Rekha S. R, M. Kulandhaivel and Hridhya K. V.
Department of Microbiology, Karpagam Academy of Higher Education, Coimbatore.
Corresponding Author E-mail: m.kulandhaivel79@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1368
Abstract
The medicinal plants are god’s gift to cure a number of diseases among the living organisms. The aim of the present study was to evaluate the antibacterial property and Minimum Inhibitory Concentration (MIC) of Azadirachta indica, Centella asiatica, Clitoria ternatea against Gram-positive (Staphylococcus aureus) and Gram-negative bacteria (Pseudomonas aeruginosa and Klebsiella species). The ethanolic extract of these plant species was prepared and demonstrated the antibacterial activity against Staphylococcus aureus, Pseudomonas aeruginosa and Klebsiella species. It exhibits broad spectrum of inhibition against the pathogens. The Minimum inhibitory concentration was determined by two fold serial dilution methods. The Concentration of plant sample of about 551.099μg of Azadirachta indica, 681.175μg of Centella asiatica, 651.175μg of Clitoria ternatea showed good activity against Staphylococcus aureus whereas 557.462μg, 623.183μg, 6137.692μg against Pseudomonas aeruginosa and 213.344μg, 611.639μg, 480.241μg against Klebsiella sp respectively. These studies prove that the plant samples exhibit good activity against the pathogen even in low concentration and it is a good antibacterial agent against the bacteria which causes wound infection.
Keywords
Antibacterial; Clitoria Ternatea and Azadirachta IndicaInhibitory Concentration (MIC); Clitoria Ternatea and Azadirachta Indica Klebsiella Species; Medicinal Plants; Minimum Inhibitory Concentration (MIC); Pseudomonas Aeruginosa Centella Asiatica; Pseudomonas Aeruginosa Centella Asiatica; Clitoria Ternatea and Azadirachta Indica Staphylococcus Aureus; Pseudomonas Aeruginosa Centella Asiatica; Clitoria Ternatea and Azadirachta Indica
Download this article as:| Copy the following to cite this article: Rekha S. R, Kulandhaivel M, Hridhya K. V. Antibacterial Efficacy and Minimum Inhibitory Concentrations of Medicinal Plants Against Wound Pathogens. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Rekha S. R, Kulandhaivel M, Hridhya K. V. Antibacterial Efficacy and Minimum Inhibitory Concentrations of Medicinal Plants Against Wound Pathogens. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18972 |
Introduction
Wounds are the result of injuries or disruption of cellular and functional continuity of living tissue. Normal wound healing is a complex process of tissue repairing response which begins with injury. The expanding bacterial resistance to antibiotics has become a growing concern worldwide (Gardam, 2000).1 Various plant materials have been used for the treatment of wounds over the years. Ethano medicine has encouraged many developments in drug discovery. Bio active compounds are normally accumulated as secondary metabolites in all plant cells but their concentration varies from parts to parts. Plants are rich in a wide variety of secondary metabolites which have been found invitro to have antimicrobial properties (Mahady, 2005).2 The use of plant extracts and photochemical both with known antimicrobial properties can be a great significance in therapeutic treatments. The medicinal plants are god’s gift to cure a number of diseases among the living organisms.3 Leaf is one of the highest accumulated plant parts of such compounds and it is preferred for therapeutic purposes. Some of the active compounds inhibit the growth of disease causing microorganisms. Authentication of the use of plants for medicinal purposes dates some 60,000 years back in both Western and Eastern culture.4 The uses of plants and plant derivatives for treating various diseases have been a common practice since years. At present about 65% of Indians are dependent on the traditional system of medicine.5 Herbal formulations are used due to their lesser side effect and low cost. For instance, the plants are generally readily available and their products are biodegradable. Antimicrobial drug resistance is a global problem today as the resistant microorganisms have emerged and spread throughout the world because of their genetic plasticity.6,7
