M. Vidyavathi1, S. K. Mobeen Farhana1, A. Sreedevi1 and R. V. Suresh kumar2
1Institute of Pharmaceutical Technology, Sri Padmavati Mahila Visvavidyalayam, Tirupati-517502, A. P, India.
2Department of surgery and radiology, College of Veterinary sciences, S.V. Veterinary university, Tirupati.
Corresponding Author E-mail: vidyasur@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/1400
Abstract
Biopolymers are used as basic compounds for design of scaffolds for different tissue engineering applications. Chitosan and sodium alginate have proven wound healing property individually. But the scaffolds prepared with single polymer have shown poor physicomechanical properties which are essential during handling, storage and application. Hence, the present study focused on development of composite scaffolds using chitosan and different concentrations of sodium alginate to enhance the physico mechanical properties and also loaded with lentil seed extract (LSE) to improve its wound healing property with the combination of antioxidant/antibacterial activities of LSE. The LSE loaded composite scaffolds were prepared with different concentrations of ethanolic extract of lentil seeds in selected best blank composite scaffolds. All the scaffolds were evaluated for various in vitro parameters like thickness, folding endurance, equilibrium swelling, antibacterial activity, tensile and texture parameters to confirm the suitability of prepared external combination. The wound healing activity was determined by in vivo studies using albino rats by estimating percentage wound contraction, histopathological properties, biochemical parameters and photography. As the concentration of sodium alginate was increased the mechanical properties of scaffolds were remarkably improved. The LSE loaded scaffolds shown significant difference in antibacterial and wound healing activity when compared to blank composite scaffolds. The present results revealed that LSE loaded prepared bioscaffolds possessed fast wound healing activity compared to blank scaffolds without losing its mechanical properties. The present study successfully designed a natural, biocompatible wound dressing at low cost for fast healing of wounds in animals and human beings.
Keywords
Chitosan; Extract Loaded Scaffolds;Lentils Extract;Sodium Alginate; Tensile and Texture Parameters Wound Healing Property;
Download this article as:| Copy the following to cite this article: Vidyavathi M, Farhana S. K. M, Sreedevi A, kumar R. V. S. Design and Evaluation of Lentil Seed Extract Loaded Bio Scaffolds for Wound Healing Activity. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Vidyavathi M, Farhana S. K. M, Sreedevi A, kumar R. V. S. Design and Evaluation of Lentil Seed Extract Loaded Bio Scaffolds for Wound Healing Activity. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19414 |
Introduction
Wound is an interruption of continuity of tissues and thus affect physiological functions. The main aim in treatment of wound is to heal the wound fast without much pain, discomfort and mark on the site of wound.1 Currently, there is a demand for materials for treatment of wound not only by closing the wound but also by antimicrobial activity for fast wound healing because delay in healing leads to bacterial infections and damage to surrounding tissues. Chitosan and different polysaccharides were tried earlier as wound healing materials.2 Polysaccharides are excellent scaffolds for cutaneous tissue regeneration but their wound healing application is limited as they are vulnerable to microbial contaminations. These bio polymers alone make the scaffolds with poor mechanical properties such as tensile strength, burst strength and elongation at break. Chitosan however is one exception. The cationic surface of chitosan provides a reasonable surface sterility for tissue regeneration.3-5 This work therefore concentrates on modification of polysaccharide end groups so that new material surfaces with improved mechanical properties by designing composite scaffolds with a blend of chitosan – sodium alginate and incorporating a natural antimicrobial agent to this blend for facilitating tissue regeneration in antibacterial environment during wound management. As LSE was proved to have good free radical scavenging activity,6,7 it was chosen for present work.
Materials and Methods
Lentil seeds were procured from general stores, Tirupati. chitosan, sodium alginate and ethylene glycol from Himedia laboratory. Agar-agar from central drug house, Mumbai. Peptone and beef extract from Qualigens fine chemicals, Mumbai, India. Glacial acetic acid, hydrochloric acid, ethanol, citric acid monohydrate, pet ether from Molychem,pvt.ltd. Sodium chloride, polyvinyl alcohol from SD fine chemicals Ltd., Mumbai, India.
Preparation of Ethanolic Extract of Lentil Seeds
Seeds of Lens Culinaris were collected from local market and authenticated by botanist Dr. K. Madhava Chetty (IAAT :357). Seeds of Lens culinaris were powdered, defatted with petroleum ether and subjected to distillation under reduced pressure. The procedure was repeated for 3 times .Then the obtained marc was macerated with ethanol for 24 hrs and refluxed for 3 hrs.. Then it was filtered, dried and stored in a desiccators.
