Department of outpatient treatment, Kazakh National Medical University named after S. D. Asfendiyarov, Abay st 87, Almaty, Kazakhstan.
Corresponding Author E-mail: mar.nogayeva@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1350
Abstract
The purpose of this paper is to conduct a clinical-laboratory and radiological evaluation of the combined administration of infliximab and methotrexate to rheumatoid arthritis patients. The research is based on a retrospective analysis of medical records of inpatients, who underwent treatment at the S.D. Asfendiyarov Kazakh National Medical University Department of Ambulatory-Out-Patient Therapy and the city rheumatologic centre (CRC) of Almaty. The main group was injected with infliximab and methotrexate, while the control group – with methotrexate as background therapy. The groups were comparable in terms of age-related, gender, and clinical-laboratory indicators. The research used DAS28 and its standard components and the Larsen index. It was established that the aggregate clinical-laboratory effectiveness of the combined background therapy is determined by a significantly greater reduction of the DAS28 (p<0.05), and a high probability of transition from the average and high to the low level of disease activity (OR – 4.90 [2.47-9.75]), compared to monotherapy. The study proved the significant effect of infliximab and methotrexate on radiological signs of osseous lesion – erosion count (p<0.05) and Larsen index (p<0.05), and the high probability of prevention of osteochondral progression, compared to a mono-component background therapy (OR – 2.66 [1.42-5.01]). The combined administration of infliximab and methotrexate for rheumatoid arthritis patients has greater clinical-laboratory and radiological effectiveness, compared to background monotherapy.
Keywords
DAS28; EULAR; larsen Index; Methotrexate; Osteochondral Destruction
Download this article as:| Copy the following to cite this article: Nogayeva M. Application of Genetic-Engineering Biological Therapy in Rheumatoid Arthritis Patients in the Republic of Kazakhstan. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Nogayeva M. Application of Genetic-Engineering Biological Therapy in Rheumatoid Arthritis Patients in the Republic of Kazakhstan. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18890 |
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory rheumatic disease with a typical peculiarity of a progressive course with deformation development, severe functional locomotor system disorders and visceral lesion, which leads to patients’ disability and reduced lifespan (Karateev et al. 2012; Lillegraven et al. 2012).
At the absence of adequate treatment the patients’ disablement can appear in the first years of disease. Due to potentially dangerous system displays and complications (vasculities, amyloid disease and etc.), and also accelerated atherosclerosis development and high frequency of the heavy cardiovascular pathology, the reduction of life duration in RA patients is observed in comparison with the general population for 3-7 year (Pahau, 2015).
Autoimmune rheumatic diseases (ARDs) encompass a wide variety of illnesses in which innate and adaptive immune responses lead to autoimmune-mediated tissue damage. In total, ARDs affect approximately 5% of the population and result in substantial morbidity, increased mortality and high financial costs. As such, measures to prevent ARDs would lead to marked improvements in public health (Deane & El-Gabalawy, 2014) 20% of patients cease their professional activity two years after being diagnosed and over 50% lose their work capacity after a decade-long illness (Shanahan, Ahern & Smith, 2002). All these factors make RA therapy the serious task. During long time RA was steadily considered to be a progressing disease, to control which course is extraordinary difficult. Since the mid of 1990-s years in approaches to RA treatment, the substantial changes, which led to the considerable prognosis improvement, were observed.
The appearance of genetically engineered biological drugs (GEBD), which are specially created immunoglobulins, specifically influencing the most important links of this disorder’s immunopathogenesis, revolutionized the treatment of RA and other inflammatory rheumatic disorders, such as ankylosing spondylitis, psoriatic arthritis (Sigidin, 2013; Nasonov, 2013). The appearance of these antirheumatic drugs allowed achieving certain success, which is determined by the elaboration of new principles and case management tactics. Updated EULAR recommendations for managing patients suffering from RA were published (Smolen et al. 2010)
GEBD creation is directly connected with views about key mechanisms of pathogenesis, on which they affect as blocking or modelling. In rheumatology GEBD take the place, analogous to target therapy in the modern oncology (Karateev & Luchikhina, 2012).
