.A. (Ed.), CRC Handbook of Methods for Oxygen Radical Research, CRC Press, Boca Raton. 1985; 283-284.
- Sastry K.V, Moudgal R.P, Mohan J, Tyagi J.S, Rao G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002;306:79-82.
CrossRef - Ellman G.L, Courtney K.D, Andres V.Jr, Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88-95.
CrossRef - Horecker B.L, Kornberg A. The extinction coefficient of the reduced band of pyridine nucleotides. J. Biol. Chem. 1948;175:385-390.
- Lowry O.H, Rosebrough N.J, Farr A.L, Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193: 265-275.
- Blake M.G, Boccia M.M. Basal Forebrain Cholinergic System and Memory. Curr. Top. Behav. Neurosci. 2017. doi: 10.1007/7854_2016_467.
CrossRef - Prast H, Fischer H, Werner E, Werner-Felmayer G, Philippu A. Nitric oxide modulates the release of acetylcholine in the ventral striatum of the freely moving rat. Naunyn Schmiedebergs Arch. Pharmacol. 1995; 352:67-73.
CrossRef - Zhao L, Chu C.B, Li J.F, Yang Y.T, Niu S.Q, Qin W, Hao Y.G, Dong, Q, Guan R, Hu W.L, Wang Y. Glycogen synthase kinase-3 reduces acetylcholine level in striatum via disturbing cellular distribution of choline acetyltransferase in cholinergic interneurons in rats. Neuroscience. 2013;255:203-211. doi: 10.1016/j.neuroscience.2013.10.001.
CrossRef - Songin M, Jesko H, Czapski G, Adamczyk A, Strosznajder R.P. GSK-3beta and oxidative stress in aged brain. Role of poly(ADP- -ribose) polymerase-1. Folia Neuropathol. 2007;45:220-229.
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346.
CrossRef - Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi K.L. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine – a PDE1 inhibitor. Eur. J. Pharmacol. 2009;620:49-56. doi: 10.1016/j.ejphar.2009.08.027.
CrossRef - Pacher P, Beckman J.S, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315-424. doi: 10.1152/physrev.00029.2006.
CrossRef - Mohan S, Wu C.C, Shin S, Fung H.L. Continuous exposure to l-arginine induces oxidative stress and physiological tolerance in cultured human endothelial cells. Amino Acids. 2012;43:1179-1188. doi: 10.1007/s00726-011-1173-y.
CrossRef