Literature survey revealed that Centella asiatica, Clitoria ternatea and Azadirachta indica has the antimicrobial, anti oxidant activity and wound healing. In this study, by preparing the organic solvent extracts of Clitoria ternatea, Centella asiatica and Azadirachta indica to check potential activity against the Staphylococcus aureus, Klebsiella species and Pseudomonas aeruginosa. Staphylococcus aureus is a gram positive, non motile and does not form spores8 which cause wound infection, food poisoning9 pyogenic infections. Klebsiella species are gram negative, non motile, oxidase negative, rod shaped bacteria with a polysaccharide based capsule10 which cause pneumonia, blood stream infection, urinary tract infection, diarrhea, wound or surgical site infection and meningitis.11,12 Pseudomonas aeruginosa is a common bacterium which causes soft tissue infection, Urinary Tract infection and pyogenic infections. Pseudomonas growth with on the human body can be asymptomatic until the bacteria from a biofilm, which overwhelms the immune system. These biofilms are found in the lungs of cystic fibrosis and primary ciliary dyskinesia and can prove fatal.13-18 The demonstration of the antibacterial activity against both Gram negative and Gram positive bacteria are an indication that the plants are potential source for production of drugs with broad spectrum of activity. The study supports the use of this herbal preparation by not only as dietary supplement but also as agents to prevent or control bacterial infections. The main aim of the present study is to evaluate the antibacterial efficacy and minimum inhibitory concentration of the plant extracts against the pathogens such as Staphylococcus aureus, Klebsiella species and Pseudomonas aeruginosa which cause wound infection.
Materials and Methods
Collection of Plant Material
The three medicinal plants Clitoria ternatea, Centella asiatica and Azadirachta indica used in this study were collected from Trivandrum, India. These three medicinal plants were used as a traditional medicine for various microbial infections and disorders. The plant samples were authenticated by Botanical Survey of India, Tamilnadu Agriculture University, Coimbatore, India. The authentication number of Clitoria ternatea, Centella asiatica and Azadirachta indica were BSI/ SRC/ 5/ 23/ 2016/ Tech/ 112, BSI/ SRC/ 5/ 23/ 2016/ Tech/ 113, BSI/ SRC/ 5/ 23/ 2016/ Tech/ 115 respectively. All the collected plant leaves were washed with water to remove the soil and dust particles. Then they were shade dried and were grind. After grinding, the collected grind plant tissue was passed through a 0.125 mesh sieve to remove debris, and stored in a sterile air tight container.
Organoleptic evaluation of Plant Material
The plant parts were organoleptically evaluated for various sensory parameters like the colour, appearance of the plant parts mainly size and shape, external texture, fracture (granular, splintery, smooth) and external marking (furrow, wrinkles, ridges, annular, out growth) of the plant part were examined. The fragrance, test for odour (aromatic, balsamic, camphoraceous, spicy, pleasant, irritating) and taste (sweet, bitter, sour, astringent, pungent, acidic, alkaline) were evaluated.19,20
Preparation of Extracts
The plant leaves were used for the preparation of extracts and they were washed thoroughly with distilled water to remove extraneous matter and dried under shade. The dried leaves were powdered using a mortar and pestle. 20 grams of powdered leaves were extracted successively with 100ml of ethanol. The extracts were evaporated drily and the resulting pasty form extracts were stored in a sterile plastic container.
Screening for Phytochemical Constituents
The dried plant extracts were screened for the presence of phytoconstituents like alkaloids, saponins, treponoids, glycosides, flavonoids, sterols and steroids, tannins and phenolic compounds, carbohydrates.21
Antimicrobial Assays
Determination of Antibacterial Activity
Selection of Bacterial Strains
The antibacterial activities of the medicinal plant extracts were determined against three predominant bacterial strains such as one from gram positive and other two from negative strains. These strains were screened through pus samples from the patients with wound infection.
