Hendry Irawan1 , I. Nyoman Semadi2 and Anita Devi3
, I. Nyoman Semadi2 and Anita Devi3
1General Surgery Department, Faculty of Medicine, Udayana University, Sanglah General Hospital, Denpasar, Bali, Indonesia.
2Thorax and Cardiovascular Surgery Department, Faculty of Medicine, Udayana University, Sanglah General Hospital, Denpasar, Bali, Indonesia.
3Hyperbaric Oxygen Therapy Department, Sanglah General Hospital, Denpasar, Bali, Indonesia.
Corresponding Author E-Mail: hendry_irawan@rocketmail.com
DOI : https://dx.doi.org/10.13005/bpj/1409
Abstract
Diabetic foot ulcers (DFUs) are the most common musculoskeletal infections and complications in patients with diabetes mellitus. DFUs require comprehensive multidisciplinary treatment and also long period time of healing. Hyperbaric oxygen therapy (HBOT) is one of adjuvant therapy for DFUs with promising results. The aim of our study was to test the hypothesis that serum album in patients with DFU is affected by HBOT. We used randomized pre- and post-test control group design with permuted block from all patients who met the inclusion criteria. We recruited 36 patients who then divided into two groups, HBOT and non HBOT group. Each patient’s blood sample was taken twice, before and after therapy. HBOT group showed significant increase in serum albumin, from 2.96 ± 0.43 g/dL to 3.51 ± 0.46 g/dL (p < 0.0001) while non HBOT group did not show any significant change, from 2.92 ± 0.51 g/dL to 3.01 ± 0.41 g/dL (p = 0.440). We also calculate the effect size of serum albumin increased level. The effect size was differed significantly between HBOT and non HBOT groups (0.55 ± 0.38 g/dL and 0.09 ± 0.49 g/dL, p = 0.007). There is a significant increase in serum albumin level in DFUs patient who underwent HBOT therapy.
Keywords
Diabetic Foot Ulcer; Hyperbaric Oxygen Therapy; Serum Albumin
Download this article as:| Copy the following to cite this article: Irawan H, Semadi I. N, Devi A. Effect of Hyperbaric Oxygen Therapy to Improve Serum Albumin for Patients with Diabetic foot Ulcers. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Irawan H, Semadi I. N, Devi A. Effect of Hyperbaric Oxygen Therapy to Improve Serum Albumin for Patients with Diabetic foot Ulcers. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19674 |
Introduction
Diabetes mellitus is a global public health threat that has increased over past two decades.1,2 An estimated 422 million adults were living with diabetes in 2014,1 177 million in 2000, 285 million in 2010,2 the prevalence increased from 4.7% to 8.5% in the adult population.1 Over the past decade, the disease burden related to diabetes is high and rising, fuelled by the global rise in the prevalence of obesity and unhealthy life style.
Diabetic complications may affect all parts of the body and increase the overall risk of dying prematurely. Its complications include heart attack, stroke, kidney failure, diabetic foot ulcer (DFUs), vision loss, and nerve damage.1 Diabetic foot ulcers are considered as a major source of morbidity and a leading cause of hospitalization in patients with diabetes, represent a huge risk to the patient’s quality life, escalating wound or infection management and cost.2,3
The risk of amputation of lower extremities in diabetic people is 10-20 times higher than non-diabetic people.1 Proper management of DFUs may reduce severity of ulcer, improve quality of life, and increase life expectancy.2-5
It is possible to reduce amputation rates through a care strategy that combine prevention, interprofessional care team, close monitoring and patient education.2,5 The standard therapy of DFUs were blood glucose control, proper antibiotics, wound debridement, wound dressing, offloading, and improved of blood flow.2,5 Hyperbaric oxygen therapy (HBOT) is one of adjuvant therapy for DFUs, it shows good outcome in enhancing wound healing and decreasing incidence of amputation.2,5-7 This therapy uses administration of 100 % oxygen at 2-3 ATA (atmosphere absolute) for 90 minutes per session per day, for total 20-30 sessions.2,5-7 During each session, patients given pure oxygen during 3 periods of 30 minutes (overall 90 minutes), intercalated by 5 min intervals in a hyperbaric chamber.2
Although exact mechanism of HBOT is not clearly explained, some studies revealed that HBOT can improve oxygenation in tissue’s hypoxia, reduce swelling, enhance perfusion, decrease inflammatory cytokines, promote fibroblast proliferation, increase collagen production, and promote angiogenesis.2,5,7
The DFUs usually have vascular compromise and it is associated with protein leakage and wound edema.8,9 The use of HBOT will reduce wound swelling and increase oxygen perfusion,5,7 but there are no previous data of study that analyses the improvement of protein levels after HBOT. The aim of our study was to test the hypothesis that serum albumin in patients with DFU is affected by HBOT.
