Shaimaa S. Mutlak1 , Vean Sabah Ali1
, Vean Sabah Ali1 and Radhwan M. Hussein2
and Radhwan M. Hussein2
1Department of Basic Science, College of Dentistry, University of Baghdad, Iraq.
2College of Pharmacy /University of Ahl AL-Bayt. Iraq.
Corresponding Author E-mail: shaimaaphdbio@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1369
Abstract
The metabolic syndrome (MS) is one of the main public fitness issues around the world. The superiority of MS was increasing in parallel with weight problems and diabetes worldwide. Many of the various standards for the identification of MS, its most important additives are atherogenic dyslipidemia, insulin resistance, high blood pressure and abdominal weight problems. A new bioactive adipokine (Apelin), which play a very important role (vital role) in controlling glucose homeostasis, dyslipdemia, cardiovascular diseases and obesity. The aim of this study is to study the relativity between apelin and metabolic syndrome in women. The research start by chosen 75 females were involved in this study (15 control and 60 had metabolic syndrome) divided into four groups and were classified according to the criteria of International Diabetes Federation 2005. All subjects were reported and it was contain anthropometric and biochemical investigation. Blood samples collected from all subjects at Baghdad teaching hospital during September 2015 to April 2016. Serum apelin was estimated using ELISA technique. While TG, HDL, HbA1C and FBG serums were measured using colorimetric methods. It was concluded that high significant elevated in apelin concentration in women with MS make suggested that apelin has potential role in metabolic syndrome.
Keywords
Apelin; BMI;; HDL; HbA1C Metabolic Syndrome; Triglyceride WC;
Download this article as:| Copy the following to cite this article: Mutlak S. S, Kadhim M. S, Hussein R. M. Apelin and some Biomarkers in Females with Metabolic Syndrome. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Mutlak S. S, Kadhim M. S, Hussein R. M. Apelin and some Biomarkers in Females with Metabolic Syndrome. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19204 |
Introduction
The metabolic of syndrome can be occurred by gathering many risk metabolic factors, along with increased plasma triglyceride (TG), decreased high density lipoprotein cholesterol (HDL-C), extended blood pressure, and elevated blood glucose.1 This aggregation is identified as a several risk issue for atherosclerotic cardiovascular disorders and T2DM.2 Metabolic risk factors are extra usual in obese. For this reason, weight problems are usually covered inside the clinical definition of metabolic syndrome.3,4 In recent years, interest has targeted at the visceral adipose tissue resulting from the presence of a dipo-cytokines synthesis and secretion from adipocytes.5,6 Apelin is a novel adipokine secreted by mature adipocytes, is thirty-six amino acid pre-peptide and endogenous ligand of APJ receptor the G-protein-coupled receptor, which discovered in different types tissues included the central nervous system, rise releasing in the hypothalamus, stomach, heart, skeletal muscle, and white adipose tissue.7-9 Apelin has paracrine function by combined and activated the apelin receptor (Apelin or APJ).10 Several types of potential apelin found involving apelin-36, apelin-17, apelin-13, and apelin-13 with the pyro glutamate form.11 Recent the system of apelin that exist to interact in the multiple conditions pathogenesis as obesity, disturbances in carbohydrate metabolism and type 2 diabetes mellitus, hypertension, cardiac failure.12,13
This adipokine was reported to be involved in physiological balance during storage which developed metabolism, and effect eat behavior and has important function in obesity-associated disorders involved type II diabetes and hypertension as well. Though apelin revealed in white adipose tissue of rats,14,15 the cells are responsible for secretion were not known. Plentiful types of cells found in this tissue, other than the adipocyte itself, there could be responsible of the detection of apelin transcripts.16,17
Syndrome metabolic has risk factors due to several conditions such diabetes and prediabetes, abdominal obesity, dyslipidemia and high blood pressure.18 Increasing clues revealed another function of apelin and related the regulation to several physiological roles such fluid balance, intake food, proliferation of cell, regulation pressure of blood, angiogenesis and utilization of glucose19,20 and therefore it can interfere in many conditions like obesity, type I and II diabetic, hypertension or cardiac diseases.21 The cardiovascular system reveals the vital role of apelin and its receptor were present in heart, precisely in both large and small conduit vessels, and endothelial cells.22
Materials and Methods
This study included 75 women were selected from Baghdad teaching hospital from September 2015 to April 2016 (There were selected according to consent papers and upon their agreement). Control group consist of fifteen healthy individual) while other four groups with metabolic syndrome were classified into: Group I (n =15; obese), Group II (n =15; obese + ↑ TG), group III (n=15; obese +↑ TG+ ↓ HDL), (n=15; obese +↑ TG + ↓ HDL + hypertension). These groups were classified according to the criteria of International Diabetes Federation 2005.23 This study was approved by the Institute Review Board at the College of dentistry, baghdad University. Each participant gave a written consent showing her agreement for the participation in this study. Abdominal obesity plus two of the other criteria are needed for the diagnosis of the metabolic syndrome. We analyzed:
Abdominal obesity (BMI > 30 kg/m2).