Isolation and Identification of Bacteria
Totally 150 pus samples were collected from wound infected patients., Among that three strains (Two gram negative and one gram positive pathogen) were isolated and identified based on Gram staining, biochemical characterization and colony morphology. These isolates were confirmed on selective medium. Then the bacterial culture were maintained in nutrient agar slant and used for further studies.
Preparation of Inoculums
Nutrient broth medium was prepared by adding the nutrient broth in the distilled water and sterilized with the help of an autoclave at a pressure of 151Lbs and temperature of 120°C for 15 minutes. The bacterial strain was inoculated in the nutrient broth medium and the cultured was maintained at 28°C ± 2°C. The culture was inoculated over the Muller Hinton Agar (MHA) plates. The determination of antimicrobial susceptibility was confirmed through medicinal plant extracts.
Antibacterial Activity by Disc Diffusion Method
Antimicrobial susceptibility was carried out by disc and well diffusion technique. First the Muller Hinton Agar (MHA) plate was prepared and after that the solidification of the culture was swabbed on the plates, and then commercially available antibiotic discs were introduced on the upper layer of the seeded agar plate. The sterile discs impregnated with methanol were used as control test discs they were aseptically applied to the surface of the agar medium. Then plates were incubated for 24 hours 37°C after the incubation period the zone of inhibition was measured. Gentamicin (30mcg), Netilmicin (30mcg), Cefazolin (30mcg), Cephalexin (30mcg), Cefuroxime (30mcg), Cefexime (5mcg), Cefotaxime (30mcg), Vancomycin (5mcg), Cloxacillin (5mcg), Penicillin- G (1 unit), Cotrimoxazole (25mcg), Ampicillin (10mcg), Ticarcillin (75mcg) and Piperacillin (100mcg) were the commercially available antibiotics were used for this study.
Assay of Antibacterial Activity by Agar Well Diffusion Method
The agar well diffusion method expresses the results as the width of the inhibition zone produced by the plant extract.22 The plant extracts were tested against bacterial strains. For agar well diffusion method, 5mm size well was prepared in the in cultural strain swabbed plates with the help of a well cutter. Then, 5µl of plant extract (ethanolic plant extract) was added in the well by using micro pipette. Methanol added well considered as a control. Then plates were incubated at 37°C 24 hours. After the incubation period the zone of inhibition was measured. A well was prepared in the plates with the help of a cork-borer (5mm).23
Minimal Inhibitory Concentration (MIC) Determination
The minimum inhibitory concentration (MIC) is the lowest concentration of a chemical which prevents visible growth of a bacterium. This is in difference to the minimum bactericidal concentration (MBC) which is the concentration resulting in microbial death as defined by the inability to re-culture bacteria. The closer the MIC is to the MBC, the more bactericidal the compound.24 Minimal inhibitory concentration (MIC) was determined by two fold serial dilution methods.25 Klebsiella, Staphylococcus and Pseudomonas were used as the indicator organisms. Samples were dissolved in DMSO (Dimethyl sulfoxide), which was a widely used commercial solvent derived from trees as a byproduct from the production of paper,26 to a final concentration of 10mg/ml and added in increasing concentration such as 31.25, 62.5, 125, 250, 500, 1000 µg respectively and incubated overnight at 37°C. Growth was observed by visual inspection and by measuring the optical density (OD) at 630 nm using a spectrophotometer. The OD was measured immediately after the visual reading. The growth inhibition for the test wells at each plant extract dilution was determined by the formula:
Percentage of inhibition = (OD of control – OD of test)/ (OD of control) × 100%
The minimum and maximum values were 0% and 100%, respectively.
Results and Discussion
The collected medicinal plants were subjected to the organoleptic evaluation which was tabulated in Table 1. The phytochemical constituents of the plant materials play a major role in antibacterial activity. It was identified by various phytochemical reactions and screen the presence of phytoconstituents like alkaloids, saponins, treponoids, glycosides, flavonoids, sterols and steroids, tannins and phenolic compounds, carbohydrates (Table 2).