Materials and Methods
Between January and December 2017, 36 patient recruited with the inclusion criteria were were patients diagnosed with DM type 2 with DFU Wagner 3-4, age more than 18 years old, no contraindication of HBOT, and provided written consent to follow the study procedure. We used randomized pre- and post-test control group design with permuted block. Each patient’s blood sample was taken twice, before and after therapy. Laboratory examinations were random blood sugar, fasting blood sugar, post-prandial blood sugar, haemoglobin, white blood count, platelet, albumin, blood urea nitrogen, and serum creatinine, taken before surgical debridement treatment. All patients received the same wound care with normal saline, sterile gauze, and elastic bandage. Then we randomly divide samples into two groups, HBOT and non HBOT.
In HBOT group, patients breathe in 100% oxygen at 2.4 ATA in a multi-place hyperbaric chamber for 90 minutes session per day, five days in a week. If the sample was unable to comply with HBOT series of therapy, they will be dropped out from the study (Figure 1). Second blood test to evaluate serum albumin was done at the end of therapy for each group respectively. For HBOT group was done after the 20th session, for non HBOT was done one month after the surgical debridement therapy.
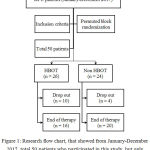 |
Figure 1: Research flow chart, that showed from January-December 2017, total 50 patients who participated in this study, but only 36 patients finished the procedure.
|
Statistical Analysis
Descriptive analysis was used to assess the baseline characteristics of all patients. The normality of data was tested with Shapiro-Wilk test and normal distribution of the data if p value more than 0.05. The variables were presented as mean ± standard deviation and percentage (%). We compared baseline variables in both groups. We compared serum albumin at baseline and the end of therapy in each group with paired t-test. The independent t-test was applied to compare serum albumin between two groups at baseline and the end of therapy. We evaluated the effect size of serum albumin between baseline and its level at the end of therapy. The p value of less than 0.05 was considered to be statistically significant. All analysis was performed using IBM SPSS statistics version 23 for Windows (IBM Corporation).
Results
In total, 50 patients who met inclusion criteria from January to December 2017. After using permuted block randomization, there were 26 patients in HBOT group and 24 in non HBOT group. The mean age in HBOT group was 54.19 years and in non HBOT group was 54.17 years. Table 1 showed baseline characteristics in this study. Duration of ulcer was similar between groups, around 4 weeks. Both of groups was similar characteristics before the therapy.