Preparation of Chitosan –Sodium Alginate Scaffolds
Chitosan 1 % (w/v) in 2 % (v/v) acetic acid solution and 1.5 % of sodium alginate (w/v) solution in distilled water were prepared after stirring overnight using a magnetic blender. All the chitosan-sodium alginate composite scaffolds (of 6 inches length and 4.5 inches width) were prepared by solvent casting technique with the following procedure.
Then different ratios of chitosan and sodium alginate solutions were mixed at high speed as shown in table no.1. When sodium alginate is blended with chitosan solution the polycationic nature of chitosan led to a strong interaction of alginate carboxylic groups with the chitosan amine groups resulted in the formation of the membrane.8 The prepared solutions were vigorously mixed by mechanical stirrers and vaccum filtered for the removal of any entrapped air bubbles. Then the solution was cast into the scaffolds on the petri plates and dried. The extract loaded scaffolds were prepared similarly, by mixing the ethanolic LSE with chitosan, sodium alginate mixture in a composition shown in table no.1. All the scaffolds were prepared by adding 0.1 ml of poly vinyl alcohol as plasticizer.
Table 1: Composition and results of physico mechanical parameters of different scaffolds
| S. no | Code of scaffold | Ratio of Chitosan – Sodium alginate | Thickness (µm.)
(Mean +S.D.) |
Folding endurance
(Mean +S.D.) |
%ESW
(Mean +S.D.) |
||
| Blank (un loaded) scaffolds | |||||||
| 1 | Sc1 | 1:0 | 52±1.42 | 202 ±17.79 | 39.5±9.1 | ||
| 2 | Sc2 | 0:10 | 56.6+0.5 | 209±14.66 | 43.4±8.32 | ||
| 3 | Sc3 | 1:10 | 57.6+0.3 | 232±4.32 | 82±0.6 | ||
| 4 | Sc4 | 1:20 | 61.3 +0.44 | 256±6.35 | 84.2±0.16 | ||
| 5 | Sc5 | 1:30 | 63+0.78 | 270±12.61 | 85.7±0.14 | ||
| 6 | Sc6 | 1:40 | 64.4+1.05 | 284±17.96 | 175.1±18.04 | ||
| LSE loaded composite scaffolds | Ratio of chitosan, sodium alginate | Amount of LSE (gms) | |||||
| 7 | Sc7 | 1:40 | 0.25 | 66.6+6.93 | 285±15.12 | 174±24.6 | |
| 8 | Sc8 | 1:40 | 0.5 | 70+5.8 | 285±5.34 | 177±0.35 | |
| 9 | Sc9 | 1:40 | 1.0 | 90+0.86 | 282±4.47 | 177±8.31 | |
| 10 | Sc10 | 1:40 | 1.5 | 124.5+11.96 | 281.3±16.02 | 177±16.7 | |
Characterization of Scaffolds
The prepared scaffolds were characterized by following methods
Physico – Mechanical Properties
All the tests were conducted in triplicate by random selection of film from three different places of the prepared scaffolds.
Thickness
The thickness of the scaffolds influence the amount of active ingredients available and also the time taken by the polymer to disappear (by absorbing into body). Thus, it was determined to find the uniformity of the scaffolds. The thickness of the scaffolds was measured using screw gauge in triplicate and the average value was determined.
Folding Endurance
This test was used to determine the flexibility of scaffolds which is required for easy and comfortable handling and application onto the wound. It was done by folding the scaffold repeatedly at same site until it cracks or up to 300folds. The number of folds can be folded without break was noted as folding endurance.
% Equilibrium Swelling (ESw)
The scaffolds were cut into circular shaped discs of 3cm. diameter. The weight of scaffolds (w0) was taken initially, then these were placed in distilled water for 24hrs. to reach equilibrium swelling. The swollen scaffolds were taken out and the excess water was wiped with filter paper and again weight (w) was taken. The %ESw was calculated using formula given below.
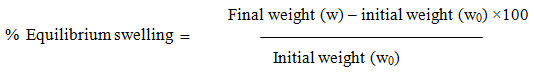
Tensile and Texture Parameters
Tensile and texture parameters measure the capacity of scaffolds to withstand the rupture, and stress or the force needed to break the scaffolds. These were determined using the TA-XT PLUS stable system analyzer and then maximum force (N), maximum elongation at break(sec), were calculated. A penetration test using a 5 mm cylinder probe was used to determine texture parameters i.e the burst strength (rupture point) and burst time using same analyzer.
