Manuscript accepted on :March 23, 2018
Published online on: --
Plagiarism Check: Yes
Ari Estuningtyas1 , Klaus Zwicker2
, Klaus Zwicker2 , Tri Wahyuni3
, Tri Wahyuni3 , Purnama Fajri3, Pustika Amalia Wahidiyat4
, Purnama Fajri3, Pustika Amalia Wahidiyat4 , Seruni K.U. Freisleben5
, Seruni K.U. Freisleben5 and Hans-Joachim Freisleben6
and Hans-Joachim Freisleben6
1Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia.
2Institute of Biochemistry I, Faculty of Medicine, Goethe-University Frankfurt, 60590 Frankfurt, Germany.
3Faculty of Pharmacy, Universitas Indonesia, Jakarta-Depok.
4Department of Paediatrics – Thalassaemia Ward, Dr. Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia.
5Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Jakarta- Depok.
6Medical Research Unit, Faculty of Medicine, Universitas Indonesia, Salemba Raya 6, 10430 Jakarta, Indonesia.
Corresponding Author E-mail: hj.freisleben@t-online.de
DOI : https://dx.doi.org/10.13005/bpj/1345
Abstract
Treatment of iron overload in thalassaemia is still a great burden for patients, their families and the health care system in developing countries like Indonesia, because of expensiveness and unwanted side effects of chemical iron-chelating therapeutics. This animal study investigates an extract from the leaves of Mangifera foedica L (EMF) and its major active compound, mangiferin, for chelating and antioxidant treatment of iron overload. Sixty rats were randomly divided into 10 groups: control, iron overload (IO), and IO with mangiferin doses between 50 and 200 mg/g BW or 2390 mg of EMF, applied via gastric tubes. For comparison, deferiprone (DFP) was used. Iron overload was induced by intraperitoneal iron dextran resembling two models, transfusion-dependent (TDT) or nontransfusion-dependent thalassaemia (NTDT). Increasing oral doses of mangiferin and EMF did not result in higher mangiferin plasma levels; however, mangiferin administered for four weeks roughly doubled blood levels compared to two weeks. In the TDT model, mangiferin significantly lowered ferritin levels by 21% and plasma iron levels by 60% (EMF by 50%), almost like DFP (by 70%) and increased iron excretion 6-fold via urine (DFP 15-fold, EMF 2-fold). In the NTDT model mangiferin and EMF decreased ferritin levels significantly by about 30%, without significantly decreasing excess plasma iron. Mangiferin increased iron excretion via urine 4-fold (EMF 2-fold) and tended to diminish Fe accumulation in liver and heart. Iron chelating effects of EMF were weaker than of mangiferin, but its in vivo antioxidant activity was stronger. In vitro, both mangiferin and the mangiferin/FeIII complex are potent superoxide radical scavengers, the iron complex being superior.
Keywords
Electron Paramagnetic Resonance Iron Overload; Iron Excretion;Mangiferin; Plasma Ferritin; Thalassaemia;
Download this article as:| Copy the following to cite this article: Estuningtyas A, Zwicker K, Wahyuni T, Fajri P, Wahidiyat P. A, Freisleben S. K. U, Freisleben H. J. Are Mangiferin and Mangiferin-Containing Plant Extracts Helpful for Iron-Loaded Transfusion-Dependent and Non-Transfusion-Dependent Thalassaemia Patients?. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Estuningtyas A, Zwicker K, Wahyuni T, Fajri P, Wahidiyat P. A, Freisleben S. K. U, Freisleben H. J. Are Mangiferin and Mangiferin-Containing Plant Extracts Helpful for Iron-Loaded Transfusion-Dependent and Non-Transfusion-Dependent Thalassaemia Patients?. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19629 |
Introduction
In Indonesia, the frequency of β-thalassaemia gene and HbE carriers ranges up to 33%, thus representing the most frequent single genetic disorder.1 Clinically, thalassemia presents as chronic anaemia with impaired erythropoiesis, intra- and extramedullary haemolysis, and iron overload due to increased gastro-intestinal (GI) iron absorption and supply of blood transfusions.2 Classification into minor, intermedia and major thalassaemias has recently been modified from the aspect of treatment into transfusion-dependent thalassaemia (TDT), which represents thalassaemia major including severe cases of HbE/ß-thalassaemia3,4 and nontransfusion-dependent thalassaemia (NTDT) mainly representing thalassaemia intermedia patients who do not (or not regularly) need blood transfusions.5-7 Either group suffers iron overload because of endogenous and exogenous uptake of excess iron. Whereas in TDT, regular blood transfusions must be accompanied by iron chelation therapy, this has not been clear in the same way for NTDT patients. Iron chelators used in therapy of TDT are deferoxamine (DFO) = desferrioxamine,8 deferiprone (DFP)9 and deferasirox (DFX).10 Meanwhile, iron-chelation of NTDT patients has been introduced, which is much more diverse6. Advantages and disadvantages of these chelators11 have been widely and even controversially discussed.12,13 Apart from administration problems and unwanted side effects14 these iron chelation therapies have one disadvantage in common: they are too expensive for most of thalassaemia patients often belonging to social classes with lower income in Africa and South-East Asia.
Overload with free iron is dangerous as a progressive cause of irreversible organ damage before clinical symptoms develop.15,16 Excess iron can generate free radicals in the circulating blood and induce tremendous oxidative stress,17,18 before it accumulates in the tissue of various organs (e.g. liver, pancreas, spleen, heart) progressively destroying them and leading to functional failure.
Our intention is to find iron chelators from domestic plants to replace these very expensive chemical drugs. Hence, we investigate mangiferin and a mangiferin-containing leaf extract whether they are suitable not only for already iron-loaded experimental animals (resembling TDT) but also in states of the developing iron overload which may be rather preventive and resembling NTDT syndromes.
Moreover, another aspect is investigated in our study, i.e., the antioxidant capacity of mangiferin in addition to the iron-chelating activity, which can have an antioxidant effect by itself, because in some chelate complexes iron is not active in generating free radicals. Antioxidant activity of mangiferin and especially its iron complex had been named “SOD-like antioxidant capacity”.19-21 This is difficult to differentiate in biological systems from endogenous activity. Hence, we confirmed the superoxide anion radical-scavenging activity of both mangiferin and the mangiferin-iron complex in an in-vitro system by electron paramagnetic resonance (EPR) spectroscopy.22,23
Materials and Methods
Mangiferin was derived from extracts of leaves of Mangifera indica L (Plamed Science Technology Inc., China) with mangiferin content of 95.8%. Mangifera foedita leaf extract (EMF) was prepared and analysed according to Wahyuni et al.24 with the analytical certificate # 4565/IPH.3/KS/X/2014 by LIPI Kebun Raya, Bogor, Indonesia. Pure mangiferin for the EPR measurements was purchased from Sigma-Aldrich, Germany.