Table 1: Organoleptic Evaluation of Medicinal Plants
| Parameteres | Azadirachta indica | Clitorea terennatea | Centella asiatica |
| Coluor | Green | Green | Green |
| Odour | Pungent | Raw | Raw |
| Appearance | Dark green when powdered | Dark green when powdered | Dark green when powdered |
| Taste | Bitter | Pleasant | Pleasant |
Table 2: Phytochemical screening of Ethanolic extract of Azadirachta indica, Centella asiatica and Clitorea terennatea
| Phytochemical
constituents |
Ethanolic extract of Medicinal plants | ||
| Azadirachta indica | Centella asiatica | Clitorea terennatea | |
| Alkaloids | + | + | + |
| Saponins | + | + | – |
| Terpenoids | + | + | + |
| Glycosides | + | + | + |
| Flavanoids | + | – | + |
| Steroids and sterols | + | + | + |
| Tannins and phenols | + | + | – |
Out of 150 samples, 131 positive cultures were isolated and identified from wound and pus samples. Among 131 positive cultures, the predominant pathogen were gram positive organisms (62.3%) followed by gram negative organism (38.15%). The isolates were subjected to biochemical characterization and carbohydrate fermentation test for the identification of the pathogens (Table 3, 4). The predominant pathogen isolated from the clinical samples was identified as Staphylococcus aureus (58.77%), Pseudomonas aeruginosa (11.45%), Klebsiella (19.08%) followed by Proteus (3.81%), E.coli (3.81%) and Streptococcus pyogenes (3.53%) (Table 5, Fig.1). From these pathogens the most frequent pathogens such as Staphylococcus aureus (58.77%), Pseudomonas aeruginosa (11.45%), Klebsiella (19.08%) were selected for the further studies.
Table 3: Biochemical Characterization of isolated pathogens
| Biochemical Reaction | S. aureus | Klebsiella | P. aeruginosa | Proteus | E.coli | S. pyogenes |
| Indole | – | – | – | – | + | – |
| Methyl Red | + | – | + | + | + | – |
| Voges Proskauer | + | + | + | – | – | – |
| Citrate Utilization | – | + | – | – | – | – |
| Urease | + | + | – | + | – | – |
| Nitrate reduction | + | – | + | + | – | – |
| Hydrogen sulfide Production | – | – | – | + | – | – |
| Oxidase | – | – | + | – | – | – |
| Catalase | + | + | + | + | + | – |
| Coagulase | + | – | – | – | – | – |
| Motility | – | – | + | + | + | – |
Table 4: Triple Sugar Iron and Carbohydrate Fermentation Test of Isolated Pathogens
| Test bacteria | TSI reaction | Carbohydrate fermentation | |||||
| Slant | Butt | Gas | H2S | Glucose | Sucrose | Lactose | |
| S. aureus | Acid | Acid | + | – | + | + | + |
| P. aeruginosa | Alkaline | Alkaline | – | – | + | – | – |
| Klebsiella Sp | Acid | Acid | + | – | – | ± | + |
| Proteus | Alkaline | Acid | + | + | + | + | + |
| E.coli | Acid | Acid | + | – | + | ± | + |
| Streptococcus | Alkaline | Acid | – | – | + | + | + |
Table 5: Organisms isolated from Wound and Pus samples
| SL.No | Isolated Oraganism | Percentage of occurence |
| 1 | Staphylococus aureus | 58.77% |
| 2 | Klebsiella species | 19.08% |
| 3 | Pseudomonas aeruginosa | 11.45% |
| 4 | Proteus species | 3.81% |
| 5 | Escherichia coli | 3.81% |
| 6 | Streptococcus pyogenes | 3.53% |
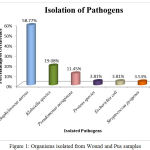 |
Figure 1: Organisms isolated from Wound and Pus samples
|
