Eyad Mallah1, Walid Abu Rayyan1, Wael Abu Dayyih1, Ibrahim S. Al-Majali3, Haitham Qaralleh4, Osama Yosef Al- Thunibat5, Nisreen Seder2, Mona Bustami1, Luay Abu Qatoosah1 and Tawfiq Arafat1
1University of Petra, Faculty of Pharmacy and Medical sciences, Amman, Jordan.
2School of Biomedical Science, Faculty of Health Sciences, University Sultan Zainal Abidin, 21300 Kuala Nerus, Terengganu, Malaysia.
3University of Mutah, College of Applied Medical Sciences, Karak, Jordan.
4Department of Medical Support, Al-karak University college, Al- Balqa' Applied University, Jordan.
5Department of Laboratory Medical Sciences, Princess Aisha Bent Al-Hussein Faculty of Nursing and Health Sciences, Al Hussein bin Talal University, Jordan.
Corresponding Author E-mail: eyad782002@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1372
Abstract
World health organization reported cardiovascular disease as the first cause of mortality for five consecutive years of 2010-2014. Smoking is an exogenous source of metals contamination in the human body and attributed to 12% of all deaths among adults aged over 30 years. This is the first study carried out to elucidate the contribution of the personal habits as smoking and the physiological process as aging on lead homeostasis and deposition in different storage sites of the human body especially on the cardiovascular system in Jordanian cadavers. 120 biological samples (coronary artery, ribs, and blood) were obtained from 40 Jordanian cadavers passed away in car accidents aged between 8 and 97. Samples were analyzed for lead levels using graphite atomic absorption. Mean lead concentrations for coronary artery (C-Pb), ribs (R-Pb) and blood (B-Pb) were 3.01 µg/g, 2.71 µg/g and 5.96 µg/dl, respectively. A significant correlation was demonstrated between both B-Pb and C-Pb levels (r= 0.273 P<0.05). A significant increment was demonstrated in lead levels in grade I and II of the atherosclerotic coronary arteries, whereas, there was no significance in the increase of lead levels in grades III, VI, and V. Duncan's test showed a significant correlation in levels of B-Pb and C-Pb with smoking and age. Eventually, lead intoxication emits an initiation effect on the endpoint cardiovascular disorders even though the blood lead levels were within recommended exposure range but still these levels are conveying deleterious side effects on the cardiovascular system.
Keywords
Atomic Absorption Cadavers;Coronary; Jordan; Ribs; Lead;
Download this article as:| Copy the following to cite this article: Mallah E, Rayyan W. A, Dayyih W. A, Al-Majali I. S, Qaralleh H, Al- Thunibat O. Y, Seder N, Bustami M, Qatoosah L. A, Arafat T. Analytical and Comparative Study About the Impact of Lead Homeostasis on Cardiovascular Disorders in Humans. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Mallah E, Rayyan W. A, Dayyih W. A, Al-Majali I. S, Qaralleh H, Al- Thunibat O. Y, Seder N, Bustami M, Qatoosah L. A, Arafat T. Analytical and Comparative Study About the Impact of Lead Homeostasis on Cardiovascular Disorders in Humans. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19492 |
Introduction
In the modern world, cardiovascular disorders (CVD) are ultimately the leading cause of mortality for five consecutive years 2010-2014. World Health Organization (WHO) in 2012 has reported the death of 17.5 million people as consequence of cardiovascular complications. 1
Overall, 12% of cardiovascular disease (CVD) deaths in adults aged over 30 years are in compliance with smoking.2 The progressive increase in numbers of cigarette smokers throughout teenagers and adults is contributed exponentially with the rate of mortality.3 Smoking is an exogenous source of heavy metals contamination as lead and cadmium. Heavy metals promote emerging of reactive oxygen species which trigger a cycle of oxidative stress and inflammation process in the biological systems. 4,5 Additionally, the abundance of heavy metals in the peripheral blood causes depletion of redox scavengers which renders the anti-cancer activity in the human body. Eventually, not only the direct smoking has a potential effect on humans health also passive smoking or exhaled smoke contains Pb-210 osculating mortality rate up to 30% for the second smokers.6,7 Lead is known toxic metal with deleterious side effects on different biological systems; predominantly found in gasoline, paints, canned food, and tobacco. In Pb intoxication; nonradioactive Pb-206 and radioactive Pb-210 induce a reduction in glomerular filtration rate leading to incidence of endpoint CVDs as hypertension, strokes, and peripheral arterial diseases.8,6 Chronic exposure to lead is reported to induce osteoporosis, renal failure and events such as lung and renal cancer in humans .9;10;11,12; The combustion of leaded gasoline was estimated to be the