In Vitro Anti Bacterial Studies
In vitro antibacterial activity was estimated by agar disc diffusion method and measuring the zone of inhibition of two gram positive and two gram negative bacteria with discs of blank composite scaffolds and LSE loaded scaffold in sterile media taken in sterile petri dishes and incubated for 24 hrs at 37±10C. This was done in triplicate for all the scaffolds with each bacteria and average diameter was noted.
In Vivo Studies
The best selected LSE loaded scaffold, blank composite scaffold and pure extract were used to determine the wound healing property in invivo using rats by “excision” wound method after obtaining IAEC approval(no 1677/PO/Re/S/2012/CPCSEA/20,6/5/2016).
Adult female albino rats of weight of 150-200gms were selected and fed with standard laboratory rat feed and water with12-hour light dark cycle and were treated as per protocol given below.
Wounds in the normal control group I were not treated and served with normal diet and water. Blank composite scaffolds (Sc6) were applied on wounds in group II , wounds in group III were treated with best selected LSE loaded composite scaffolds (Sc10). The group IV animals were treated with only extract. Excision wound of 2 cm2 area was created after anesthetization and shaving of the fur at the region of inter scapula on the back of rats. After confirming the homeostasis, the created fresh wound was blotted with sterile gauge. Then the scaffolds were placed on the wounds in groups II, III, IV then covered with sterile gauze. Then the different parameters estimated at specific time periods are given below.
% Wound Contraction
It was measured by graphical method to find the % of decrease in area of wound at specific periods of treatment i.e on 7th,14th day by counting the number of squares of retraced area of wound on graph paper. The % wound contraction was estimated using following formula 9
%wound contraction = 1-(Ad/A0) Χ100
Ao – area of wound on zero day
Ad – area of wound on different days.
Biochemical Studies
Tissue was collected from all groups at site of wound on 0 and 11th day and cleaned with saline to wash off the blood clots and stored in normal saline at -200C for further estimation of hydroxyproline (mg/gm) and hexosamine(µg/gm) as both amino acids are present in collagen fiber of granulation tissue during healing.
Hydroxy proline was determined after acid hydrolysis of tissue and neutralization of excess acid. The absorbance of purple colour formed by treating with Chloramine –T solution and para dimethyl amino benzaldehyde was measured at 557 nm using colorimetry. The amount of hydroxy proline was expressed in mg/gm. wt of tissue using a standard curve.10
Hexosamine is an amino sugar forms a chromogen on heating in an alkaline solution, which further produces a purple coloured compound when reacted with N, N-dimethyl-p-aminobenzaldehyde (Ehrlich reagent) and its intensity was measured using a colorimeter at 530nm.11 The amount of hexosamine was determined in μg / gm wt. of the tissue by comparing with a standard curve.
Histopathological studies
Biopsy specimens for histopathological examination were collected at 7th,14th day post treatment from all the groups and stored in buffered 10%formalin. These were treated by paraffin embedding technique. preserved in 10% buffered formalin. They were processed by routine paraffin embedding technique. Then 5 to 6 microns thick sections were cut and stained with haematoxylin and eosin 12 and microphotographs were taken.
Photography
The images of wounds of rats of different groups were taken for visual comparison.
Tensile strength
The tensile strength of healthy skin and healing skin was determined to find the extent of healing in terms of strength of treated skin in comparison with normal skin
Statistical Analysis
The results were compared using paired t-test and ANOVA test . Statistical confidence levels were set at p =0.05.
Results and Discussion
The natural polymers are preferred, as these overcome the drawbacks of synthetic polymers and degrade into biologically accepted compounds.13 Natural polymers are useful in controlled release systems, tissue engineering and wound healing. Many wound dressings for closing and treating wounds were developed such as fibrin glue,14 sheets of gelatin,15 films of chitosan,2 collagen 16 are commonly used for fast healing of wound. The polymers in combination like gelatin-fibrin, chitosan-fibrin15 shown improved results when compared to treatment with single polymer.
Biopolymers are used as basic compounds for design of scaffolds for different tissue engineering applications. Chitosan and sodium alginate have proven wound healing property individually. But the scaffolds prepared with single polymer have shown poor physico mechanical properties which are essential during handling, storage and application. Hence, the present study focused on development of composite scaffolds using chitosan and different concentrations of sodium alginate to enhance the physico mechanical properties and also loaded with lentil seed extract (LSE) to improve its wound healing activity with its anti oxidant /anti bacterial properties.