The Antibiotic susceptibility pattern of screened pathogens was determined using commercial antibiotics by disc diffusion method. S. aureus, the predominant pathogen was sensitive to Gentamicin, Netilmicin, Cefazolin, Cephalexin, Cefuroxime, Vancomycin and Cloxacillin. In other hand gram negative organisms, the Klebsiella sp show resistance to the antibiotics such as Gentamicin, Netilmicin, Cefazolin, Cephalexin, Cefuroxime, Cefexime, Cefotaxime, Penicillin-G, Cotrimoxazole and Ampicillin. In case of Pseudomonas aeruginosa, it shows sensitivity to Ticarcillin, Piperacillin and resistance towards all other antibiotics (Table 6). The antimicrobial activity of the selected plant was determined by agar well diffusion method and all the three medicinal plant showed good activity against the pathogens. Clitoria ternatea, Centella asiatica and Azadirachta indica showed good activity against the gram positive organism Staphylococcus aureus followed by gram negative organisms Klebsiella sp and Pseudomonas aerginosa (Table 7, Fig.2, Fig.3, Fig.4). The mimimum inhibitory concentration is the lowest concentration of an antimicrobial agents like plant extracts or bacteriostatic or bactericidal or an antifungal agent that inhibit the growth of microbial pathogen after overnight incubation. The MIIC of the medicinal plants Clitoria ternatea, Centella asiatica and Azadirachta indica inhibits the growth of bacterial pathogens in the lowest concentration of about 213.344μg to 681.175μg (Table 8).
Table 6: Antibiotic Susceptibility Pattern of Pyogenic Bacterial Isolates
| Antibiotics | Staphylococcus | Klebsiella | Pseudomonas | |||
| %of S | %of R | %of S | %of R | %of S | %of R | |
| Gentamicin | 56.32 | 43.6 | 37.93 | 62.06 | 6.66 | 93.33 |
| Netilmicin | 50.57 | 49.42 | 41.37 | 58.62 | 33.33 | 66.66 |
| Cefazolin | 66.66 | 33.33 | 27.58 | 72.41 | 13.33 | 86.66 |
| Cephalexin | 52.87 | 47.12 | 10.34 | 89.65 | 6.66 | 93.33 |
| Cefuroxime | 62.06 | 37.93 | 34.49 | 65.51 | 20.00 | 80.00 |
| Cefexime | ND | ND | 27.58 | 72.41 | 13.33 | 86.66 |
| Cefotaxime | 43.67 | 56.32 | 24.13 | 75.86 | 26.66 | 73.33 |
| Vancomycin | 94.25 | 5.74 | ND | ND | ND | ND |
| Cloxacillin | 70.11 | 29.88 | ND | ND | ND | ND |
| Penicillin- G | 2.29 | 97.70 | 3.44 | 96.55 | 0 | 100 |
| Cotrimoxazole | 36.78 | 63.21 | 17.24 | 82.75 | 13.33 | 86.66 |
| Ampicillin | 1.14 | 98.85 | 3.44 | 96.55 | 0 | 100 |
| Ticarcillin | ND | ND | ND | ND | 60.00 | 40.00 |
| Piperacillin | ND | ND | ND | ND | 53.33 | 46.66 |
R: Resistant, S: Sensitive, %: Percentage, ND: Not Done
Table 7: Antimicrobial activity of the Ethanolic extract of A. indica, C. asiatica and C. terennateaia against the pyogenic organisms
| Ethanol Extract | Concentration (mg/mL) | Zone of inhibition (mm) | ||
| Staphylococcus aureus | Pseudomonas aeruginosa | Klebsiella spp | ||
| Azadirachta indica | 10 | 26 | 19 | 22 |
| 5 | 16 | 18 | 17 | |
| 2.5 | 11 | 14 | 15 | |
| 1.25 | 8 | 10 | 11 | |
| Centella asiatica | 10 | 22 | 18 | 20 |
| 5 | 16 | 17 | 21 | |
| 2.5 | 14 | 14 | 19 | |
| 1.25 | 10 | 8 | 9 | |
| Clitoria ternateaia | 10 | 24 | 17 | 21 |
| 5 | 18 | 16 | 19 | |
| 2.5 | 12 | 12 | 16 | |
| 1.25 | 6 | 10 | 8 | |
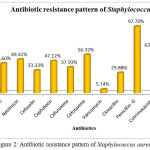 |
Figure 2: Antibiotic resistance pattern of Staphylococcus aureus
|
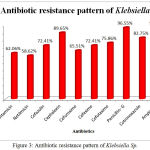 |
Figure 3: Antibiotic resistance pattern of Klebsiella Sp.