largest environmental pollutant in the modern world.13;14 Nowadays, the major risk seems to be batteries leakage, water pipes and lacking hazardous knowledge in small enterprises and in some large industries where adequate industrial hygiene programs do not exist or difficult to be implemented.15,16;17;18 Although all of the previous sources of Pb contamination are preventable it is still a major environmental hazard in highly exposed communities.8 Even after 100 years of reporting a link between lead and cardiovascular disorders, the contribution of lead is not precisely understood in the pathogenesis of cardiovascular disorders 19,20 In this study, we have addressed the question of “Is the role of lead focused on the initiation or development process of cardiovascular disorders in human beings”. Therefore, in order to give an answer to the proposed question, we decided to analyze lead levels in the biological samples of the coronary artery, ribs, and blood from Jordanian cadavers passed recently in car accidents and to study the association between lead levels in different storage sites, pro-atherogenic and atherogenesis process. Several factors were taken into consideration as age, sex, smoking and atherosclerotic grad. A questioner has carried out to collect the personal data and medical history. Data were analyzed with SPSS software to elucidate the correlation of lead deposition in different tissues during life development. While reviewing the literature for the levels of lead in the coronary artery in of Jordanian citizens; we could not find any study implemented in elucidation the levels of lead in the coronary artery of Jordanian citizens and neither a decisive answer about the exact role of lead in the atheroma pathogenesis.
Material and Methods
Study Population
The study was conducted on a group of 40 cadavers were recently passed away in car accidents, 22 males and 18 females aged between 8 and 97 years old. The cadavers were divided into five subgroups according to age and atherosclerotic grade. In addition, we adjusted the results for smoking habit as smokers and nonsmokers. During postmortem autopsy at forensic department of Jordan university hospital, three samples were obtained; coronary artery, ribs, and blood from each cadaver.
The study has been approved by the Faculty of Pharmacy and Medical Sciences at the University of Petra and the Faculty of Forensic Medicine and Toxicology at the University of Jordan and was performed in compliance the Helsinki Declaration following the approval of an independent ethics committee at the Hospital of University of Jordan in Amman, Jordan. This study is conducted on the behalf of toxicological investigations for cadavers with unknown medical history or passed away in a car accident. The permission to use the biological samples in forensic investigations was signed by cadaver’s first degree relatives.
Sample Collection
Samples were collected by pathologists at the Hospital of the University of Jordan following a standard protocol. After obtaining the ribs and coronary artery from the thoracic cavity, the contaminating blood was washed away with ultrapure water. Tissue fragments (approximately 1 cm3) were collected from the fourth ribs and the left coronary artery. Ribs and coronary samples were normalized against weight in gram before the analysis. Blood samples were obtained from cadavers in 10 ml plain polypropylene tubes and labeled. Samples were stored in sterile polypropylene tubes (Sestet, Germany) at -20 ºC until analysis.
Sample Pre-Treatment Blood
Sample digestion refers to the destruction of the entire matrix by complete oxidation of the organic compound in the sample.21 Sample preparation was carried out by adding 1 ml of blood into 50 ml glass beaker. 10 ml of concentrated nitric acid was added to each beaker, each beaker was covered with watch glass and heated to approximately 120˚C on hot plate under fume hood until most of the liquid evaporated, 5 ml of nitric acid were also added to each beaker and digestion continued until the digests were clear and 1-2 ml of acid remain. After cooling for 1 hour, the residue was transformed into 5 ml falcon polyethylene tube and brought to a final volume of 5 ml by adding deionized water.
Sample Pre-Treatment Coronary Artery and Ribs
40 coronary artery and ribs digestion was carried out by adding approximately 1.0 g of ribs and coronary artery individually into 50 ml glass beaker, prepared in the same manner as mentioned in blood digestion,
Lead Determination
Coronary artery, ribs, and blood digested samples were analyzed using graphite atomic absorption spectroscopy (Shimadzu 6800) (Departments of Forensic Toxicology, University of Jordan Hospital, and Amman, Jordan).
All samples were analyzed in triplicates; the mean level was adopted for results.