GEBD substantially improved the results of treatment of earlier untreated patients. It is well known that only 50-60% patients (at primary RA, when the duration of a disease doesn’t exceed nearly a year, the results can be better) respond satisfactory to the standard therapy with basic anti-inflammatory drugs (BAID), such as methotrexate, leflunomide and sulfasalazine in combination with glucocorticoids (Karateev & Luchikhina, 2012). Thus, nearly half of patients turn out to be resistant to BAIDs.
For RA treatment during last 20 years nearly 10 innovative GEBDs – monoclonal antibodies (mAB) and recombinant proteins, inhibiting the activity of the most important anti-inflammatory cytokines and pathological activation of T- and B-lymphocytes, which participate in RA immunopathogenesis were specially developed (Bhati & Bandyopadhyay, 2016).
The results of remicade application looks impressive according to the ASPIRE data (Active Controlled Study of Patients Receiving Infliximab for Treatment of Rheumatoid Arthritis of Early Onset), in which 1004 patients participated from 122 research centres in RA patients with the disease age up to 3 years, the frequency of remission on 54th week of observation made 21.2-31% in dependence on the medicinal drug dose (3 or 6 mg/kg accordingly), and the possibility appeared to attain the substantial slowing down of the joint destruction progressing, than on the background of methotrexate monotherapy (Sigidin, 2013; Nasonov, 2013; Stoffer et al. 2016). The attainment of clinical remission generally is typical peculiarity of biological therapy. The results of randomized study BeSt (Behandel Strategienn), show that the frequency of steady remission at the treatment of combination of methotrexate with each from TNF blocker-α (infliximab, adalimumab and etanercept) at observation during 2-3 years was compared and made nearly 50 % (Smolen et al. 2016). DAS28 was used to assess RA activity and the level of clinical-laboratory remission, including the following parameters: tender joint count (TJC), swollen joint count (SJC) out of 28, erythrocyte sedimentation rate (ESR), C-reactive protein level (CRP), and the visual analogue scale score (VAS) (Smolen et al. 2010; Smolen et al. 2016).
GEBD application allowed substantially improving the prognosis in RA patients and expanding the views about pathogenic mechanisms, which lie in the basis of disease progressing. However, presently it became evident that radical improvement at RA prognosis depends not only on the application of innovation medicinal drugs, but from the treatment strategy improvement (Bhati & Bandyopadhyay, 2016). This strategy is based on the early diagnosis that determines the possibility of initiation of the earliest active carefully controlled anti-inflammatory therapy, directed on maximally quick remission attainment (conception “Treatment to target attainment ”) (Treat to Target)) (Smolen et al. 2016). It finds its reflection in the development of new RA classification criteria, directed on the early diagnosis of disease and remission criteria (Funovits et al. 2010; Neogi et al. 2010; Kuriya et al. 2012).
Earlier researches studied the treatment efficiency only of methotrexate compared to using rituximab (Karlsson et al. 2012). The research has shown that taking only methotrexate is not as effective, as in conjunction with rituximab. 161 patients were selected and divided into 2 groups – control and experimental. The control group, with patients, who took only methotrexate, showed that effect from treatment was much lesser then in the second group. That shows that the use of methotrexate alone is not effective in controlling the disease (Edwards et al. 2004).
Medicines, containing rituximab, are relatively cheaper than products based on infliximab, but the latter has fewer admixtures that can have side effects, such as dermatitis, hypoxia, nausea, and therefore are safer.
Thus, the ultimate goal was to go into remission. The longer the remission period and the weaker the disease activity, the better the long-term prognosis. Strategic studies showed that the achievement of low disease activity or remission by correcting the therapy every 1-3 months in combination with a strict monitoring ensures better clinical, radiological, and functional results than unsystematic observation.
RA patients should be regularly examined for key clinical-laboratory components of disease activity, which carry information on the need to enhance treatment or, maybe, to reduce carefully the intensity of treatment if the disease becomes controllable.
The aim of the study is to conduct a clinical-laboratory and radiological evaluation of the effectiveness of combined administration of infliximab and methotrexate to RA patients.
Materials and Methods
The retrospective analysis of clinical trials was conducted in clinics and Almaty rheumatoid center. The researchers studied medical records of patients, computer database and primary medical documentation (stationary discharge diseases, diseases of the extract from other clinics and hospital records at the place of permanent residence). They analyzed the patient data before treatment, during and after 38 weeks of the drug use.