Table 1: Baseline characteristics in each group
| Variable | HBOT
(n = 26) |
Non HBOT
(n = 24) |
p value |
| Age (years) | 54.19 ± 6.46 | 54.17 ± 5.98 | 0.988 |
| Duration of ulcer (weeks) | 4.88 ± 2.72 | 4.00 ± 2.72 | 0.226 |
| Duration of DM (years) | 5.87 ± 6.30 | 7.58 ± 5.93 | 0.108 |
| Body mass index (kg/m2) | 23.15 ± 4.71 | 23.37 ± 3.56 | 0.415 |
| Gender (%) | |||
| Male | 15 (57.7) | 10 (41.7) | 0.258 |
| Female | 11 (42.3) | 9 (58.3) | |
| Smoking (%) | 6 (23.1) | 4 (16.7) | 0.418 |
| Hypertension (%) | 11 (42.3) | 10 (41.7) | 0.963 |
| Random blood sugar (mg/dL) | 264.19 ± 73.97 | 269.58 ± 122.68 | 0.705 |
| Fasting blood sugar (mg/dL) | 177.81 ± 97.45 | 189.92 ± 77.48 | 0.466 |
| Post prandial blood sugar (mg/dL) | 248.00 ± 110.95 | 241.25 ± 84.77 | 0.969 |
| Hemoglobin (g/dL) | 10.21 ± 1.32 | 10.42 ± 1.58 | 0.603 |
| White blood count (103 cells/µL) | 15.07 ± 5.77 | 15.34 ± 6.27 | 0.831 |
| Platelet (103 cells/µL) | 363.04 ± 94.53 | 345.42 ± 121.66 | 0.568 |
| Serum albumin (g/dL) | 2.91 ± 0.43 | 2.90 ± 0.50 | 0.950 |
| Blood urea nitrogen (mg/dL) | 13.81 ± 5.49 | 13.92 ± 3.99 | 0.937 |
| Serum creatinine (mg/dL) | 0.86 ± 0.22 | 0.86 ± 0.23 | 0.573 |
avalues in mean ± standard deviation
Patients who did not comply to the HBOT therapy were drop out. At the end of therapy, there were 16 patients in HBOT group and 20 patients in non HBOT group (Table 2). We evaluated and compared serum albumin baseline and the end of therapy. Both groups showed increased of serum albumin, but in HBOT group we can see that the albumin level was significantly increased (2.96 ± 0.43 g/dL to 3.51 ± 0.46 g/dL, p < 0.0001), while the non HBOT group only showed 2.92 ± 0.51 g/dL to 3.01 ± 0.41 g/dL (p = 0.440).
Table 2: Baseline and end of therapy values between groups
| HBOT (n = 16) | Non HBOT (n = 20) | |||||||||||
| Variables | Baseline | End of therapy | p value | Baseline | End of therapy | p value | ||||||
| Serum albumin (g/dL) | 2.96 ± 0.43 | 3.51 ± 0.46 | < 0.0001 | 2.92 ± 0.51 | 3.01 ± 0.41 | 0.44 |
All values analysed with paired t-test, mean ± standard deviation
Table 3 defined the delta or the effect size of serum albumin (value at baseline minus value at the end of therapy). There is significant differences (p = 0.007) between groups. The serum albumin baseline between groups, was not significantly differ in value (p = 0.825), but at the end of therapy it was increase significantly (p = 0.004) for HBOT group than in non HBOT group. We showed the boxplot diagram of this study (Figure 2).
Table 3: The difference value between group
| Value baseline minus end of therapy | HBOT | Non HBOT | p value |
| Serum albumin (g/dL) | -0.55 ± 0.38 | -0.09 ± 0.49 | 0.007 |
All values analysed with independent t-test, mean ± standard deviation
 |
Figure 2: Boxplot diagram of serum albumin between groups before and after therapy.
|
It showed increasing of serum albumin in HBOT group and non HBOT group.