The sample digests were diluted (100-fold) with 0.2% (v/v) HNO3 solution before injection into the graphite tube. Sample solutions were measured in triplicate. For results with RSD≥10%, an additional two injections were performed.
Calibration standards were also prepared by diluting a commercial multi-element standard solution
(PerkinElmer Pure; Part No. N9300244) with 0.2% (v/v) HNO3 solution.
Analytical Quality Control
We had adequate accuracy and precession during the study time (correlation= 0.98%; mean difference= 0.37 µg/dl; standard deviation = 0.0058).
Statistical Analysis
The data was expressed as Mean ± SD and analyzed using the SPSS computer software (Statistical Package for the Social Sciences, version 19.0, SPSS Inc., Chicago, IL, USA). Statistical analysis was done using (ANOVA) analysis, independent t-test analysis and Duncan’s test for studying the correlation of lead concentration in the different biological samples (coronary artery, ribs, and blood). Correlation between lead levels in coronary, ribs, blood and age was determined by Pearson’s correlation. P<0.05 was considered significant.
Results
Levels of Lead in Coronary, Ribs and Blood
Mean blood lead level of cadavers was 5.96 µg/dl and a median of 4.9 µg/dl. Coronary lead levels were ranged from (1.25 to 8.6) µg/g with a mean of 3.01 µg/g and a median of 2.76 µg/g. Ribs lead levels were ranged from (0.98 to 5.85) µg/g with a mean of 2.71 µg/g and a median of 2.5 µg/g (Table 1).
The cadaver’s population was divided into five subgroups according to their ages and atherosclerotic grades. There was age interval of 20 years starting with 1 year and ending with 100 years. Mean lead concentrations of the coronary artery, ribs, and blood for different age groups was illustrated in (Table 1, Figure 1,2).
Table 1: Mean lead concentration in coronary, ribs, and blood samples.
| N | Mean Coronary Lead ±SD (µg/g) | Mean Ribs Lead ±SD (µg/g) | Mean Blood
Lead ± SD (µg/dL) |
||
| Sex | Male | 22 | 3.18 ± 1.57 | 5.79 ± 4.34 | 2.90 ± 1.65 |
| Female | 18 | 2.31 ± 1.24 | 6.1 ± 4.13 | 2.47 ± 1.07 | |
| P- value | < 0.05 | < 0.05 | < 0.05 | ||
| Age | 0-20 | 5 | 2.11 ± 0.78 a | 2.06 ± 0.827 a | 1.63 ± 0. 013 |
| 20-40 | 11 | 2.44 ± 0.96 a | 3.38 ± 1.014 a | 1.77 ± 0.017 | |
| 40-60 | 14 | 2.94 ± 0.82 b | 5.99 ±0.821 b | 2.8 ± 0.027 | |
| 60-80 | 8 | 4.19 ± 1.021 b | 9.51 ±1.153 b | 3.31 ± 0.021 | |
| 80-100 | 2 | 4.58 ± 1.025 b | 15.45 ±1.396 c | 3.48 ± 0.025 | |
| P- value | > 0.05 | > 0.05 | > 0.05 | ||
| Smoking | Smokers | 21 | 3.74 ± 1.57 | 5.21 ± 4.41 | 1.36 ± 0.53 |
| Nonsmokers | 19 | 2.25 ± 1.04 | 4.57 ± 3.56 | 2.79 ± 0.41 | |
| P- value | > 0.05 | < 0.05 | > 0.05 | ||
| Grade | I | 5 | 2.74 ± 0.78 a | 1.46 ± 0.78 | 8.44 ± 0.78 |
| II | 9 | 4.84 ± 0.96 b | 1.65 ± 0.96 | 4.57 ± 0.96 | |
| III | 6 | 5.07 ± 1.72 b | 2.11 ± 1.72 | 7.22 ± 1.72 | |
| VI | 13 | 5.35 ± 1.021 b | 2.49 ± 1.021 | 9.89 ± 1.021 | |
| V | 7 | 6.12 ± 1.025 b | 2.86 ± 1.025 | 4.38 ± 1.025 | |
| Total | 40 | 3.01 ± 2.05 | 5.96 ± 4.90 | 2.71 ± 1.41 | |
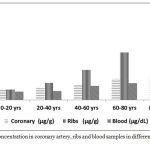 |
Figure 1: Lead concentration in coronary artery, ribs and blood samples in different age groups.
|
There was a significant exponential increase in coronary ribs and blood lead levels in different age groups.