|
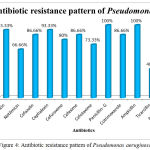 |
Figure 4: Antibiotic resistance pattern of Pseudomonas aeruginosa
|
Table 8: Minimum Inhibitory Concentration (MIC) of Azadirachta indica, Centella asiatica and Clitoria ternateaia against the pyogenic organisms
| Plants | Minimum Inhibitory Concentration | ||
| Staphylococcus aureus | Pseudomonas aeruginosa | Klebsiella spp | |
| Azadirachta indica | 551.099μg | 557.462μg | 213.344μg |
| Centella asiatica | 681.175μg | 623.183μg | 611.639μg |
| Clitoria ternateaia | 651.175μg | 637.692μg | 480.241μg |
Antibiotic resistance has become a global concern. There has been an increasing incidence of multiple resistances in human pathogenic microorganisms in recent years, largely due to indiscriminate use of commercial antimicrobial drugs commonly employed in the treatment of infectious diseases. Such increase has been attributed to indiscriminate the use of broad spectrum antibiotics, immune suppressive agents and ongoing epidermis of Human Immuno deficiency virus infections. Antimicrobial resistance leads to bmillions of death every year.27 This has forced scientists to search for new antimicrobial substances from various sources like the medicinal plants.28 The selection of plant material for this study was based on ethno botanical data on the traditional use of the plants in treatment of bacterial diseases. Table 1 shows the antibacterial activity of medicinal plants against Gram-positive and Gram-negative bacteria.
Conclusion
In the present study, plants like Centella asiatica, Azadirachta indica, Clitoria ternatea were selected that could accelerate the wound healing. The results showed that ethanolic extracts inhibited the growth of Pseudomonas, Klebsiella to a great extent followed by Staphylococcus aureus. The selection of plant material for this study was based on ethno botanical data on the traditional use of the plants in treatment of bacterial diseases. The antibacterial effect may be attributed to the phytoconstituents present in the herbal extracts and further studies will be focused on formulations against the microbes using these extracts. This may lead to find the effective role of medicinal plant as alternative medicine.
References
- Gardam M.A. Is methicillin-resistant Staphylococcus aureus an emerging community pathogen? A review of the literature. J. Infect. Dis. 2000;11:202-211.
- Mahady G.B. Medicinal plants for the prevention and treatment of bacterial infections. Pharm. Des. 2005;11:2405-2427.
CrossRef - Bushara Beegum N.R, Devi G.T. Antibacterial activity of selected seaweeds from Kovalam south west coast of India. Asian J Microbiol Biotechnol Environ Exp Sci. 2003; 319-22.
- Gossell-Williams M,Simon O.R,West M.E. The past and present use of plants for medicines, West India Med J. 2006;55:217-8.
CrossRef - Bhatt D.C, Mitaliya K.D, Patel N.K, Ant H.M. Herbal remedies for renal calculi. Adv Plant Sci. 2002;15(1):1-3.
- Kunin C.M. Resistance to antimicrobial drugs- a aworld wide calamity. Ann Int Med 1993;118:557-561.