The author analysed 87 medical records of inpatients, who were administered a combination of infliximab and methotrexate (group 1). As the control group, proceeding from the abovementioned criteria, 95 patients’ medical records were chosen, where methotrexate was administered as background therapy (group 2). The average disease duration was 6.2±3.7 years with an insignificant difference between groups by age-related, gender, and clinical-laboratory indicators.
DAS28 was used to assess RA activity and the level of clinical-laboratory remission, including the following parameters: tender joint count (TJC), swollen joint count (SJC) out of 28, erythrocyte sedimentation rate (ESR), C-reactive protein level (CRP), and the visual analogue scale score (VAS) (Smolen et al. 2010; Smolen et al. 2016).
The radiological study of both hands, knee and ankle joints was conducted with the ARMAN-32 apparatus (Aktyubrentgen, Kazakhstan). An individual research assessed the effectiveness of background therapy in groups 1-2 by the Larson method, which allows taking into account the presence and severity of destructive joint changes (Kirwan et al. 2000).
All clinical-laboratory parameters were analysed before the start of treatment and after 38±2.4 weeks. At the same time, the assessment of the tender joint count, swollen joint count, and morning stiffness duration was conducted after 2, 6, 14, 22, 30, and 38 weeks.
The inclusion criteria for the study were as follows: 1) the presence of RA according to the American College of Rheumatology (ACR) criteria (2010), diagnosed at least 6 months prior to the start of therapy; 2) the presence of an active disease stage (morning stiffness at least 45 minutes, ESR>15 mm/hr, DAS28<5.1).
The following were excluded from the study: 1) disabling forms of RA; 2) cases with systemic inflammatory diseases in the setting of RA; 3) cases of decompensated lesion of kidneys, liver, lungs, heart, and nervous system; 4) endocrine and haematological diseases; 5) cases of tuberculosis in past medical history.
Methotrexate was administered in both groups at 10-15 mg/week, with the average dose of 13.7±1.9 mg. Group 1 also used infliximab (Netherland), infusions in an amount of 3 mg/kg intravenously by drop infusion in a saline solution (250.0 ml) during 2 hours after 0, 2 or 6 weeks after the start of therapy, and then every eight weeks. Methotrexate was administered in both groups at 10-15 mg/week. In the first group, infliximab infusions were used additionally in an amount of 3 mg/kg on the 1st, 2nd, and 6th weeks after the start of the therapy, and then every eight weeks. The overall period of controlled observation was 38 weeks. Symptomatic and pathogenic therapy in both groups included NSAID aceclofenac at 200 mg/day or nimesulide at 200 mg/day, GC methylprednisolone at 4-8 mg/day.
The study was approved by the institutional review board or the ethics committee at each study site. All patients gave written informed consent (Association GA, 2014). The statistical analysis was conducted by means of the STATISTICA 8.0 software package for Windows OS. The data were presented as M±m. The assessment of treatment effectiveness also included the treatment results – absolute (AE) or relative (RE) therapeutic effectiveness and the odds ratio (OR) of drugs, with a calculation of confidence intervals and validity test regarding RE and OR. At p<0.05, differences were considered statistically insignificant. The DAS28 and its standard components were used to control the clinical-laboratory remission. The Larsen index was used for the radiological assessment.
Results
The first objective was to conduct a comparative assessment of the aggregate clinical effectiveness by DAS28 between the combined background therapy and the mono-component background therapy. The DAS28 dynamics is presented in Figure 1. A significant reduction of DAS28 (p<0.05) was observed in both groups. However, in group 1, the difference before and after treatment was 3.22±0.81 points, while in group 2 it was 2.22±0.67 points (p1<0.05). Thus, in terms of the aggregate clinical index, combined background therapy was significantly more effective. The analysis of DAS28 constituents showed a dominating impact of combined background therapy on clinical determinants – swollen joint count (p<0.05), tender joint count (p<0.05), morning stiffness duration (p<0.05), and the visual analogue scale score (p<0.05).
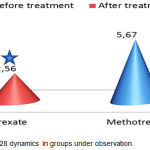 |
Figure 1: DAS 28 dynamics in groups under observation.
|
* –significant difference in indicators after treatment;
** –significant difference between indicators of groups 1-2.
It is known that the lower the disease activity, the better the long-term prognosis for the disease. The analysis of the probability of transition to a low level of activity, based on DAS28 data, provided the following results (Table 1).