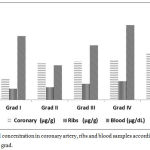 |
Figure 2: Lead concentration in coronary artery, ribs and blood samples according to atherosclerotic grad.
|
There was a significant increase in coronary lead concentration in grades I and II, whereas, the increase in grades III, IV and V was not significant.
Discussion
Lead levels according to atherosclerotic grad
Until the beginning of the 21 century, lead combustion from car emissions and the usage of leaded gasoline had the highest impact on lead contamination in the human body.22;23 In our study, we demonstrated lower levels of lead in all biological samples (coronary artery, ribs, and blood) in the younger age groups when compared with older age groups. These findings could be a result of adopting the usage of unleaded gasoline in petrol stations of Jordan.24 In addition, the short period of exposure to pollutants in the younger age groups has minimized the amount of lead in the biological tissues.25 The levels of lead in the coronary artery of our study were consistent with lead levels of aorta 2.95 µg/g reported by Alves et al (2014) in Portugal. Barry (1975) showed levels of 2.56 µg/g for the atherosclerotic aorta and 1.82 µg/g for non-atherosclerotic aorta for the British population.26 This indicates normative levels of lead in the cardiovascular system of the Jordanian population when compared with European countries. Furthermore, an oscillation was found in the lead concentration during the pathogenesis of an atherosclerotic artery, for instance, there was a significant increase in lead levels in the coronary artery between grade I and II , whereas, the increase in grades II, VI, and V was not significant. These results point to the prognostic role of metals (especially lead) in the initiation of the atherosclerotic process and not literally in the development of the disease. While reviewing literature, Navas et al., 2007 showed that higher concentrations of lead deposited in the veins and arteries of the cardiovascular system may lead to a tendency for atherosclerosis.27 Moller and Kristensen (1992) showed a positive but not statistically significant association between lead levels and the incidence of strokes.28
In addition, we found a weak significant association between levels of B-Pb and C-Pb (r= 0.297, p<0.05) in the study population. Cardiovascular system and other components of soft tissues are in rapid exchange with blood lead contents. In average, 90 to 99% of the body lead are associated with red blood cells, whereas, the other free fraction correlates closely with the recent environmental exposure.29 The free accessible fractions have tremendously toxic effects on the different biological systems as it induces an acute intoxication on the level of organs. 30 Lead in the accessible portion has a mean half-life of about 30 days, whereas, it has a half-life of 50 days in the soft tissues.31 This implicates a deleterious effect on the increase of lead exposure on the hemostasis of cardiovascular disorder through a direct effect of lead on cell injury and depletion of antioxidant activity. 8
Lead Levels According to Age
There was a significant increase in lead levels in blood and coronary among different age groups, there was a proportional increase of 57% in coronary lead levels in cadavers aged ≥ 80 years when compared to cadavers aged ≤ 20 years. On the other hand, there was a higher increase of 87% in blood lead levels in cadavers aged ≥ 80 years than cadavers aged ≤20 years. Pearson correlation coefficient revealed a significant correlation between lead deposition in a coronary artery during different life stages, a direct increase in lead levels was demonstrated with a spontaneous increase in age (Table 1, Figure 1). These findings could be highly indicative of a contributing role of lead in the pathogenesis of cardiovascular system especially with the increase in CVD in older ages.32 This increase in lead concentration is a multifactorial process duo cumulative exposure to contaminant as car emitted gasses, occupational exposure, smoking and residence during different life stages.33 Weiss et al. (2009) reported a positive correlation in lead levels in blood among veterans 17. Not only high blood lead levels can emit the occurrence of CVD; for instance Taylor et al 200 reported even BLL of 3.62 µg/dl – having more than double the risk of cardiovascular mortality than individuals with a blood lead of less than 1.94 µg/dl.6
According to lead toxicokinetics, lead is circulating between different storage sites in a process of concentration shifting in the human body until equilibrium is reached. 34 Many publications agree that skeletal system considered being the ultimate reservoir for metals storage in mammalians.35 Evan et al. (2002) demonstrated a significant correlation between blood lead levels and age in Sweden.33 Alomary et al., 2006 found that there is an increase in lead concentration with age.36
Lead Levels According to Smoking
There was a significant increase in the lead levels in smokers than nonsmokers concerning lead deposition in coronary artery and blood. Ma et al., (2009) reported that there is a slight difference in lead distribution among lead levels in smokers than nonsmokers habits.37 In a previous study, we depicted higher levels of lead in smokers than non-smokers in a population of university students in Jordan.38 Likewise, Alves 2014 reported a significant difference among lead levels between cadaver smokers and nonsmokers.39 One of the limitations of our study is we could not control the internal variables between the age groups such as the type of work and residence.