LSE was proved to have more free radical scavenging activity and antioxidant activity thus selected for present work to test wound healing activity by loading into best selected blank scaffolds. All the scaffolds were evaluated for different physicomechanical properties, in vitro antibacterial activity and in vivo wound healing activity. The thickness, folding endurance and equilibrium swelling of different blank composite scaffolds (Sc1-Sc6) was significantly different (p<0.05) (table no.1) and were increased with increase in concentration of sodium alginate. Which indicated that the sodium alginate increasing the flexibility of scaffolds. Increased equilibrium swelling might be due to hydrophilicity and swelling property of sodium alginate 17,18 which is consistent with results of chitosan& gelatin.3,17 It was also observed that the tensile and texture parameters were increased with increase in sodium alginate concentration (table no 2) which confirmed the enhanced mechanical property by composite scaffolds. Then, Sc6 was selected for loading the extract, as it has the maximum tensile strength, high equilibrium swelling and folding endurance. The extract was loaded into Sc6 scaffolds at a concentration of 0.25, 0.5,1.0,1.5 gm./0.1 sq.cms. of scaffold as shown in table no.1. A significant difference in thickness of LSE loaded scaffolds (p<0.05) was observed at different concentrations of extract when compared with blank composite scaffold. It indicated that the loading of extract added the thickness to scaffold.
Table 2: The results of tensile and texture parameters of scaffolds
| S.No | Code of scaffold | Maximum force (N) | Maximum elongation at break (sec) | Tensile strength (mpa) | Burst strength (kg) | Burst time (sec ) |
| Blank scaffold | ||||||
| 1 | Sc1 | 0.082 | 2 | 0.084 | 0.0098 | 0.9 |
| 2 | Sc2 | 1.569 | 2.5 | 1.765 | 0.0759 | 1.0 |
| 3 | Sc3 | 2.15 | 2.32 | 2.25 | 0.15 | 1.9 |
| 4 | Sc4 | 2.45 | 2.6 | 2.5 | 0.20 | 2.8 |
| 5 | Sc5 | 4.61 | 2.46 | 4.31 | 0.20 | 2.85 |
| 6 | Sc6 | 5.29 | 2.49 | 5.59 | 0.238 | 2.9 |
The folding endurance, % equilibrium swelling and tensile parameters of LSE loaded scaffolds was not significantly changed (p<0.05) as the composition of polymers is same.
The inhibition of growth of selected bacteria in agar disc diffusion technique with different LSE loaded scaffolds was different. As the quantity of extract in the scaffold was increased, the diameter of zone of inhibition was also significantly increased with all LSE loaded scaffolds (table no.3) and also compared to blank scaffolds. Among LSE loaded scaffolds, Sc10 has shown highest zone of inhibition against selected bacteria hence it was confirmed that LSE has anti bacterial activity as LSE loaded scaffolds shown more antibacterial activity than blank scaffolds.
Table 3: Antibacterial activity against different organisms with best selected blank scaffold and all LSE loaded scaffolds
| name of scaffold | Diameter of zone of inhibition (in cm) (Mean±S.D.) | |||
| B. subtilis | Staph. aureus | E. coli | Pseud. aeruginosa | |
| Sc 6 | 0.67±0.13 | 0.85±0.16 | 0.98±0.15 | 0.76±0.14 |
| Sc7 | 1.2±0.2 | 1.24±0.58 | 1.54±0.34 | 1.72±0.16 |
| Sc8 | 1.4±0.05 | 1.89±0.20 | 1.72±0.23 | 1.96±0.02 |
| Sc9 | 1.5±0.00 | 2.54±0.16 | 2.05±0.08 | 2.07±0.04 |
| Sc10 | 2.2±0.40 | 3.34±0.62 | 3.24±0.64 | 2.48±0.27 |
From the invitro anti bacterial studies, Sc10 has shown maximum inhibitory action against all selected organisms, so, Sc10 was selected for in vivo studies.
In vivo studies were conducted with best blank composite scaffolds (Sc6) and selected LSE loaded scaffolds (Sc10). The % wound contraction represent the reduction in the wound area (table no.4). There was a significant (p<0.05) between untreated (group I) and treated groups (Group-II ,III, IV) and also between wounds treated with blank scaffolds and treated with LSE loaded scaffolds. 100% of wound contraction was observed in groups III treated with LSE loaded scaffolds (Sc10) within 14 days. It revealed that blank scaffolds and LSE loaded scaffolds have more wound healing property than extract alone (group IV).