Fasting plasma glucose ≥ 110 mg/dl .
Systolic (SBP) ≥ 130 or diastolic (DBP) ≥ 85 mmHg.
Level of high density lipoproteins (HDL) < 40 mg/dl.
Level of triglycerides (TG) ≥ 150 mg/dl.
Blood samples were taken in morning from fasting women (12 hours fasting) and were gathered in plane tube, and centrifugation the samples at 3200 rpm for 10 min to isolate the serum and after that measurements were taken to fasting blood like glucose, triglyceride and HDL-Cholesterol using colorimetric methods and apelin (Mybiosource apparatus, USA) using ELISA technique.
Statistical analyses to all obtained data were done using convenient methods for each analysis (Rosner B, 2006) and computer program SPSS v.16.0. The data were expressed in mean± value with standard error (mean ± SEM). Results were compared by one way analysis of variance (ANOVA) and the least significant differences (LSD) were calculated. Correlations between variables were evaluated using Pearson’s correlation coefficient and simple regression analysis. The association or difference was considered statistically significant when P-value ≤ 0.05.24
Results
The totals of 75 samples (participants) were consisting of control group which has 15 samples (participants), while the four metabolic syndromes were 60 participants. All metabolic syndrome and control groups were match by age. BMI differ significantly between MS and control group. It was clear from Table I the reveals the mean value measurements of waist circumference was significantly in all metabolic syndrome group than control group. While the systolic and diastolic blood pressure shows highly significant differences in group (IV) MS compared with control and other MS groups. Table (2) shows the mean value of apelin significantly increase as MS components number increase (p<0.001).
Table 1: Descriptive clinical data and anthropometric parameters of control and metabolic syndrome groups.
| Features | control | Group I | Group II | Group III | Group V |
| Age (years) | 39.7±1.64 | 39.7±2.42 | 39.9±1.21 | 39.2±1.93 | 39.5±1.12 |
| BMI (kg/m2) | 24.6±0.45 | 27.5±0.47 | 34.3±0.93a***, b*** | 34.3±0.93a***, b*** | 34.8±0.79a***, b*** |
| SBP (mmHg) | 121.1±0.64 | 121.2± 0.64 | 121.3±0.54 | 121±0.62 | 136.9±0.64a***,b***,c***,d*** |
| DBP (mmHg) | 80.7±0.69 | 80.1±0.6 | 80.9±0.67 | 82.4±0.78 | 90.4±0.35 a***,b***,c***,d*** |
| WC (cm) | 70.5±1.26 | 116.7±1.6a*** | 117.2±1.73 a*** | 119±1.8a*** | 121±1.79a*** |
aANOVA test: Group I,II,III, and IV vs Control group: ***p<0.001.
bANOVA test: Group II,III and IV vs Group I: ***p<0.001
cANOVA test: Group III and IV vs Group II: ***p<0.001.
dANOVA test: Group III vs Group IV: ***p<0.001.
Table 2: Biomarkers feature of studies groups.
| Features | control | Group I | Group II | Group III | Group V |
| HbA1C % | 4.6±0.22 | 4.4±0.18 | 5.2±0.18 a*, b**, | 5.04±0.24 b*, | 5.7±0.13 a***, b***, d* |
| FBS (mg/dl) | 83.5±2.39 | 78.3±2.89 | 86.07±3.03 b***, | 88.5±2.54 b***, | 89.6±2.03 b***, |
| HDL-cholesterol (mg/dl) | 50±1.56 | 47.73±1.64 | 43.3±1.86 a**, b*, | 34.7±0.83 a***, b***, c***, | 34.7±0.83 a***, b***, c***, |
| Triglycerides (mg/dl) | 107.5±6.46 | 119.8±5.24 | 180.2±10.6 a***, b***, | 198.4±10.3 a***, b***, | 196.9±12.4 a***, b***, |
| Apelin ng/ml | 1.27±0.08 | 1.94±0.15 a** | 2.7±0.06 a***, b*** | 2.72±0.19 a***, b*** | 3.14±0.12 a***, b***, c*, d* |
aANOVA test: Group I,II,III, and IV vs Control group: ***p<0.001, **p<0.01, *p<0.05
bANOVA test: Group II,III and IV vs Group I: ***p<0.001, **p<0.01, *p<0.05
cANOVA test: Group III and IV vs Group II: ***p<0.001, **p<0.01, *p<0.05
dANOVA test: Group III vs Group IV: ***p<0.001, **p<0.01, *p<0.05.