Table 1: Probability of transition of RA into low process activity according to DAS28 with different types of background therapy
| AE,% | RE | OR | |
| Infliximab + methotrexate | 83.0 | 1.67 [1.34-2.09] | 4.90 [2.47-9.75] |
| Methotrexate | 49.0 |
After 38 weeks of observations, 83.0% of group 1 patients and 49.0% of group 2 patients showed a reduction of DAS28 from >5.1 and higher to <3.2 points. Thus, the relative clinical effectiveness (RE) and odds ratio (OR) of group 1 in terms of reduction of RA activity were significantly higher (1.67 [1.34-2.09] and 4.90 [2.47-9.75]) than those of group 2.
The next objective of the study was to conduct a component assessment of DAS28 in the studied groups. The analysis of changes in TJC, SJC, and morning stiffness duration in groups under observation provided results that are presented in Figures 2-4.
In particular, the tender joint count of RA patients with different types of background therapy reduced from 14.2±1.8 to 2.7±0.7 in group 1, and from 13.9±2.7 to 4.2±0.9 in group 2. Despite the fact that the TJC significantly reduced (p<0.05) in both groups, group 1 showed changes that were more significant (p1<0.05) (see Figure 2).
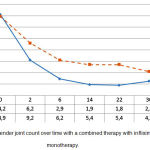 |
Figure 2: Tender joint count over time with a combined therapy with infliximab and monotherapy.
|
The swollen joint count over time reduced from 12.4±1.5 to 1.6±0.4 (p<0.05) with a combined background therapy, and from 12.7±1.9 to 4.2±0.7 (p<0.05) with mono-component background therapy. This proves the greater effectiveness of combined administration of infliximab and methotrexate, compared to the administration of methotrexate only (p1<0.05) (see Figure 3).
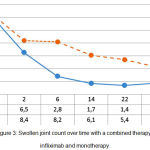 |
Figure 3: Swollen joint count over time with a combined therapy with infliximab and monotherapy.
|
A similar regularity was also observed while analysing the morning stiffness duration over the course of treatment (Figure 4). With the administration of infliximab and methotrexate, morning stiffness, according to the patients’ subjective assessment, reduced from 172.0±31.2 to 34.4±12.5 minutes (p<0.05). Administration of methotrexate reduced morning stiffness duration from 165.0±35.8 to 54.0±15.9 minutes (p<0.05) with a significantly greater effectiveness in group 1 (p1<0.05) (see Figure 4).
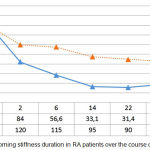 |
Figure 4: Morning stiffness duration in RA patients over the course of treatment.
|
Significant VAS results were obtained in both background therapy groups (Table 2) (p<0.05). However, the analysis of effectiveness by VAS showed that combined background therapy, compared to monotherapy, provides a more significant clinical effect by the patient’s self-assessment (reduction by 64.5 points versus 50.2 points, p1<0.05), assessment of general weakness (41.0 points versus 28.7 points, p1<0.05), general pain (65.1 points versus 38.9 points, p1<0.05), and the doctor’s assessment of the patient’s state (62.5 points versus 44.7 points, p1<0.05).
Table 2: Visual analogue scale over time with various types of background treatment for RA patients
| Group 1 n=87 | Group 2 n=95 | |||
| Before treatment | After 38 weeks | Before treatment | After 38 weeks | |
| Patient global scale | 77.9±3.8 | 13.4±4.19* | 74.9±5.67 | 24.7±8.84* ** |
| General weakness | 53.9±2.41 | 12.9±3.23* | 52.4±4.54 | 23.7±6.45* ** |
| General pain | 76.9±1.53 | 11.8±3.85* | 75.1±6.51 | 36.2±4.21* ** |
| Doctor’s assessment of the patient’s state | 73.6±1.77 | 11.1±4.93* | 72.8±5.81 | 28.1±3.27* ** |
* – significant difference in indicators after treatment (p<0.05);
** – significant difference between indicators of groups 1-2 (p1<0.05).
The analysis of laboratory indicators in studied groups showed significant changes of both combined and mono-component background therapy (p<0.05). The differences between groups 1 and 2 in terms of ESR and CRP after 38 weeks of observation were expressed as p1>0.05 (Table 3).