Study Design: Treatment of Animals
Sixty male Sprague Dawley rats were randomly divided into ten groups: normal controls (C), the groups with iron overload (IO), and therapy groups that obtained mangiferin (50 mg/kg BW), EMF (2930 mg/kg BW), and a group with DFP as positive control. Hoffbrand et al.9 had given the dose of DFP as 75 mg/kg BW in humans and Reagan-Shaw et al.25 converted this dose to 462.5 mg/kg BW in rats.
In a first set of experiments, the plasma levels of mangiferin were determined, both from mangiferin after oral administration of 50, 100, and 200 mg/kg BW and of EMF 2.93, 5.86, and 11.72 g/kg BW.
In the main experiments, the state of iron overload was induced by intraperitoneal (IP) injection of iron dextran (50 mg Fe/mL), twice a week. Two different experimental models were applied as shown in Tables 1 and 2. In the first experimental procedure (resembling TDT), iron overload was established by excess iron for 3 weeks and subsequently, DFP, mangiferin or EMF were administered orally during week 4. In the second experimental procedure, mangiferin or EMF were given from the beginning in parallel with inducing iron overload (resembling NTDT).
Table 1: Animal treatment in the transfusion-dependent thalassaemia (TDT) model
| Groups | Week 1 through week 3 | Week 4 | ||||||||||||
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| N | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| IO | · | – | – | · | – | – | – | – | – | – | – | – | – | – |
| IO+DFP | · | – | – | · | – | – | – | ◊ | ◊ | ◊ | ◊ | ◊ | ◊ | ◊ |
| IO+M75 | · | – | – | · | – | – | – | □ | □ | □ | □ | □ | □ | □ |
| IO+EMF | · | – | – | · | – | – | – | § | § | § | § | § | § | § |
Note: N = normal control; IO = iron overload, 15 mg iron IP/kg BW, twice a week (negative control); IO+DFP = iron overload + deferiprone 462.5 mg/kg BW (positive control); IO+M = iron overload + mangiferin 75 mg/kg BW; IO+EMF = iron overload + Mangifera foetida leaf extract 2930 mg/kg BW; · = administration of iron dextran; ◊ = administration of deferiprone; □ = administration of mangiferin; §= administration of Mangifera foetida leaf extract. All animals were sacrificed at the end of week 4.
Table 2: Animal treatment in the nontransfusion-dependent thalassaemia (NTDT) model
| Groups | Week 1 through week 4 | ||||||
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| N | – | – | – | – | – | – | – |
| IO | · | – | – | · | – | – | – |
| IO+M50 | · □ | □ | □ | · □ | □ | □ | □ |
| IO+EMF | ·§ | § | § | · § | § | § | § |
Note: N = normal control; IO = iron overload, 7.5 mg iron IP/kg BW, twice a week (negative control); IO+M50 = iron overload + mangiferin (M) 50 mg/kg BW; IO+EMF = iron overload + Mangifera foetida leaf extract 2930 mg/kg BW; · = administration of iron dextran; □ = administration of mangiferin; § = administration of Mangifera foetida leaf extract (EMF). All animals were sacrificed at the end of week 4. In both cases, experiments were terminated after 4 weeks. Mangiferin, EMF, and DFP were given orally via a syringe connected to a gastric tube.26,27
The entire experimental procedure was conducted in two phases, both with approval by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia and Dr. Cipto Mangunkusumo Hospital, 205/H2.F1/ETIK/2013 and 738/UN2.F1/ ETIK/2014. During all phases of experiments the animals were kept according to the Helsinki and Tokyo Declarations in their updated international version of year 2000 and European Directives for Animal Experiments 1986/609/EEC and 2010/63/EU.
Blood samples were taken from the tail vein for measurement of plasma mangiferin, plasma ferritin levels, plasma iron concentration and the activity of superoxide dismutase (SOD),28 urine samples to determine the Fe excretion and samples of liver, spleen and heart to determine levels of Fe in the respective organs. During the entire time course of adaptation and experiments, the rats were treated according to prevailing standards and monitored daily and noted signs of general toxicity such as weight loss, diarrhoea and death.
Assay of Plasma Mangiferin
Measurements of plasma mangiferin were performed by HPLC (Waters) with a Symetri C18 column and a PDA detector 2998, at a wavelength of l = 257 nm. The mobile phase used was a mixture of methanol and formic acid 0.5% (30:70). The procedure followed Estuningtyas et al.26
Assay of Fe levels in Plasma and Urine
Iron concentrations in plasma and urine were measured as published29 with slight modifications. Plasma (100 µL) or urine (250 µL) samples were destroyed with 1 mL HNO3 conc. Further procedure followed the assay of Fe levels in organs.30
Assay of Fe Levels in Organs
For analysis of Fe content in organs tissue samples weighing 500-1500 mg were destroyed in an Erlenmeyer flask by 2 mL conc. HNO3 and 1-2 drops of perchloric acid. Distilled water was added to a volume of 50 mL for measurement with atomic absorption spectrometer (AAS, Varian) in BGC-D2 lamp mode at a wavelength l = 248.3 nm.30
Determination of Plasma Ferritin Levels
Determination of plasma ferritin levels was done with plasma samples of 20 µL vs. a standard curve according to manufacturer instructions.31 Absorbance was read in an ELISA reader (Bio-Rad) at a wavelength of l = 450 nm.
Electron Paramagnetic Resonance Measurements of Superoxide Radical Scavenging
The superoxide scavenging capacities of mangiferin and mangiferin-iron-complex were estimated upon superoxide generation by xanthine/xanthine-oxidase system and detection of a spin trap adduct of superoxide radical by EPR. The adduct of superoxide anion radical with DEPMPO (5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide) provides a longer lifetime than the superoxide radical itself and allows detection of its specific EPR signal for several minutes.
Typically, EPR samples contained DEPMPO 40 mM, xanthine 0.5 mM and increasing concentrations of mangiferin or mangiferin-iron complex, respectively. The reaction was started by addition of xanthine-oxidase, final concentration 0.02 U/mL, and the mixture was transferred into a 50 µL glass-capillary which was inserted into the resonator of the EPR spectrometer. Spectra were recorded every minute after start of the enzymatic reaction for a total time of 5 min. To improve signal to noise ratios in quantitative determinations only the fourth peak of the total spectrum was recorded separately, and its amplitude taken as a measure of spin adduct concentration. X-band EPR spectra were obtained at room temperature with a Bruker EMX-AA spectrometer equipped with an ER4103 TM resonator (Bruker Biospin, Rheinstetten, Germany).