Table 4: % wound contraction in different groups
| Group | (%)Wound contraction (Mean± S.D.) | |||
| On 7th day | On 14th day | On 21st day | On 28th day | |
| I Group (untreated) | 12.28±10.23 | 64.9±6.56 | 82.8±3.75 | 97.9±0.5 |
| II Group (treated with blank scaffold) Sc6 | 29.82±4.23 | 82.42±0.73 | 95.2±2.55 | Completely healed |
| III Group (treated with extract loaded scaffold) Sc10 | 73.68±10.23 | 100±5.13 (completely healed) | Completely healed | Completely healed |
| IV Group (treated with pure extract) | 56.14±4.38 | 84.6±2.2 | 93.1±1.5 | Completely healed |
As per table no.5, the hydroxyproline and hexosamine values in the present study were increased significantly from the base value to day-7 and day-11 in group-I, group-II and group-IV animals which indicated increased amount of collagen deposition. Increased collagen content at wound site is directly correlated with number of accumulated fibroblasts and suggested early healing process. But in group-III, hydroxyproline value was increased significantly from the base value to day-7 only followed by significant decrease on day-11 which indicated early completion of healing process. These results are in accordance with the results of other scaffolds reported.1,19-20
Table 5: Hydroxyproline and hexosamine quantities in different groups
| Groups | Quantity of Hydroxyproline
mg/gm (Mean ±S.D.) |
Quantity of Hexosamine
mg/gm (Mean ±S.D.) |
||
| Day 0 | Day 11th | Day 0 | Day 11 th | |
| Group I | 3.84±0.01 | 14.72±0.64 | 3.46±0.04 | 11.42±0.40 |
| Group II | 3.92±0.01 | 12.82±0.006 | 3.54±0.01 | 10.54±0.11 |
| Group III | 3.98±0.03 | 10.68±0.70 | 3.98±0.13 | 8.96±0.41 |
| `Group IV | 3.74±0.04 | 13.03±0.07 | 3.36±0.07 | 9.93±0.09 |
The wound healing efficacy can also be supported by observations of microscopic changes. The histo pathological characteristics were studied using skinn samples collected from different groups at different periods.
On 7th day, photomicrographs shown destructed cells in epidermis and surrounding the wound in untreated wounds and treated wounds as wound was fresh one (fig.no.1). Untreated wounds, wounds treated with blank scaffolds and extract have shown edema of dermis and infiltration of neutrophils and on 7th day. But presence of numerous fibroblasts and fibrous tissue on 7th day in wounds treated with LSE loaded scaffolds indicated fast regeneration of tissue and negligible presence of neutrophils and necrosis on 14th day indicated complete healing wound. This suggested that LSE loaded scaffolds might possess high ability to heal the wound fast and rapid epithelialization, as wounds in other groups shown delay in wound healing. It might be due to its action against bacteria which do not cause further damage by infections, hence supported for rapid growth of epithelial tissue and fast wound healing.
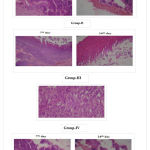 |
Figure 1: Photomicrograpphs of wounds
|
In the process wound healing the damaged tissue will rebuilt to normal tissue and shrinks the area of the wound which depends on the restoring ability of tissue. Sometimes, it may be delated or stopped due to bacterial attack. Thus, the increased repairing ability of tissues by LSE loaded scaffolds is proved in present study.
Based on the photography, the wound size was reduced in all groups on 7th day, 14th day when compared to 0 day. (fig .no.2). The wound was completely healed by 14th day in group III, confirmed the early healing of wound by LSE loaded scaffolds.
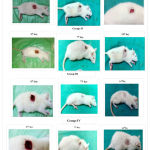 |
Figure 2: Photographs of wounds
|
The tensile parameters were measured for the samples of normal skin and treated skin. (table no.6) which revealed the positive results for treated skin. Maximum force and tensile strength of treated skin was less than normal skin indicated that the strength of treated skin is slowly increasing / changing near to normal skin.
The early wound healing by LSE loaded scaffolds supported that the loading of extract into composite scaffolds enhanced the healing of wound might be due to presence antioxidants in lentil extract, which scavenge the free radicals to large extent and thus improved the healing of wound. But extract alone could not cover wound in absence of scaffold might lead to delay in wound healing whereas, blank scaffolds can support only the regeneration tissue.