Table 3: Level of ESR and CRP with different types of background therapy over time in RA patients
| Group 1 n=87 | Group 2 n=95 | |||
| Before treatment | After 38 weeks | Before treatment | After 38 weeks | |
| ESR, mm/hr | 63.0±12.9 | 17.0±5.72* | 67.8±10.1 | 18.2±4.24* |
| CRP, mg/% | 58.1±13.1 | 7.11±3.81* | 55.6±9.82 | 6.87±2.78* |
* – significant difference in indicators after treatment (p<0.05).
Although the groups’ laboratory indicators did not differ significantly after 38 weeks of observation, significant discrepancies were discovered during the analysis of radiological progression rate (Table 4).
Table 4: Radiological indicators over time with different types of background therapy for RA patients
| Group 1 n=87 | Group 2 n=95 | |||
| Before treatment | After 38 weeks | Before treatment | After 38 weeks | |
| Larsen index | 73.2±26.5 | 76.0±27.9 | 72.4±31.9 | 78.3±19.6** |
| Erosion count | 9.0±9.4 | 9.8±7.4 | 8.7±8.5 | 11.0±9.1** |
| Level of joint space narrowing in points | 40.8±33.8 | 42.1±32.5 | 39.4±32.4 | 42.2±28.2** |
** – significant difference between indicators of groups 1-2 (p1<0.05).
With comparable (p>0.5) initial values of joint destruction indicators, it was found that the main and control groups showed a rising osteochondral destruction after 38 weeks of observation. However, its rate of rise in patients, who underwent combined background therapy (infliximab + methotrexate), was lesser than in patients, who underwent monotherapy with methotrexate. This was demonstrated by the Larsen index (2.81±0.61 rate of rise versus 6.67±2.34) and the erosion count (0.51±0.51 versus 2.07±0.87), where this difference was statistically significant (p<0.05).
The probability of prevention of osteochondral destruction, according to the Larsen index, in observed groups is presented in Table 5. With combined background therapy, the probability of preventing osteochondral destruction, i.e. the absolute therapeutic effectiveness by the Larsen index, was 46.0%; with a mono-component background therapy, it was 24.0%. Significant indicators of RE and OR were obtained. Thus, the application of combined background therapy was significantly more effective in preventing osteochondral destruction, compared to the mono-component background therapy (see Table 5).
Table 5: Probability of prevention of osteochondral destruction in RA patients with different types of background therapy
| AE,% | RE | OR | |
| Infliximab + methotrexate | 46.0 | 1.90 [1.24-2.89] | 2.66 [1.42-5.01] |
| Methotrexate | 24.0 |
The conducted retrospective study with a long observation period of RA patients confirms that the combination of infliximab and methotrexate is a promising method of treatment, which allows achieving stable clinical-laboratory remission by the DAS28 scale, improving the patients’ life quality by VAS, and helps prevent osteochondral destruction by the Larsen index.
Discussion
Over the past year, the quality of the treatment of RA has much increased, which led to long-term remission in patients. Modern researches allow prolonging remission and tracking the activity of the disease (Gabay et al. 2013). In Russia we conducted a retrospective analysis of data on the treatment of patients with RA from 1970 to 1999, which studied the entire history of illness of patients, up until their death. We compared the indicators of two groups – main (fatal) and control, patients of which have lived more than 15 years since the detection and early treatment of disease. Treatment of these patients was carried out by means of basic therapy, based on methothrexate (Karateev & Luchikhina, 2012). Our study was conducted at the same time as the treatment, which means that we received information about the patient’s condition directly during treatment and monitored the slightest changes.
As of today, a number of trials proved that biological therapy significantly improves the treatment of several forms of RA. In particular, the SWEFOT trial, showed that the application of two types of therapy – “triple therapy” and a combination of methotrexate and infliximab – for patients with early stages of RA, whose response to methotrexate was insufficient, provided better results in the methotrexate+infliximab group in the 1st year of treatment, but in the 2nd year, results evened out. The radiological progression was also less significant in the methotrexate+infliximab group.
The ATTRACT (Anti-TNF Therapy in Rheumatoid Arthritis patients on Concomitant Therapy) researches state that infliximab, combined with methotrexate, blocks the structural lesion progression of early-stage RA patients in the course of a 2-year treatment. Early prescription of infliximab into the active RA therapy, despite methotrexate therapy, can ensure long-term advantages by preventing radiographic progression and preserving joint continuity.