Preparation of Mangiferin-Iron-Complex
The complex of mangiferin and iron was formed by incubation of mangiferin and FeCl3 in a 2:1 ratio according to described procedures21.
Data Processing and Statistical Analysis
Results obtained in the form of numerical data comparing the results of more than two groups were statistically analysed by one-way ANOVA parametric test for normal distribution and homogeneous variance. Limit of significance was set to p = 0.05. To compare two groups, post hoc multiple comparison test LSD followed. If the above ANOVA hypothesis did not qualify, Kruskal-Wallis test was applied followed by post hoc Mann-Whitney.32
Results
Body Weight of the Rats
In the beginning of the study, mean weight of the rats was 200 g and 250 g by the end of the study, i.e., all groups gained about 50 g BW, except for the iron overloaded group (IO) without any further treatment, which slightly lost BW. This means, mangiferin contributed to maintain the normal weight gain of rats although they were iron overloaded and would not have gained weight without mangiferin treatment.
Mangiferin Plasma Concentration
As shown in Table 3, mangiferin was administered orally between 50 mg/kg BW and 200 mg/kg BW for 2 and 4 weeks. In these preliminary experiments, mangiferin did not exhibit linear dose-dependent pharmacokinetics, but time-dependence. After 4 weeks, average plasma levels (about 400 ng/mL) were roughly doubled as compared with 2 weeks of administration (almost 200 ng/mL). These values were independent of the amount of mangiferin administered between 50 and 200 mg/kg BW. Similarly, administration of EMF did not exhibit dose-dependent plasma levels of mangiferin (Table 3). For this reason, further experiments were evaluated with the administration of 50-75 mg mangiferin per kg BW without differentiation and with 2.93 g of EMF.
Table 3: Mangiferin plasma levels [ng/mL ± SD]
| Dosage of pure mangiferin | 50 mg / kg BW | 100 mg / kg BW | 200 mg / kg BW | Increase (average) |
| 2 weeks | 177.6 ± 75.8 | 184.8 ± 57.3 | 164.7 ± 25.9 | |
| 4 weeks | 416.1 ± 112.0 | 310.6 ± 134.2 | 450.1 ± 166.0 | ~ 2-fold vs week 2 |
| Dosage | 2.93 g / kg BW | 5.86 g / kg BW | 11.72 g / kg BW | |
| of EMF | ||||
| 4 weeks | 212.0 ± 78.4 | 116.9 ± 45.7 | 145.3 ± 39.3 |
Note: SD, standard deviation; BW, body weight; EMF, Mangifera foetida leaf extract.
Experimental Iron Overload and Plasma Concentration
In the main experiments, two different experimental models were applied; rats were iron overloaded experimentally by IP injection of i) 0.3 ml iron dextran (50 mg Fe/mL) containing 15 mg Fe, for 3 weeks, twice-a-week with subsequent iron-chelating therapy (resembling TDT) and ii) 7.5 mg Fe for 4 weeks, twice a week with simultaneous iron-chelation (resembling NTDT). Control animals had a measurable plasma iron concentration of 3.09 ± 1.44 µg/mL. During the procedure of experimental iron overload, mean iron values were 115.2 ± 51.7 µg per mL plasma in the NTDT model and after iron overload had been established for 3 weeks in the TDT model, average plasma iron levels of 342.3 ± 152.5 µg/mL were reached (Table 4).
Application of mangiferin in the NTDT model only slightly lowered plasma iron levels by about 10% to 103.1 ± 43.2 µg Fe/mL, whereas the administration of mangiferin after the experimental iron overload in the TDT model diminished plasma iron by about 60% to 135.8 ± 92.1 µg/mL (Table 4).
Table 4: Iron levels in plasma and excretion via urine
| Iron chelator | IO + DFP 462.5 mg / kg BW | IO + M 75 75 mg / kg BW | IO + EMF 2930 mg /kg BW | IO + M 50 50 mg /kg BW | IO + EMF 2930 mg / kg BW |
| Model | TDT | TDT | TDT | NTDT | NTDT |
| IO plasma level Fe | 342.3±152.5 µg/mL | 342.3±152.5 µg/mL | 342.3±152.5 µg/mL | 115.2±51.7 µg/mL | 115.2±51.7 µg/mL |
| Decreased plasma iron levels vs. IO (100%) | |||||
| 102.6±45.7 µg/mL =
(-70 %) |
135.8±92.1 µg/mL =
(-60 %) |
167.1±88.4 µg/mL =
(-50 %) |
103.1±43.2 µg/mL =
(-10 %) |
No difference measured | |
| Increased iron excretion via urine vs. control | |||||
| 15-fold | 6-fold | 2-fold | 4-fold | 2-fold | |
Note: IO, iron overload; BW, body weight; TDT, transfusion-dependent thalassaemia; NTDT, nontransfusion-dependent; EMF, Mangifera foedita leaf extract; DFP, deferiprone.
Total Iron Content in Organs
Experimental iron overload in rats for 4 weeks increased total iron content in liver almost six-fold, in spleen about 5-fold, and in the heart almost four-fold. In liver and heart, mangiferin reduced iron levels by 13-15%, but in spleen, no clear reduction of iron content could be detected.
Total Iron Excretion Via Urine
Experimental iron overload in rats for 4 weeks and simultaneous administration of 50 mg mangiferin/kg BW in the NTDT model, iron excretion via urine within 24 hours increased almost 4-fold vs. controls (Table 4). Administration of mangiferin (75 mg/kg BW) after experimental iron overload in the TDT model increased iron excretion/mL urine about six-fold, whereas DFP as a positive control increased iron excretion by 15-fold. It should be mentioned that iron overload without any further treatment almost doubled iron excretion via urine in rats.
Ferritin plasma concentration
In our experimentally iron-overloaded rats, ferritin plasma concentration increased about 10-fold over controls after 3 weeks of experimentally induced iron overload in the TDT model and about 7-fold after 4 weeks in the NTDT model. Mangiferin reduced ferritin levels significantly by 21% in the TDT model (EMF was not determined) and by about 30% in the NTDT model, similar with the effect of EMF in this model (Table 5).