Conclusion
The present study successfully designed an effective extract loaded medicated scaffolds for early wound healing and better wound management by ease of application externally on wounds without the need of further removal of scaffold after healing.
References
- Ansari M. A., Jadona N. S., Singh S. P. Amresh kumar and Harpalsingh. Effects of approaches to wound management. Micro Rev. 1997;14:244-269.
- Daniela E., Camelia E. O. Functionalised Chitosan and its use in Pharmaceutical, Biomedical and Biotechnological Research. Chem Engg Communi. 2008;195(10):1269-1291.
CrossRef - Su C. H., Sun C. S., Juan S. W., Hu C. H., Ke W. T and Sheu M. T. Fungal mycelia as the source of chitin and polysaccharides and their application as skin substitutes. Biomaterials. 1997;18(17): 1169-1174.
CrossRef - Ueno H., Yamada H., Tanaka I., Kaba N., Matsuura M., Okumara M., Kadosawa T and Fuginaga T. Accelerating effects of chitosan for healing at early phase of experimental open wounds in dogs. Biomaterials. 1999;20:1407-1414.
CrossRef - Koide S. S., Chitin-Chitosan. Properties Benefits and Risks. Nutrition Res. 1998;18:1091-1101.
CrossRef - Amarowicz R.,Karamać M., Shahidi F. Antioxidant activity of phenolic fractions of lentil (Lens culinaris). J of Food Lipids. 2003;10:1–10.
CrossRef - Amarowicz R., Estrella I., Hernández T., Robredo S., Troszyńska A., Kosińska A.,Pegg R. B. Free-radical scavenging capacity antioxidant activity and phenolic composition of green lentil (Lens culinaris). Food Chem. 2009;121:705-711.
CrossRef - Tanwar Y. S. Formulation and evaluation oftransdermal films of salbutamol sulphate. The Dhaka University J of Phar. Sci. 2005;4. DOI: http://dx.doi.org/10.3329/dujps.v4i2. 208.
- Himabindu T. V. L., Vidyavathi M., Kavitha K., Sastry T. P., kumar S. R. V. Preparation and evaluation of ciprofloxacin loaded chitosan-gelatin composite films for wound healing activity. Int J of Drug Deliv. 2010;2:173-182.
CrossRef - Woessner J. F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino aci. Arch of Biochem and Biophy. 1961;93:440- 447.
CrossRef - Blix G. The determination of he xosamines according toElson and Morgan. Acta Chemica Scandinavica. 1948;2:467-473.
CrossRef - Carleton H. M and Drury R. A. B. Histological technique for normal and pathological tissue 2nd edition, Oxford University Press, London. 1965;250.
- Cullen T. V. Advances in drug delivery systems. Nanotechnology feature article. 2001;4:49-52.
- Brown D. M., Barton B. R., Young V. L. Decreased wound contraction with fibrin glue treated skin grafts. Arch of Surgery. 1992;127:404-406.
CrossRef - Sinha R. N., Varma D. K and Madan P. Burn treatment with gelatin sheets. J of plastic and Recons Surgery. 1972;5:56-61.
- Doillon C. J., Deblois C., Cote M. F., Fournier N. Bioactive collagen sponge as connective tissue substitute. Mat Sci and Eng. 1994;2:43-49.
CrossRef - Nagwa F., Hanan E., Mina T. Implantable biodegradable sponges: Effect of inter polymer complex formation of Chitosan-gelatin on the release behavior of tramadol HCl. Drug Dev and Ind Phrm. 2007;33:7-17.
CrossRef - Mingyu C. h., Jinguang D., Fei Y., Yandao G., Nanming Z and Xiufang Z. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871-2880.
CrossRef - Gomathi K., Gopinath D., Ahmed R. M., Jayakumar R. Quercetin incorporated collagen matrices for dermal wound healing processes in rat. Biomaterials. 2003;24(16):2767-2772.
CrossRef - Kumar R. K., Reddy S . B., Sreenu M., Babu V. V. V., Kumar D. B and Sekhar R. S. Characterization of Deshydroxy Posaconazole. Curr. 2016;5:167, doi:10.4172/2161-0401.1000167.
CrossRef - Nayak S., Poorna N.,Steve S.,Vidyasagar.,Andrew A. Evaluation of wound healing activity of allamanda cathartica.L. and laurus nobilis.L. extracts on rats. BMC Compl and altern med. 2006;6:12. doi: 10.1186/1472-6882-6-12.
CrossRef