Conclusion and Recommendations
From our present comprehensive study, we provide further evidence of the role of lead in the initiation of atherosclerosis and not literary in the disease development. We can conclude that although the levels of lead in blood samples from Jordanian cadavers were less than 10µg/dl which is reported as acceptable level of lead in blood according to the ASTRD, there is a high contribution of these levels on the chronic deposition of metals in the storage sites such as coronary artery and ribs. The highest age-related tendency for increased lead levels due to smoking habit was found in a coronary artery. There is a direct significant correlation between blood and coronary lead concentrations. No significant gender-related difference was observed in lead levels. We believe that one of the major sources of lead contamination in Jordan arise from smoking as it was significant in our study so we highly recommend enforcing the law of smoking prohibition as all and especially in the public places.
Acknowledgment
The authors are grateful to member staff of the University of Jordan hospital for providing biological samples and the faculty of pharmacy at the University of Petra for their support in conducting this study.
Conflict of Interest
There is no conflict of interest
References
- WHO. Cardiovascular diseases (cvds). World Health Organization. 2016.
- Abed K. Aluminum, cadmium and microorganisms in female hair and nails from riyadh saudi arabia. J Med Sci. 2007;7:263-6.
- Ozden T. A., Gokcay G., Ertem H. V., et al. Elevated hair levels of cadmium and lead in school children exposed to smoking and in highways near schools. Clinical biochemistry. 2007;40:52-6.
CrossRef - Navas-Acien A., Guallar E., Silbergeld E. K., Rothenberg S. J. Lead exposure and cardiovascular disease: A systematic review. Environmental health perspectives. 2007;472-82.
- de Fost M., Langeveld M., Franssen R., et al. Low hdl cholesterol levels in type i gaucher disease do not lead to an increased risk of cardiovascular disease. Atherosclerosis. 2009;204:267-72.
CrossRef - Taylor R., Roberts A., by Elizabeth P. C. Tobacco and lead toxicity. Reported to The LEAD Group Inc, Australia. 2010.
- Omu A. E., Dashti H., Mohammed A. T., Mattappallil A. B. Cigarette smoking causes impairment of spermatozoal quality: Andrological and biochemical evaluation. Medical principles and practice. 1998;7:47-53.
CrossRef - Sharma P., Purohit P. Lead exposure exacerbates cardiovascular risk. Indian J Clin Biochem. 2014;29:117-8.
CrossRef - García-Esquinas E., Pérez-Gómez B., Fernández-Navarro P., et al. Lead mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC public health. 2013;13:1.
CrossRef - Faramawi M. F., Delongchamp R., Lin Y. S., et al. Environmental lead exposure is associated with visit-to-visit systolic blood pressure variability in the us adults. International archives of occupational and environmental health. 2014.
- Ettinger A. S., Tellez-Rojo M. M., Amarasiriwardena C., et al. Effect of breast milk lead on infant blood lead levels at 1 month of age. Environmental health perspectives. 2004;112:1381-5.
CrossRef - Iqbal M. P. Lead pollution a risk factor for cardiovascular disease in asian developing countries. Pak J Pharm Sci. 2012;25:289-94.
- Masri A. T., Badran E. F., Saleem M. M., Al-Qudah A. A. Lead levels in children with developmental delay. A hospital-based study. Neurosciences. 2009;14:302-3.
- Peters J. L., Kubzansky L. D., Ikeda A., et al. Lead concentrations in relation to multiple biomarkers of cardiovascular disease: The normative aging study. Environmental health perspectives. 2012;120:361-6.
CrossRef - Massadeh A., Al-Sharif L., Dalale’h R., Hassan M. Analysis of lead levels in local jordanian and imported sheep meat and organs using atomic absorption spectrometry. Environmental monitoring and assessment. 2006;115:87-93.
CrossRef - Schwartz J. Lead, blood pressure, and cardiovascular disease in men and women. Environmental health perspectives. 1991;91:71-5.