Table 5: Ferritin levels [µg/mL±SD]
| Model | TDT | NTDT | ||
| Control | IO | IO+M | IO | IO+M/EMF |
| 714±219* | 7051±1368 | 5544±1226 | 5038±772 | 3461±1170 |
| Increase vs C | 9.9-fold | ** | 7.1-fold | ** |
| Decrease vs IO | 21% | 30% |
Note: SD, standard deviation; C, control; IO, iron overload; EMF, Mangifera foedita leaf extract; M, mangiferin; * all IO values are significantly different from C (p<0.05); ** significantly different from IO (p<0.05).
SOD Activity in Plasma
By the end of the experimental procedure in the NTDT model, SOD activity – defined as inhibition of epinephrine oxidation – in blood was about two-fold in controls vs. iron overloaded rats. Mangiferin increased SOD activity about 1.5-fold vs. iron overload. SOD activity (units/mL) calculated through 50% inhibition of epinephrine oxidation was 1.49 U/mL in controls and 1.29 U/mL in mangiferin treated rats, whereas treatment with DFP as positive control had only 0.81 U/mL SOD activity (Table 6).
Table 6: SOD activity in plasma [U/mL±SD]
| Model | TDT | TDT | TDT | NTDT | NTDT | |
| Control | IO | IO + DFP | IO + M75 | IO + EMF | IO + M50 | IO + EMF |
| 1.49±0.24 | 0.75±0.21 | 0.81±0.24 | 1.29±0.30 | 1.47±0.34 | 1.12±0.26 | 1.16±0.27 |
| 1 | 0.5 | 0.54 | 0.87 | 0.99 | 0.75 | 0.78 |
Note: IO, iron overload; BW, body weight; TDT, transfusion-dependent thalassaemia; NTDT, nontransfusion-dependent; EMF, Mangifera foedita leaf extract 2930 mg/kg BW; DFP, deferiprone 462.5 mg / kg BW; M75, mangiferin 75 mg/kg BW; M50, mangiferin 50 mg/kg BW.
SOD activity was low in the blood of rats, possibly due to the epinephrine oxidation assay, which may not be suitable to determination of SOD activity in biological samples, and thus raised our curiosity to test “SOD-like” activity in an in-vitro system measuring as directly as possible, i.e. by electron paramagnetic resonance (EPR).
Electron Paramagnetic Resonance Measurements of Superoxide Radical Scavenging In Vitro
Figure 1 shows a typical spectrum of the spin adduct formed from superoxide anion radical and DEPMPO. For quantitative estimation of the radical concentration the amplitude of the fourth peak (inset Figure 1) was used.
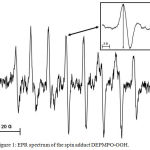 |
Figure 1: EPR spectrum of the spin adduct DEPMPO-OOH. Click here to View figure |
The amplitude of the fourth peak (inset) was taken for quantitative evaluations. Instrumental settings: microwave frequency 9.5 GHz; microwave power 20 mW; modulation amplitude 1G.
Although the spin adduct formation in reference samples (without mangiferin) could be followed for several minutes showing linear time dependence, this was not the case for mangiferin concentrations >10 µM. Therefore, the intensities after 3 min reaction time were used for evaluation of the scavenging effect of mangiferin and mangiferin-iron complex (Fig. 2)
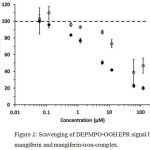 |
Figure 2: Scavenging of DEPMPO-OOH EPR signal by mangiferin and mangiferin-iron-complex.
|
Mangiferin (open circles) reveals significant effects at concentrations above 1 µM. The scavenging effect of the mangiferin-iron-complex (filled circles) is more pronounced, showing more explicit concentration dependence. (100 % intensity corresponds to the signal amplitude in the absence of any scavenging compound; error bars represent standard deviations of at least 3 independent measurements.)
In general, reduction of signal intensity occurred above 1.2 µM concentration. The mangiferin/ FeIII complex was more active with the highest difference of two-fold over mangiferin alone at 6 µM concentration. This might be attributed to an increased “SOD-like” activity of the mangiferin-iron-complex scavenging superoxide.
Effects of Mangiferin-Containing Leaf Extract from Mangifera Foetida L.
Chemical analysis of the ethanolic leaf extract from Mangifera foedica L showed flavonoids, saponins and triterpenoids. Total flavonoid content was up to 1.1 % (w/w); mangiferin was identified as the major flavonoid by thin layer chromatography (TLC) with a content of about 1%.24
Soediro et al.33 had estimated 2.56 % of mangiferin in Mangifera foetida leaves. From this value, we calculated in our study design 2930 mg/kg BW to be equivalent to 75 mg/kg of mangiferin. Afterwards, it turned out that we had only about 1% of mangiferin in our leaf extract; hence, the actual amount was about 30 mg/kg instead of the estimated 75 mg/kg BW.
The extract (2930 mg/kg BW) reduced plasma iron levels in iron-overloaded rats (TDT model) by 50% and doubled iron excretion in urine. SOD activity in extract-treated iron overloaded rats was the same as in controls, that means that SOD activity which decreased in iron-overloaded rats by 50% was fully restored to normal.
In the NTDT model, we did not see effects on plasma iron, but iron in urine was doubled and ferritin levels were reduced by one third (>30%) vs IO. SOD-like activity vs IO increased from 50 % (IO) to 78% of controls (100%). Iron content in spleen was about 10% lower than in IO, spleen weight increased in the IO group by about 10% during the experimental procedure, but remained normal (even slightly below controls) under treatment with the extract.
Discussion
Mangiferin Pharmacokinetics and Plasma Concentration
Mangiferin plasma levels obtained with oral administration did not show a linear dose response relationship. Increasing oral dosage from 50 to 200 mg/kg BW was not proportional to the resulting blood levels, which did not increase, accordingly. In other words, mangiferin has non-linear pharmacokinetics in rats after oral administration.
Lai et al.34 have proven that mangiferin given intravenously provides linear dose-response relationship in rats at doses of 10-30 mg/kg BW, while doses of 30-100 mg/kg BW show non-linear pharmacokinetics. There is a possibility that increasing concentrations of mangiferin may cause conglomerations, both after intravenous and oral administration. Ex-vivo studies with bovine serum albumin demonstrated that mangiferin-binding was not saturable and induced conformational changes at high concentrations.35 Higher oral doses of mangiferin may form larger-sized particles in the gastrointestinal (GI) tract which makes the absorption more difficult and irregular. Human plasma levels were investigated after oral administration and it was concluded that mangiferin exerts the phenomenon of first-pass metabolism and its pharmacokinetics are not linear.36
Without solubilizing adjuvants mangiferin may not dissolve well in the fluid of the GI tract, thereby limiting absorption, especially if conglomeration occurs. Hence, tests have been carried out to improve mangiferin solubility and absorption. Wang et al.37 prepared homogeneous suspension of mangiferin (micelles with CMC 0.4%) to be administered to rats. Comparable results were obtained by phospholipid complexation of mangiferin.38
On the other hand, there was a clear time effect in our experiments; blood levels after 4 weeks of mangiferin administration were roughly doubled as compared to 2 weeks of treatment. In conclusion, the most effective doses of mangiferin were 50 mg and 75 mg/kg BW. Follow-up long-term studies are planned to investigate how pharmacokinetics of mangiferin after oral administration can be improved and whether mangiferin further accumulates if the intake is longer than 4 weeks.