The data, obtained in this study, confirm and supplement the recommendations of the European League Against Rheumatism (EULAR), which provide for a possibility of administering biological disease-modifying drugs in cases with insufficient response to methotrexate8. In the author’s opinion, biological therapy reflects the progress in understanding the pathogenesis of rheumatic diseases, and the regularities of the autoimmunity and targeted effect on these systems, in particular.
FDR of NAN patients with RA have a higher prevalence of joint symptoms compared to individuals with no family history of autoimmune disease. This finding is only partially explained by a high prevalence of RA autoantibodies in the FDR 20. The combined use of methothrexate with adalimumab increases the therapy’s efficacy12. However, the combined use of methothrexate with infliximab is safer than methothrexate with adalimumab as the maximum permissible dose of the latter is not known and it has a wide range of contraindications and side effects, and therefore is not widely applied. The comparative monotherapy with adimulab and tocilizumab has shown the high efficacy of the latter. The efficacy of monotherapy with methothrexate is unsatisfactory (O’Dell et al. 2013). Methothrexate has little effect in slowing the disease (Scott, 2012), so we need to combine the treatment with methothrexate with other substances.
Infliximab treatment at early RA stages leads to more a frequent remission development at the early therapy stages, compared to advanced illness stage results. A distinct dose-depending effect of infliximab is observed with patients who were administered more than 4 infusions of the drug. Bone destruction was inhibited more distinctly, compared to the patients who were administered fewer infusions. In a number of cases, destruction inhibition was combined with clinical improvement. A significant therapeutic effect was observed in cases of prescription of both a yearlong infliximab course and “average” (5-7 infusions per annum) drug doses, which is proven by the given research.
The efficacy of infliximab is proven by comparing its effect to other drugs, such as adalimumab, rituximab, abatacept, against which infliximab shows better results (Singh et al. 2010). As methothrexate is not as effective as previously thought, there was a need for a study of its joint action with other substances to enhance its effect.
It was important to investigate the difference between treatments at an early stage and the advanced ones. Early detection of rheumatoid arthritis in individuals, genetically susceptible to it, will allow more effective use of drug therapy and will slow the progression of the disease. According to our research, earlier application of infliximaba in conjunction with methotrexate can significantly slow down the destruction of bone tissues. In future we can explore them in more detail and create a new drug based on these two agents, as well as a more effective treatment of RA in its early stages, preventing further complications of the disease.
Conclusion
It was shown that the combined administration of infliximab and methotrexate as a background therapy for rheumatoid arthritis patients has a significantly higher clinical-laboratory and radiological effectiveness, compared to mono-component background therapy with methotrexate.
The aggregate clinical-laboratory effectiveness of combined background therapy is expressed in a significantly greater reduction of DAS28 (p<0.05) and a high probability of transition from the average and high into the low level of disease activity (OR – 4.90 [2.47-9.75]), compared to a mono-component background therapy.
The analysis of DAS28 constituents showed a dominating impact of combined background therapy on clinical determinants – swollen joint count (p<0.05), tender joint count (p<0.05), morning stiffness duration (p<0.05), and the visual analogue scale score (p<0.05), compared to a mono-component background therapy.
The study showed a significant effect of infliximab and methotrexate on radiological signs of osseous lesion – erosion count (p<0.05) and Larsen index (p<0.05), and the high probability of prevention of osteochondral progression, compared to a mono-component background therapy (OR – 2.66 [1.42-5.01]), compared to a mono-component background therapy.
The analysis of real clinical practice in Kazakhstan shows that the combined administration of infliximab and methotrexate to RA patients has a favourable long-term prognosis, inducing in most cases a quick and pronounced reduction of disease activity, facilitating the deceleration of osteochondral destruction, and helping to go into remission at the early stage of therapy.
Acknowledgment
The study did not involve any third parties other than the authors of the article
Conflict of Interest
There is no conflict of interest
Fundiing Source
The article was financed by the authors of the article
References
- Karateev D.E, Luchikhina E.L, Muraviov Y.V, Demidova N.V, Grinyova G.I, Novikova D.S, et al. The first Russian strategic study of pharmacotherapy of rheumatoid arthritis. Sci Pract Rheumatol. 2012;2(51):21.