† Albumin baseline with albumin end of therapy in HBOT groups, p < 0.0001;
‡ Albumin baseline of HBOT group with albumin baseline of non HBOT group, p = 0.825;
* Albumin end of therapy of HBOT group with albumin end of therapy of non HBOT group, p = 0.004;
# Albumin baseline with albumin end of therapy in non HBOT group, p = 0.440
Discussion
Diabetic patients with DFUs require multidisciplinary team to manage metabolic disturbance, improve wound healing, and prevent complication of DFUs.1,2,5-7 Hyperglycemia condition in DFU induce the production of advanced glycation end products, oxidative stress, and endothelial inflammation. This condition generates deterioration of ulcer, macrovascular and microvascular disease, decreased blood flow, and edema due to increased capillary permeability, and infection.7,8
Based on the consensus of the European Committee for Hyperbaric Medicine, the use of HBOT on DFUs patients is a type 2 recommendation and a level of evidence B. This suggested that HBOT is recommended due to its good influence on the final outcome of the.10 Hyperoxygenation can increase the amount of dissolved oxygen in plasma. People who breathe with 100% oxygen at normal atmospheric pressure (1 ATA) will have plasma dissolved oxygen of 2.09 ml%. If the same condition is applied to more than 1 ATA, there will be an increase in plasma dissolve oxygen level, for example in 2 ATA is 4.44 ml% and 3 ATA is 6.8 ml%.11,12
The mechanisms of HBOT are enhancing immune system, increasing angiogenesis and neovascularization, increasing proliferation of fibroblasts, increasing collagen, increased phagocytic leukocytes, decreasing inflammatory cytokines, inhibitors of toxins, bacteriocidic effects, reducing edema, and vasoconstriction effects.5,7,11,12 In normal tissue hyperoxia state, vasoconstriction will reduce tissue edema. This vasoconstrictive condition will not cause hypoxia but is a compensation of increased plasma oxygen and microvascular blood flow.11 Increased and improved microvascular blood flow will increase capillary density so that reperfusion in the ischemic region.5,6,13
Zhang et al 9 evaluated factors affecting the outcome in patient with DFUs based on Wagner classification, body mass index, serum albumin, severity infection, and nutritional status. This study shows that 62% patients with DFUs Wagner 1-5 were malnourished and this nutritional status causing poor outcome for DFUs patients. In DFUs patients, malnutrition is characterized by protein deficiency due to kidney failure, protein loss from chronic wound, gluconeogenesis from amino acid, and poor diet intake.14 Moreover, increased matrix metalloproteinases levels due to high proteolytic activity and low growth factors in chronic wound of DFUs may prolong wound healing.8
Our study showed significant increase in serum albumin after 20 sessions of HBOT (< 0.0001) compared to the non HBOT group (p = 0.440). No previous studies have studied the effect of HBOT on serum albumin levels. Zhang et al 9 showed that the higher the Wagner grade, the lover the serum albumin level. For instance, the found that serum albumin of 3.69 ± 3.9 g/dL for Wagner 1 and serum albumin of 2.52 ± 5.4 g/dL is found in group of Wagner 5, that study showed that there were significant difference in serum albumin level within Wagner classification (p < 0.001). Tubili et al14 revealed hypoalbuminemia occurred in 40% patient with DFUs. Patient with uninfected DFUs were not severely malnourish, but severe infected DFUs patients were 43.2 % severely malnourish.9
Serum albumin also reduced during inflammatory condition. In infected DFUs patient who need amputation, they have significant decreased of albumin levels compared to patient who did not need amputation. The patient with albumin levels > 3.5 g/dL healed better than other.15 In Lipsky et al16 study, albumin levels is associated with the severity of DFUs infection.
Akinci et al 17 stated that patient who undergo amputation in DFU had lower serum albumin. In that study, Wagner grade 4 and 5 (OR 14.924, p < 0.001), Wagner 3 (OR 12.137, p < 0.001), and serum albumin (OR 4.343, p = 0.002) had significant association with increased risk of amputation.17 Jiang et al [18] also found that albumin levels median is significantly differ between groups (p < 0.001), in non-amputation group was 3.8 g/dL, in minor amputation group was 3.4 g/dL, and in major amputation group was 3.23 g/dL.
Serum albumin has its half time about 17 days, and if it is assumed if the patient is in a steady state, the measurement of serum albumin is ideally done within 2-3 weeks.19
Hyperbaric oxygen therapy can increase systemic tissue oxygen levels. Elevated tissue oxygen level can increase growth factors and decrease inflammatory cytokines.5,11,20 Patient with severe infection required more energy intake and so is wound healing, that requires more energy.9
Conclusion
Diabetic patients with DFUs require multidisciplinary team to manage metabolic disturbance, improving wound healing, and preventing complication of DFUs. The standard therapy of DFUs were blood glucose control, proper antibiotics, wound debridement, wound dressing, offloading, and improving the blood flow. Our study showed a significant increasing of serum albumin in patients with DFUs treated by HBOT. Standard therapy added with HBOT can help improve the condition of the patient. Further study must be done to evaluate HBOT ability in improving patient condition with DFUs including clinical condition, biochemical markers, and biomolecular markers.