Iron and Ferritin Plasma Concentrations
Administration of excess iron increased total plasma iron levels almost 40-fold and ferritin levels up to 10-fold compared with the normal group. This situation proves that excessive iron administration for 3 weeks in our TDT model or 4 weeks in our NTDT model increased plasma ferritin levels significantly resembling the condition of thalassaemia intermedia and major patients.
In the TDT model, mangiferin (50-75 mg/kg BW) lowered both plasma ferritin levels and excess plasma iron significantly, almost as much as positive control DFP at 462.5 mg/kg BW. In the NTDT model mangiferin reduced ferritin levels significantly by 30%, but lowered excess plasma iron by only 10%, which was statistically not significant. This result indicates that ferritin level appears more sensitive in our NTDT model than plasma iron levels. In this model administration of mangiferin was started still in normal condition, in parallel with the experimental induction of iron overload. In this condition iron is bound by transferrin and not in a free state that can be complexed by mangiferin. Plasma ferritin levels are an indicator of iron overload. Clinically, iron chelating therapy in TDT is normally started at ferritin levels above 1000 mg/mL or if ferritin has increased about 10 times over normal condition, which was just reached in our TDT model, but intentionally not in our NTDT model, even by the end of the experimental course.
Giving chelating agents to male patients with serum ferritin levels > 3000 mg/mL or about 30 times higher than normal Porter et al.39 reported that the decrease in iron concentration was larger and faster than at lower ferritin levels. This shows that the effectiveness of iron chelators is higher in TDT condition than in NTDT and matches the result of our experiments. Since in the latter model iron overload still develops to become manifest, the effects of mangiferin and EMF have also a preventive aspect.
In NTDT, it is possible that the iron is still bound to iron-binding proteins such as ferritin and transferrin, which may not yet be fully saturated. This situation causes low efficacy of iron chelators. In our experiments, the results obtained show the tendency of mangiferin to reduce iron levels in the body more effectively in the TDT model than in NTDT.
In ex-vivo experiments, interaction between mangiferin and ferritin has been reported40, possibly like the mangiferin-bond on albumin subdomain IIA shown by Freitas et al.35 Mangiferin-binding to plasma proteins may interfere with iron chelation and reduce the latter activity, if free plasma iron is not sufficiently available as in the NTNT model.
Total Iron Excretion Via Urine
Total Iron excretion through urine in the NTDT model increased 4-fold with 50 mg mangiferin and 6-fold in the TDT model with 75 mg/kg BW. Positive control DFP with 462.5 mg/kg BW increased total iron excretion via urine 15-fold.
In the NTDT model, iron excretion via urine seems in contrast with the decrease in plasma iron concentration, which, in turn, is in contrast with plasma ferritin levels. Supposedly, there is a positive correlation between ferritin levels and plasma iron concentration. In the NTDT model, low plasma ferritin levels should mirror low plasma Fe and high iron excretion. However, although iron excretion in urine was 4-fold higher, iron in plasma only decreased by 10%. In NTDT condition, it can be assumed that after iron excretion, it takes a certain time until the concentration in plasma decreases, although ferritin levels already indicate a decrease.
In the TDT model, the decrease of total plasma iron by 60% mirrors the 6-fold increased excretion by mangiferin via urine.
Total Iron Content in Organs
Measurement of iron concentrations in liver, spleen and heart is necessary to determine whether mangiferin can prevent free iron to enter and accumulate in organs. In the NTDT model, mangiferin tends to prevent the accumulation of iron in liver and heart, but not in spleen. Most important in this context is the cardioprotective effect of mangiferin41 in addition to its capability to diminish iron overload in the heart, because myocardium is very sensitive to excess iron and heart failure is a major reason of deaths in transfused thalassaemic patients.
SOD Activity
SOD-activity in plasma, which is mainly determined by erythrocyte SOD, was highest in controls (1.49 U/mL). This value was set to 100% activity. In iron-overloaded rats SOD activity decreased to about 50% in either model. Positive controls with DFP did not show significantly higher values of 54%, whereas mangiferin reached 87% and the EMF 99% SOD activity. In the NTDT model, mangiferin treated rats exerted 75% plasma SOD activity.
In either model, TDT and NTDT, mangiferin exerts “SOD-like” antioxidant capacity as postulated by Pardo-Andreu et al.20,21 In literature, measurement of SOD activity in biological systems has been widely discussed42,43 and antioxidant capacity of iron chelating agents was questioned, in particular, whether it was due to scavenging reactive oxygen species (ROS) or by chelating iron, thus preventing the generation of ROS44. Although the iron-chelating mechanism of action has been shown45-47 an important question remained concerning the “SOD-like” antioxidant activity of mangiferin, whether being due only to chelating iron or whether the mangiferin-iron complex still exerts superoxide radical scavenging capacity as suggested.20
We tried to answer this question using EPR spin-trapping with an in-vitro system. At all concentrations of mangiferin in general and between 1.2 and 12 µM in particular, the mangiferin-iron complex exerts almost twice the superoxide scavenging capacity of mangiferin alone and shows clearer concentration dependence. In other words, mangiferin does not only chelate iron and thus prevent the formation of ROS, but its iron complex exerts also higher “SOD-like” radical scavenging activity than mangiferin alone. This is now shown in an EPR in-vitro system; it would be much more difficult to show this in biological systems or in vivo.
It should be mentioned that both mangiferin and mangiferin-iron complex exert flattening of the linear time dependence of the reaction at higher concentrations. This means that there are additional superoxide radical lowering effects and a direct inhibiting effect on xanthine oxidase in the radical generating system might be suggested. Another explanation could be some influence on the spin trap-adduct at high concentrations of mangiferin. Although the spin adduct is temporarily stable, it may further react with mangiferin or be more rapidly degraded under the influence of mangiferin.
In total, the EPR experiment shows that mangiferin does not only act as an iron chelator but in addition as a radical scavenger. The mangiferin iron complex is even a stronger scavenger than mangiferin alone and the iron complexing effect of mangiferin increases its capacity as an antioxidant.