- Lillegraven S, Prince F.H.M, Shadick N.A, Bykerk V.P, Lu B, Frits M.L, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Rheum. Dis. 2012;71(5):681–686.
CrossRef - Pahau H. Cardiovascular disease in rheumatoid arthritis. PhD Thesis,UQ Diamantina Institute, The University of Queensland, Woolloongabba, Australia. 2015.
- Deane K.D, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Rheumatology 2014;10(4):212.
CrossRef - Shanahan E.M, Ahern M.J, Smith M.D. Which patients stop working because of RA? Rheum. Dis. 2002;61(9):859.
CrossRef - Sigidin Y.A. On the generalized analysis of the results of genetic engineering of biological therapy – the search for new patterns. Sci Rheumatol. 2013;2(51):21.
- Nasonov E.L. Genetically engineered biological agents in the treatment of rheumatoid arthritis. Rheumatol. J. 2013;3:33-40.
- Smolen J.S, Landewé R, Breedveld F.C, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Rheu. Dis. 2010;69(6):964-975.
CrossRef - De K, Luchikhina E.L. Modern rheumatoid arthritis treatment strategy. Med. J. 2012;20: 30.
- Bhati M, Bandyopadhyay S. Efficacy and safety of an anti-CD20 monoclonal antibody for the treatment of patients with moderate to severe rheumatoid arthritis following the failure of conventional synthetic disease-modifying anti-rheumatic drugs. Rheumatol. 2016;35(8):1931–1935.
CrossRef - Stoffer M.A, Schoels M.M, Smolen J.S, Aletaha D, Breedveld F.C, Burmester G. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Rheum. Dis. 2016;75(1):16–22.
CrossRef - Smolen J.S, Emery P, Fleischmann R, van Vollenhoven R.F, Pavelka K, Durez P, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. The Lancet. 2016;383(9914):321–332.
CrossRef - Funovits J, Aletaha D, Bykerk V, Combe B, Dougados M, Emery P, et al. European League Against Rheumatism classification criteria for rheumatoid arthritis: methodological report phase. Rheum.Dis. 2010;69(9):1589–1595.
CrossRef - Neogi T, Aletaha D, Silman A.J, Naden R.L, Felson D.T, Aggarwal R, et al. The 2010 American College of Rheumatology. European League Against Rheumatism classification criteria for rheumatoid arthritis. Rheum. 2010;62(9):2582–2591.
- Kuriya B, Sun Y, Boire G, Haraoui B, Hitchon C, Pope J.E, et al. Remission in early rheumatoid arthritis—a comparison of new ACR/EULAR remission criteria to established criteria. Rheumatol. 2012;39(6):1155–1158.
CrossRef - Karlsson J.A, Neovius M, Nilsson J.Å, Petersson I.F, Bratt J, van Vollenhoven R.F, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in early rheumatoid arthritis: 2-year quality-of-life results of the randomised, controlled, SWEFOT trial. Rheum. Dis. 2012.
- Edwards J.C.W, Szczepański L, Szechiński J, Filipowicz-Sosnowska A, Emery P, Close D.R, et al. Efficacy of B-cell–targeted therapy with rituximab in patients with rheumatoid arthritis. New England. Med. 2004;350(25):2572–2581.
- Kirwan J.R. Using the Larsen index to assess radiographic progression in rheumatoid arthritis. Rheumatol. 2000;27(1):264–268.
- Association G.A of the W.M. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Am.Coll. Dent. 2014;81(3):14.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, Green J. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. The Lancet. 2013;381(9877):1541–1550.
CrossRef - Dell O.J.R, Mikuls T.R, Taylor T.H, Ahluwalia V, Brophy M, Warren S.R, Keystone E. Therapies for Active Rheumatoid Arthritis after Methotrexate Failure. New Eng. J. Med. 2013;369(4):307-318.
CrossRef - Scott D.L. Biologics‐Based Therapy for the Treatment of Rheumatoid Arthritis. Clinical Pharmacol. Therapeut. 2012;91(1):30–43.
CrossRef - Singh J.A, Christensen R, Wells G.A, Suarez-Almazor M.E, Buchbinder R, Lopez-Olivo M.A, Tugwell P. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Sao Paulo Med. J. 2010;128(5):309–310.
CrossRef