CrossRef - Abdullah W.B, Anowa H, Doli C, Amina T.S, Kamrun N, Korshed A,et al. Bacteremic typhoid fever in children in an Urban slum, Bangladesh. Emerg .Infect. Dis. 2005;11(2): 326-329.
CrossRef - “Pathogen Safety Data Sheet – Infectious Substances.” Staphylococcus cells have a diameter of 0.7-1.2 um. Staphylococcus Aureus. Public Health Agency of Canada. 2011. Web
- “Staphylococcal Food Poisoning”. cdc.gov. hhs.gov. 4 October 2016. Retrieved 23.
- Ryan K. J. Sherris Medical Microbiology, 4th ed, Ray CG (editors), McGraw Hill. 2004; 370. ISBN0-8385-8529-9.
- Ristuccia, Patricia A,Burke C .A. “Klebsiella”. Topics in Clinical Microbiology. 1984;5(7): 343–348. JSTOR 30144997.
- Podschun R, Ullmann U. Klebsiella as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603.
CrossRef - Funke G.C . Microbiology: An Introduction (12th ed.). Pearson Education. 2016;54. ISBN978-0-321-92915-0.
- Hassett D.J. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. Journal of Bacteriology. 1996;178(24):7322–5.
CrossRef - Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer K.C, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas J.R, Randell S, Boucher R.C, Döring G, “Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients”. The Journal of Clinical Investigation. 2002;109(3):317–25.
CrossRef - Cooper M, Tavankar G.R, Williams H.D.Regulation of expression of the cyanide-insensitive terminal oxidase in Pseudomonas aeruginosa. Microbiology. 2003;149(Pt 5): 1275–84.
CrossRef - Williams H.D, Zlosnik J.E, Ryall B. “Oxygen, cyanide and energy generation in the cystic fibrosis pathogen Pseudomonas aeruginosa”, Advances in Microbial Physiology. 2007; 52:1–71.
- Richard L, Kevin M,Derek B. Oxford Desk Reference: Acute Medicine. Oxford University Press. 2016;244. ISBN 9780191007149.
- Wang R.W, Zhou H.Y ,Hand W,Chow F. Effects of green tea extract on the quality of bread by unfrozen and frozen dough process. J. Science of food and agriculture. 2006;86: 857-864.
CrossRef - Wang R.W,Zhou H, Yu H and Chow W. F. Kinetic study of the thermal stability of tea catechin in aqueous system using a microwave reactor. J. Agri. Food. Chem. 2006;86: 857-864.
- Harborne S.B and Baxter H. Phytochemical dictionary. A Handbook of Bioactive Compounds from Plants. Taylor and Francis, London. 1995;279.
- Eloff J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? Ethanopharmacol. 1998;60:1-8.
CrossRef - Perez C, Pauli M, Bazerque P, An antibiotic assay by agar well-diffusion method. Acta Biologiae et Medecine Experimentaalis. 1990;15:113-115.
- Tripathi K.D. Essentials of Medical Pharmacology(7th ed.), New Delhi, India: Jaypee Brothers Medical Publishers. 2013;696-697.
- Fabry W, Okemo P.O, Ansorg R. Antibacterial activity of East African medicinal plants. J Ethnopharmacol. 1998;60:79–84.
CrossRef - Gregg D.W, (1998). DMSO and MSM – The Biochemical Oxygen Transport Pair – A new insight into their biochemistry that explains the claims for their wide-ranging health and energy benefits. Obtained from the internet: http://www.krysalis.net/dmso.html
- WHO (April 2014). “Antimicrobial resistance: global report on surveillance 2014″. WHO. WHO. Retrieved May 9,2015.
- Karaman I, Sahin F, Gulluce M, Ogutcu H, Sengul M, Adiguzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J. Ethnopharmacol. 2003; 85:231–235.
CrossRef