Effects of Mangifera Foetida Leaf Extract
Around the world, Mangifera indica is the standard mango plant for food and medicinal purpose. The species has many variations and cultivars.48 Soediro et al.33 investigated a broad range of Indonesian variations and cultivars and estimated 2.56% of mangiferin in a cultivar with the Indonesian name ‘bacang’. Purwaningsih et al.49 identified closely related Mangifera foetida as the most suitable source of mangiferin and determined 1.13% mangiferin in their extract from the leaves.
Extracts were prepared from leaves, bark, fruit flesh and peel, and kernels of Mangifera indica using a broad variety of extraction methods and with different yields.50-57 Barreto et al.58 found around 10% in Brazilian cultivars of Mangifera indica with highest amounts so far reported in young leaves.
In our TDT model, the extract was not as effective as mangiferin in its activity to decrease plasma iron and excrete iron via urine, but the antioxidant capacity of the extract fully restoring SOD-like activity was stronger than that of mangiferin. This is certainly due to the composition of the extract, which contains various antioxidants in addition to mangiferin.48,56,58,59
In the NTDT model, EMF did not decrease plasma iron level, although it decreased ferritin levels more than 30% and doubled the iron in urine. This is certainly due to the time effect also observed with pure mangiferin. Spleen was moderately protected by EMF towards excess iron60 and SOD activity increased to 78% in the NTDT model.
The Mangifera foedita leaf extract is well tolerated by mice; LD50 showed no deaths and no differences in blood analysis up to the highest dose of more than 13 g/kg BW.24 Mangiferin absorption from the GI tract after oral administration may be gradually better from plant extracts than as pure mangiferin as was reported from honeybush tea in pigs.61 Finally, the importance of this research is demonstrated by the fact that other working groups in Indonesia investigate alternative candidates for iron chelation, e.g., Caesalpinia sappan.62
Summary and Conclusion
Mangiferin was investigated in two different models of experimental iron overload in rats, one resembling TDT and the other one resembling NTDT.
Oral administration of mangiferin showed low GI absorption and non-linear dose-dependent pharmacokinetics in rats, however, time-dependence between 2 and 4 weeks.
The decrease in plasma iron and excretion via urine by mangiferin were more expressed in the TDT model than in the NTDT model, in which ferritin levels were significantly reduced.
The adequate dose in our rat models was 50 to 75 mg mangiferin/kg BW.
Mangiferin and the EMF exert strong antioxidant capacity via iron complexing and free radical scavenging activities.
In the NTDT model, mangiferin showed tendency to moderately diminish the iron content in liver and heart, but not in spleen. The cardioprotective effect is most important to protect iron-overloaded thalassaemia patients from myocardial failure.
Mangiferin is a suitable and potent candidate as iron chelator and antioxidant in the therapy of both, TDT and NTDT patients. In our experiments, EMF exerts gradually lower chelating but stronger antioxidant effects than pure mangiferin.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This research received funding from Directorate of Research and Community Service (DRPM) Universitas Indonesia and Beasiswa Pendidikan Pasca Sarjana (BPPS) Kementrian Riset, Teknologi dan Pendidikan Tinggi Indonesia. The authors are also grateful to the Goethe-University in Frankfurt am Main, Germany for providing the laboratory facilities for the EPR measurements.
References
- Wahidiyat I and Wahidiyat P. A. Genetic problems at present and their challenges in the future: Thalassemia as a model. Presentation at the XIIIth National Congress Ilmu Kesehatan Anak [Child Health Science] 2005;4-7. Bandung, Indonesia.
- Rund D and Rachmilewitz E. b-Thalassemia. N. Eng. J. Med. 2005;353(11):1135-1145.
CrossRef - Berdoukas V., Farmaki K., Carson S., Wood J and Coates T. Treating thalassemia major-related iron overload: the role of deferiprone. J. Blood Med. 2012;3:119–129.
CrossRef - Saliba A. N., Harb A. R and Taher A. T. Iron chelation therapy in transfusion-dependent thalassemia patients: current strategies and future directions. J. Blood Med. 2015;6:197–209.
- Taher A. T., Porter J., Viprakasit V., Kattamis A., Chuncharunee S., Sutcharitchan P., Siritanaratkul N., Galanello R., Karakas Z., Lawniczek T., Ros J., Zhang Y., Habr D and Cappellini M. D. Deferasirox reduces iron overload significantly in nontransfusion-dependent thalassemia: 1-year results from a prospective, randomized, double-blind, placebo-controlled study. Blood. 2012;120(5):970-977. DOI: 10.1182/blood-2012-02-412692.
CrossRef - Taher A. T., Viprakasit V., Musallam K. M. and Cappellini M. D. Treating iron overload in patients with non-transfusion-dependent thalassemia. Am. J. Hematol. 2013;88:409–415.
CrossRef - Taher A. T., Porter J. B., Viprakasit V., Kattamis A., Chuncharunee S., Sutcharitchan P., Siritanaratkul N., Galanello R., Karakas Z., Lawniczek T., Habr D., Ros J., Zhu Z and Cappellini M. D. Deferasirox effectively reduces iron overload in non-transfusion-dependent thalassemia (NTDT) patients 1-year extension results from the THALASSA study. Ann. Hematol. 2013;92:1485–1493. DOI: 10.1007/s00277-013-1808-z.
CrossRef - Hussain M. A., Green N., Flynn D. M and Hoffbrand A. V. Effect of dose, time, and ascorbate on iron excretion after subcutaneous desferrioxamine. Lancet. 1977;309(8019):977–979. https://doi.org/10.1016/S0140-6736(77)92279-6.
CrossRef - Hoffbrand A. V., Cohen A and Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood. 2003;102:17-24.
CrossRef - Taher A and Cappellini M. D. Update on the use of deferasirox in the management of iron overload. Ther. Clin. Risk Manag. 2009;5:857–868.
CrossRef - Maggio A. Light and shadows in the iron chelation treatment of haematological diseases. Br. J. Haematol. 2007;138:407-421.
CrossRef - Kontoghiorghe C. N and Kontoghiorghes G. J. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with nontransfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016;10:465–481.
CrossRef - Taher A. T., Porter J. B., Kattamis A., Viprakasit V and Cappellini M. D. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with nontransfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016;10:4073–4078 (Letter).
CrossRef - Porter J. B., Jaswon M. S., Huehns E. R., East C. A and Hazell J. W. Desferrioxamine ototoxicity: evaluation of risk factors in thalassaemic patients and guidelines for safe dosage. Br. J. Haematol. 1989;73(3):403-409.