CrossRef - Weisskopf M. G., Jain N., Nie H., et al. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the department of veterans affairs normative aging study. Circulation. 2009;120:1056-64.
CrossRef - Oumeish E. M. S., Abu W. D., Hamad M., Abu W. R., Bustami M., Arafat T. Serum concentrations of magnesium among jordanians: Effect of cardiovascular disease on magnesium levels. Journal of Chemical and Pharmaceutical Research. 2015;7:499-503.
- Lustberg M, Silbergeld E. Blood lead levels and mortality. Archives of internal medicine. 2002;162:2443-9.
CrossRef - Bhatnagar A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circulation research. 2006;99:692-705.
CrossRef - Massadeh A. M., Al-Safi S. Analysis of cadmium and lead: Their immunosuppressive effects and distribution in various organs of mice. Biological trace element research. 2005;108: 279-86.
CrossRef - Vaziri N. D., Gonick H. C. Cardiovascular effects of lead exposure. Indian J Med Res. 2008;128:426-35.
- Rayyan A. W. Influence of smoking duration on cadmium deposition in blood and scalp hair among university students in jordan. Iranian journal of public health. 2016;45:266-7.
- Safi J., Fischbein A., El Haj S., et al. Childhood lead exposure in the palestinian authority, israel, and jordan: Results from the middle eastern regional cooperation project. 1996-2000. Environmental health perspectives. 2006;114:917-22.
CrossRef - Silveira E. A., Siman F. D., de Faria T. O., et al. Low-dose chronic lead exposure increases systolic arterial pressure and vascular reactivity of rat aortas. Free Radic Biol Med. 2014;67:366-76.
CrossRef - Barry P. S. A comparison of concentrations of lead in human tissues. British journal of industrial medicine. 1975;32:119-39.
CrossRef - Navas-Acien A., Guallar E., Silbergeld E. K., Rothenberg S. J. Lead exposure and cardiovascular disease–a systematic review. Environmental health perspectives. 2007;115: 472-82.
CrossRef - Moller L., Kristensen T. S. Blood lead as a cardiovascular risk factor. American journal of epidemiology. 1992;136:1091-100.
CrossRef - Olchowik G., Widomska J., Tomaszewski M., et al. The influence of lead on the biomechanical properties of bone tissue in rats. Annals of agricultural and environmental medicine : AAEM. 2014;21:278-81.
CrossRef - Schwartz J. Lead, blood pressure, and cardiovascular disease in men. Archives of environmental health. 1995;50:31-7.
CrossRef - Braun J. M., Wright R. J., Just A. C., et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from mexico city: A cross-sectional study. Environmental health a global access science source. 2014;13:50.
- Bloch M. J., Basile J. N. Is there accord in accord? Lower blood pressure targets in type 2 diabetes does not lead to fewer cardiovascular events except for reductions in stroke. J Clin Hypertens (Greenwich). 2010;12:472-7.
CrossRef - Evans M., Fored C. M., Nise G., et al. Occupational lead exposure and severe ckd: A population-based case-control and prospective observational cohort study in sweden. American journal of kidney diseases the official journal of the National Kidney Foundation. 2010;55:497-506.
CrossRef - Alaraj M., Al-Tamimi N., Rayyan W. A., et al. Role of age and uric acid levels on dialysis efficacy among end stage renal disease patients in saudi arabia. Journal of Research in Medical and Dental Science. 2016.
- Kim R., Hu H., Rotnitzky A., Bellinger D., Needleman H. Longitudinal relationship between dentin lead levels in childhood and bone lead levels in young adulthood. Archives of environmental health. 1996;51:375-82.
CrossRef - Alomary A., Al-Momani I. F., Massadeh A. M. Lead and cadmium in human teeth from jordan by atomic absorption spectrometry: Some factors influencing their concentrations. The Science of the total environment. 2006;369:69-75.
CrossRef - Ma Y., Yang Z. Cardiovascular disease may lead to female sexual dysfunction. Med Hypotheses. 2009;72:482.
CrossRef - Rayyan W. A. Obesity influence on lead levels in blood and scalp hair among teenage smokers in jordan. Scholars Journal of Applied Medical Sciences. 2016;4:244-50.
- Alves A. S. P. Evidences from the comparative postmortem analysis of tissues. 2014.