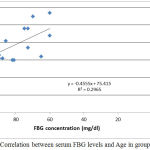 |
Figure 1: Correlation between serum FBG levels and Age in group I.
|
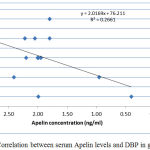 |
Figure 2: Correlation between serum Apelin levels and DBP in group I.
|
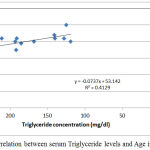 |
Figure 3: Correlation between serum Triglyceride levels and Age in group II.
|
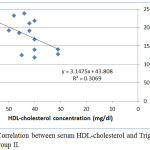 |
Figure 4: Correlation between serum HDL-cholesterol and Triglyceride levels in group II.
|
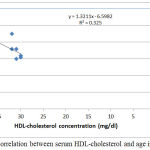 |
Figure 5: Correlation between serum HDL-cholesterol and age in group III.
|
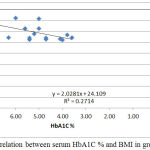 |
Figure 6: Correlation between serum HbA1C % and BMI in group III.
|
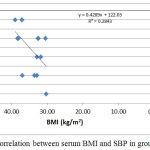 |
Figure 7: Correlation between serum BMI and SBP in group IV.
|
Discussion
Metabolic syndrome like insulin fight, diabetes, hypertension, fatty liver disease (FLD), polycystic ovarian syndrome, and many types of cancer were caused by severe adiposity.21 Adipose tissue secreted several adipokine such as apelin has an important role in complications of obesity.9,19] High plasma apelin has been reported by different authors in severe obesity and correlated with adiposity.22,25 Obesity propagation was increased actively in the latest 30 years.26 The data obtained from present study shows that the level of apelin was significantly increased in all MS group rather than control group. This finding agrees with the results of Yu et al.27 In that study, they investigate patients with diabetes and obesity and reported significantly higher apelin levels in the patients compared to the control group.
Data of current study did not discover any significant correlation with BMI, as was illustrated in other studies also,25,28 this could be due to the causes of fat in the tissue which was not the only source of apelin synthesis and secretion, in addition to the other places such as the vascular endothelium which can visor less release from there.28-30 Furthermore, low level of apelin was elucidating after weight decreased.31
Our data shows that obese women had significantly increased in triglycerides concentration and HDL-C levels, when comparison to control individuals. The same findings are reported by many other authors.32-34 Many studies describe acute effects of apelin on metabolism of lipid. In both processes extraction adipocytes and differentiated 3T3-L1adipocytes, adipokine like apelin is was noticed that to inhibition of isoproterenol (b-adrenergicagonist)-induced lipolysis through a pathway involving Gq,Gi, and AMPK.35 This data was confirmed by (Than A and his co-authors, 2012),36 the researchers also discovered that apelin reducing the liberating of FFA through 3T3-L1 adipocytes via AMPK initiation and by growing the amount of periling nearby the lipid vacuoles, and it was supporting with additional constancy and a lipase resist.36 Certainly (Higuchi and his co-authors, 2007) displayed that regular apelin doses throughout 2 weeks will reduce the triglycerides quantities in adipose soft tissues and the bulk of diverse fat contents in normal mice and in HFD fed mice.15
Conclusion
It was noticed that a significant and remarkable increases in serum apelin level in the all metabolic syndrome classes which confirm additional evidence which approve the role of apelin in playing a potential role in women with metabolic syndrome.
In this study we focused on the effect of metabolic syndrome on the level of apelin in women.
Further studies should be taken a place using more instruments and gathered more data and measurements could be useful to clarify the effect of MS on apelin levels and the mechanisms involved in this interaction.
Acknowledgements
We highly appreciate the cooperation and help of all members of Laboratory Teaching Unit/ Baghdad Teaching Hospital for their technical.
Conflict of Interest
There is no conflict of interest.
References
- Grundy E., et al. The metabolic syndrome. Lancet. 2005;365:1415-1428.
CrossRef - Zimmet P., Alberti K. G., Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782-787.
CrossRef - Alberti K. G. M. M., Eckel R. H., Grundy S. M., et al., Harmonizing the metabolic syndrome a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart Lung and Blood Institute American Heart Association World Heart Federation; International Atherosclerosis Society and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645.
CrossRef - Gami A. S., Witt B. J., Howard D. E., et al., Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am CollCardiol. 2007;49:403-414.
CrossRef - Maury E., Brichard S. M. Adipokinedys regulation adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 2010;314:1-16.
CrossRef - Galic S., Oakhill J. S., Steinberg G. R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129-139.
CrossRef - Tatemoto K., Hosoya M., et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6.