CrossRef - Fleming R. E and Ponka P. Iron overload in human disease. N. Engl. J. Med. 2012;366:348-359.
CrossRef - Atmakusuma D. T, Klinis M., Diagnosis P., dan Intermedia T. In: Sudoyo A. W.,et al. (eds.) Ajar B., Penyakit I. D., Jilid I. I., Ed. V. I. Jakarta, Indonesia: Interna Publishing. 2014;2632-2638. (Indonesian) [Thalassaemia: Clinical manifestation, diagnostic approach and thalassaemia intermedia].
- Livrea M. A., Tesoriere L., Pintaudi A. M., Calabrese A., Maggio A., Freisleben H. J., D’Arpa D., D’Anna R and Bongiorno A. Oxidative stress and antioxidant status in ß-thalassemia major. Iron overload and depletion of lipid soluble antioxidants. Blood. 1996;88:3608-3614.
- Laksmitawati D. R., Handayani S., Udyaningsih-Freisleben S. K., Kurniati V., Adhiyanto C., Hidayat J., Kusnandar S., Dillon H. S. D., Munthe B. G., Wirawan R., Soegianto R. R., Ramelan W. and Freisleben H. J. Iron status and oxidative stress in ß-thalassemia patients in Jakarta. BioFactors. 2003;19:53-62.
CrossRef - Leiro J. M., Alvarez E., Arranz J. A., Siso I. G and Orallo F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-a and transforming growth factor b-genes. Biochem. Pharmacol. 2003;65:1361-1371.
CrossRef - Pardo-Andreu G., Delgado R., Velho J. A., Curti C and Vercesi A. E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibit mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005;513:47-55.
CrossRef - Pardo-Andreu G. L., Cavalheiro R. A., Dorta D. J., Naal Z., Delgado R., Vercesi A. E and Curti C. Fe(III) shifts the mitochondria permeability transition eliciting capacity of mangiferin to protection of organelle. J. Pharmacol. Exp. Ther. 2007;320:646-653.
CrossRef - Zhao B. L., Shen J. G., Li M., Li M. F., Wan Q and Xin W. J. Scavenging effect of chinonin on NO and oxygen free radicals and its protective effect on the myocardium from the injury of ischemia-reperfusion. Biochim. Biophys. Acta. 1996;1315:131-137.
CrossRef - Jiang L. Y., He S., Pan Y. J and Sun C. R. Bioassay-guided isolation and EPR-assisted antioxidant evaluation of two valuable compounds from mango peels. Food Chem. 2010; 119:1285–1292. DOI: 10.1016/j.foodchem.2009.09.005.
CrossRef - Wahyuni T., Sari S. P., Estuningtyas A and Freisleben H. J. Toksisitas Ekstrak Etanol Mangifera foetida L. sebagai Pengkelat Besi Ditinjau dari LD50 dan Komponen Sel Darah. Pharm. Sci. Res. 2015;2(3):124-134. (Indonesian) [Toxicity of an ethanolic Mangifera foetida L. extract as iron chelator observed from LD50 and blood cell components].
CrossRef - Reagan-Shaw S., Nihal M and Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661.
CrossRef - Estuningtyas A. Efek preventif mangiferin pada tikus yang diberi preparat besi berlebih. Dissertation, Doctoral Study Program Biomedical Science, Faculty of Medicine, Universitas Indonesia, Jakarta (Indonesian) [Preventive effect of mangiferin to rats, which are given excess iron preparation]. 2015.
- Wahyuni T. Pengaruh mangiferin dan ekstrak air daun Mangifera foetida L. sebagai zat pengkelat besi dan antioksidan secara in vivo pada tikus Sprague Dawley. Thesis, Study Program Biomedical Science, Faculty of Medicine, Universitas Indonesia, Jakarta (Indonesian) [The influence of mangiferin and an aqueous leaf extract from Mangifera foetida L. as iron chelators and antioxidants on Sprague Dawley rats in vivo]. 2013.
- Misra H. P and Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10): 3170-3175.
- Fajria M. A., Wuyung P. E., Handayani S., Freisleben S. K. U and Freisleben H. J. Effect of Red Spinach Amaranthus gangeticus vs Commercial Iron Tablets to Increase Blood Levels of Iron & Hemoglobin in Iron-adequate Mice. Asian J. Biochem. Pharmaceut. Res. (AJBPR). 2015;5(2):1-8.
- Papanastasiou D. A., Vayenas D. V., Vassilopoulos A and Repanti M. Animal and in vitro models in human disease concentration of iron and distribution of iron and transferrin after experimental iron overload in rat tissues in vivo: Study of the liver, the spleen, the central nervous system and other organs. Pathol. Res. Pract. 2000;196(1):47-54.
CrossRef - Genway. Immunoperoxidase assay for determination of ferritin in rat samples. San Diego, CA: Genway Instructions. 1-4.
- Dahlan M. S. Statistik untuk Kedokteran dan Kesehatan, 5th Edn., Jakarta, Indonesia: Salemba Medika, (Indonesian) [Statistics for Medicine and Health]. 2011.
- Soediro S., Iwang S., Kosasih P and Asep W. Isolasi dan Karakterisasi Mangiferin dari Daun Mangga Arumanis dan perbandingan kadarnya pada daun tujuh kultivar Mangifera indica. Acta Pharm. Indones. 1991;4(16):26-35. (Indonesian) [Isolation and characterisation of mangiferin from Mangga Arumanis leaves and comparison of its content in the leaves of seven Mangifera indica cultivars].
- Lai L., Lin L. C., Lin J. H and Tsai T. H. Pharmacokinetic study of free mangiferin in rats by microdialysis coupled with microbore high-performance liquid chromatography and tandem mass spectrometry. J. Chrom. A. 2003;987:367–374.
CrossRef - Freitas P. G., Barbosa A. F., Saraiva L. A., Camps I., da Silveira N. J. F., Veloso M. P., Santos M. H. and Schneedorf, J.M. Mangiferin binding to serum albumin is non-saturable and induces conformational changes at high concentrations. J. Lumin. 2012;132:3027-3034.
CrossRef - Hou S., Wang F., Li Y., Li Y., Wang M., Sun D and Sun C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012;132(1): 289-294. https://doi.org/10.1016/j.foodchem.2011.10.079.
CrossRef - Wang Z., Deng J., Wang Q., Li X and Wei H. Improvement in the solubility of mangiferin by HP-β-CD inclusion. Chin. Trad. Pat. Med. 2008;30:1123-1126.
- Ma H., Chen H., Sun L., Tong L and Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia. 2014;93:54–61. http://dx.doi.org/10.1016/j.fitote.2013.10.016.