Acknowledgments
Author contributions: Hendry Irawan contributed to the data analysis, interpretation of findings, and drafting of the manuscript. I Nyoman Semadi contributed to the study design, conduct, data interpretation, and drafting of the manuscript. Anita Devi contributed to data interpretation and drafting of the manuscript. The authors would like to thank the many investigators and patients for their participation, without which this study would not have been possible.
Funding Supports
There is no funding supports
Disclosure Statement
All the authors have no relevant conflict of interest to disclose.
Ethics Statement
All the participants provided informed consent. The research was approved by our local research ethics committees, Institutional Review Board of Medical Faculty of Udayana University and Sanglah General Hospital, Denpasar.
References
- World Health Organization. Global Report on Diabetes. Switzerland: WHO Press. 2016.
- Yazdanpanah L., Nasiri M., Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6:37-53.
CrossRef - Chidiac C., Bru J. P., Choutet P., et al. Clinical practice guidelines: Management of diabetic foot infections. Medicine et maladies infectieuses. 2007;37:14-25.
CrossRef - Frykberg R. G., Zgonis T., Armstrong D. G., et al. Diabetic foot disorders: A clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45:1-66.
CrossRef - Irawan H. K. Terapi Oksigen Hiperbarik sebagai Terapi Adjuvan Kaki Diabetik. Cermin Dunia Kedokteran-245. 2016;43:782-785.
- Kessler L., Bilbault P., Ortéga F., et al. Hyperbaric oxygenation accelerate the healing rate of nonischemic chronic diabetic foot ulcers: A prospective randomized study. Diabetes Care. 2003;26:2378-2382.
CrossRef - Flood MS. Hyperbaric oxygen therapy for diabetic foot ulcers. The Journal of Lancaster General Hospital. 2007;2:140-145.
- Ho T. K., Leigh R. D., Tsui J. Diabetic foot disease and oedema. The British Journal of Diabetes and Vascular Disease. 2013;13:45-50.
CrossRef - Zhang S. S., Tang Z. Y., Fang P., et al. Nutritional status deteriorates as the severity of diabetic foot ulcers increases and independently associates with prognosis. Exp Ther Med. 2013;5:215-222.
CrossRef - Mathieu D. 7th European Consensus Conference on Hyperbaric Medicine. Lille: European Committee for Hyperbaric Medicine. 2004.
- Bhutani S., Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45:316-324.
CrossRef - Sahni T., Singh P., John M. J. Hyperbaric Oxygen Therapy: Current Trends and Applications. J Assoc Physicians India. 2003;51:280-284.
- Lipsky BA, Berendt AR. Hyperbaric oxygen therapy for diabetic foot wounds. Diabetes Care. 2010;33:1143-1145.
CrossRef - Tubili C., Carnevale S., Gianni S., et al. Nutritional Status in diabetic patients with foot ulcers: Bioelectrical Analysis in routine evaluation. Mediterranean Journal of Nutrition and Metabolism. 2014;7:181-184.
- Hobizal K. B., Wukich D. K. Diabetic foot infections: current concept review. Diabet Foot Ankle. 2012;3:18409.
CrossRef - Lipsky B. A., Armstrong D. G., Citron D. M., et al. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP) prospective, randomised controlled double-blinded multicentre trial. Lancet. 2005;366:1695-1703.
CrossRef - Akinci B., Yener S., Yesil S., et al. Acute phase reactants predict the risk of amputation in diabetic foot infection. J Am Podiatr Med Assoc. 2011;101:1-6.
CrossRef - Jiang Y., Ran X., Jia L., et al. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in China. Int J Low Extrem Wounds. 2015;14:19-27.
CrossRef - Levitt D. G., Levitt M. D. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurement. Int J Gen Med. 2016;9:229-255.
CrossRef - Aydin F., Kaya A., Karapinar L., et al. IGF-1 Increases with Hyperbaric Oxygen Therapy and Promotes Wound Healing in Diabetic Foot Ulcers. J Diabetes Res. 2013;2013:567834.
CrossRef