CrossRef - Pitkin S. L., Maguire J. J., Bonner T. I., et al. International union of basic and clinical pharmacology. LXXIV. Apelin receptor nomenclature distribution pharmacology and function. Pharmacol Rev. 2010;62:331–42.
CrossRef - Boucher J., Masri B., Daviaud D., et al. Apelin a newly identified adipokine upregulated by insulin and obesity. Endocrinology. 2005;146:1764–71.
CrossRef - Ishida J., Hashimoto T., Hashimoto Y., et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–9.
CrossRef - Castan-Laurell I., Dray C., Attané C., et al. Apelin diabetes and obesity. Endocrine. 2011;40:1–9.
CrossRef - Kuba K., Zhang L., Imai Y. Impaired heart contractility in apelin gene-deficient mice associated with aging and pressure overload. Circ Res. 2007;101:e32–42.
CrossRef - Yue P., Jin H., Aillaud M., et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E59–E67.
CrossRef - Sorli S. C., den v., Berghe L., Masri B., et al. Therapeutic potential of interfering with apelin signalling. Drug Discov Today. 2006;11:1100–6110.
CrossRef - Higuchi K., Masaki T., Gotoh K., et al. Apelin an APJ receptor ligand regulates body a diposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology. 2007;148:2690-7.
CrossRef - AL-Suhaimi A., Shehzad A., resistin L and visfatin. the missing link between endocrine metabolic disorders and immunity. Euro. J. Med. Res. 2013;18:12.
CrossRef - Castan-Laurell I., Dray C., Attane´ C., et al. Valet Apelin diabetes and obesity Endocrine. 2011;40(1):1–9.
CrossRef - Malik S., Wong N. D., Franklin S. S., et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250.
CrossRef - Garcia-Diaz J. C., Milagro F et al., Adiposity dependent apelin gene expression relationships with oxidative and inflammation markers. Mol. Cell. Biochem. 2007;305:87–94.
CrossRef - Assaad N., El-Aghoury A., El-Sharkawy E., et al. Study of serum apelin and its relation to obesity-associated hypertension. Egypt J. Obes. Diabetes Endocrinol. 2015;1:28–35.
CrossRef - Gomez-Ambrosi J., Salvador J., Silva C., et al. Increased cardiovascular risk markers in obesity are associated with body adiposity: role of leptin, Thromb. Haemost. 2006;95(6):991–996.
CrossRef - Falcao-Pires I and Leite-Moreira A. Apelin A Novel Neurohumoral Modulator of the Cardiovascular System: Pathophysiologic Importance and Potential Use as a Therapeutic Target. Revista Portuguesa de Cardiologia. 2005;24(10):1263-1276.
- The IDF Consensus Worldwide Definition of the Metabolic Syndrome. 2005.
- Rosner B. Fundamentals and biostatistics. 6th, Thomson Brooks/ cole. canada. 2006;1-76.
- Soriguer F., Garrido-Sanchez L., Garcia-Serrano S., et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. Nov. 2009;19(11):1574–1580.
CrossRef - World Health Organization Obesity and Overweight. 2013.
- Shan Y., Zhang Y., Li M. et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J. 2012;125(19):3440-3444.
- Wallace T and Matthews R. The Assessment of Insulin Resistance in Man. Diabetic Medicine. 2002;19( 7):527-534.
CrossRef - Reinehr T., Woelfle J and Roth C. Lack of Association between Apelin Insulin Resistance Cardiovascular Risk Factors and Obesity in Children A Longitudinal Analysis. Metabolism—Clinical and Experimental. 2011;60(9):1349-1354.
CrossRef - Beltowski and A. Kedra. Asymmetric Dimethylarginine (ADMA) as a Target for Pharmacotherapy. Pharmacological Research. 2006;58:159-178.
- Dray C., Knauf C., Daviaud D., et al. Apelin Stimulates Glucose Utilization in Normal and Obese Insulin-Resistant Mice. Cell Metabolism. 2008;8(5):437-445.
CrossRef - Samatha P., Venkateswarlu M., Prabodh V. Lipid profile levels in type 2 diabetes mellitus from the tribal population of Adilabad in Andhra Pradesh. India. J. Clin. Diagn. Res. 2012;6:590–592.
- Idogun S., Unuigbe E., Ogunro P., et al. Assessment of the serum lipids in Nigerians with type 2 diabetes mellitus complications. Pak. J. Med. Sci. (Part 1). 2007;23:708–712.
- Despre´ s. J., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887.
CrossRef - Yue P., Jin H., Xu S., et al. Apelin decreases lipolysis via G(q), G(i) and AMPK-Dependent Mechanisms. Endocrinology. 2011;152(1):59–68.
CrossRef - Than A., Cheng Y., Foh L. C., et al. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell. Endocrinol. 2012;362:227–241.
CrossRef