CrossRef - Porter J., Galanello R., Saglio G., Neufeld E. J., Vichinsky E., Cappellini M. D., et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur. J. Haematol. 2008;80:168-176. DOI: 10.1111/j.1600-0609.2007.00985.x.
CrossRef - Pohan A. P. N., Purwaningsih E. H and Dwijayanti A. Efek kelasi ekstrak etanol daun Mangifera foetida pada feritin serum penderita talasemia di RS Cipto Mangunkusumo, tahun 2012. e. J. Kesehatan Indonesia. 2013;1(1):45-52. (Indonesian) [Chelating effect of an ethanolic extract from Mangifera foetida leaves on serum ferritin of thalassaemia patients at Cipto Mangunkusumo Hospital, year 2012].
- Jiang D. J., Dai Z and Li Y. J. Pharmacological Effects of Xanthones as Cardiovascular Protective Agents. Cardiovasc. Drug Rev. 2004;22(2):91–102.
CrossRef - Nandi A and Chatterjee I. B. Assay of superoxide dismutase activity in animal tissues. J. Biosci. 1988;13(3):305–315.
CrossRef - Serra J. A., Marschoff E. R., Domınguez R. O., de Lustig E. S., Famulari A. L., Bartolomé E. L and Guareschi E. M. Comparison of the determination of superoxide dismutase and antioxidant capacity in neurological patients using two different procedures. Clin. Chim. Acta. 2000;301:87–102.
CrossRef - Deng W., Fang X and Wu J. Flavonoids function as antioxidant: By scavenging reactive oxygen species or by chelating iron? Radias. Phys. Chem. 1997;50(3):271-276.
CrossRef - Ghosal, S. and Rao, G. A plausible chemical mechanism of the bioactivities of mangiferin. Indian J. Chem., 1996;35B:561–566.
- Leopoldini M., Russo N and Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288-306.
CrossRef - Masibo M and He Q. Major Mango Polyphenols and Their Potential Significance to Human Health. Compr. Rev. Food Sci. F. (CRFSFS). 2008;7:309-319.
CrossRef - Ramirez J. E., Zambrano R., Sepúlveda B and Simirgiotis M. J. Antioxidant Properties and Hyphenated HPLC-PDA-MS Profiling of Chilean Pica Mango Fruits (Mangifera indica L. Cv. piqueño). Molecules. 2014;19:438-458. DOI: 10.3390/molecules19010438.
CrossRef - Purwaningsih, E.H., Hanani, E., Amalia, P. and Krisnamurti, D.G. The chelating effect of Mangifera foetida water extract on serum thalassemic patient. J. Indones. Med. Assoc. 2011;61(8):321-325.
- Kim H., Moon J. Y., Kim H., Lee D. S., Cho M., Choi H. K., Kim Y. S., Mosaddik A and Cho S. K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010;121:429–436. DOI: 10.1016/j.foodchem.2009.12.060.
CrossRef - Kobayashi M., Matsui-Yuasa I., Fukuda-Shimizu M., Mandai Y., Tabuchi M., Munakata H and Kojima-Yuasa A. Effect of mango seed kernel extract on the adipogenesis in 3T3-L1 adipocytes and in rats fed a high fat diet. Health. 2013;5(8A3):9-15. http://dx.doi.org/10.4236/health.2013.58A3002 .
CrossRef - Kulkarni V. M and Rathod V. K. Extraction of mangiferin from Mangifera indica leaves using three phase partitioning coupled with ultrasound. Ind. Crop. Prod. 2014;52:292–297. http://dx.doi.org/10.1016/j.indcrop.2013.10.032.
CrossRef - Kulkarni V. M and Rathod V. K. Mapping of an ultrasonic bath for ultrasound assisted extraction of mangiferin from Mangifera indica leaves. Ultrason. Sonochem. 2014;21:606–611. http://dx.doi.org/10.1016/j.ultsonch.2013.08.021.
CrossRef - Prado I. M., Prado G. H. C., Prado J. M and Meireles M. A. A. Supercritical CO2 and low-pressure solvent extraction of mango (Mangifera indica) leaves: Global yield, extraction kinetics, chemical composition and cost of manufacturing. Food Bioprod. Process. 2013;91:656–664. http://dx.doi.org/10.1016/j.fbp.2013.05.007 .
CrossRef - Ruiz-Montañez G., Ragazzo-Sánchez J. A., Calderón-Santoyo M., Velázquez-de la C., Ramírez de G., León J. A and Navarro-Ocaña A. Evaluation of extraction methods for preparative scale obtention of mangiferin and lupeol from mango peels (Mangifera indica L.). Food Chem. 2014;159:267–272. http://dx.doi.org/10.1016/j.foodchem.2014.03.009 0308-8146/.
CrossRef - Sogi D. S., Siddiq M., Greiby I and Dolana K. D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013;141:2649–2655. http://dx.doi.org/10.1016/j.foodchem.2013.05.053.
CrossRef - Zou T. B., Xia E. Q., He T. P., Huang M. Y., Jia Q and Li H. W. Ultrasound-Assisted Extraction of Mangiferin from Mango (Mangifera indica L.) Leaves Using Response Surface Methodology. Molecules. 2014;19:1411-1421. DOI: 10.3390/molecules19021411.
CrossRef - Barreto J. C., Trevisan M. T. S., Hull W. E., Erben G., de Brito E. S., Pfundstein B., Würtele G., Spiegelhalder B and Owen R. W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008;56(14):5599–5610. DOI:10.1021/jf800738r.
CrossRef - Dorta E., González M., Lobo M. G., Sánchez-Moreno C and de Ancos B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014;57:51–60. http://dx.doi.org/10.1016/j.foodres.2014.01.012.
CrossRef - Fajri P., Estuningtyas A., Louisa M and Freisleben H. J. The preventive effect of Mangifera foetida L. leaf extract administered simultaneously to excess iron on markers of iron overload in Sprague-Dawley rats: a preliminary study. Med. J. Indones. 2018. in press.
CrossRef - Bock C., Waldmann K. H and Ternes W. Mangiferin and hesperidin metabolites are absorbed from the gastrointestinal tract of pigs after oral ingestion of a Cyclopia genistoides (honeybush tea) extract. Nutr. Res. 2008;28:879–891.
CrossRef - Maskoen A. M., Safitri R., Milanda T., Reniarti L and Fauziah P. N. Iron Chelation Ability of Granule Sappan Wood (Caesalpinia Sappan, L.) Extract on Iron-Overloaded. Int. J. PharmTech Res. 2016;9(5):299-305.







