Manuscript accepted on :February 23, 2018
Published online on: --
Plagiarism Check: Yes
Kim Outhoff1 and Oppel B. W. Greeff1,2
and Oppel B. W. Greeff1,2
1Department of Pharmacology, University of Pretoria, South Africa.
2Cytespace, Pretoria, South Africa.
Corresponding Author E-mail: kim.outhoff@up.ac.za
DOI : https://dx.doi.org/10.13005/bpj/1354
Abstract
The 15-25% of breast cancers that overexpress human epidermal growth factor receptor type 2 (Her-2) are aggressive and more difficult to treat with conventional chemotherapy than their oestrogen receptor positive (ER+) counterparts. Adjuvant trastuzumab, a specific Her-2 targeting monoclonal antibody, has significantly improved the prognosis of women with metastatic and early Her-2 positive breast cancer. Yet clinically relevant cardio-toxicity continues to undermine its gains. This study investigated the unexplored potential of aspirin, β-oestradiol and calcipotriol to attenuate the antibody’s cardio-toxicity in an adult female Balb/c mouse model using serial echocardiography to assess left ventricular function at baseline and after treatment. Mean changes in left ventricular function were compared within and between treatment groups. Trastuzumab demonstrated statistically significant left ventricular dysfunction, detectable by reductions in speckle tracking echocardiographic parameters (global radial strain) from baseline. Calcipotriol did not abrogate these cardio-toxic effects. Conversely, β-oestradiol, high and low dose aspirin attenuated these early and subtle signs of trastuzumab-induced cardiac dysfunction. The findings of this pilot study suggest that β-oestradiol or aspirin may provide cardio-protection against trastuzumab in-vivo, and larger definitive studies are justified.
Keywords
Aspirin;Calcipotriol; Cancer; Cardio-Protection Her-2 Positive Breast β-oestradiol; Trastuzumab;
Download this article as:| Copy the following to cite this article: Outhoff K, Greeff O. B. W. An In-Vivo Investigation of the Cardio-Protective Potential of Aspirin, β-oestradiol and Calcipotriol for Trastuzumab Treatment of Her-2 Positive Breast Cancer. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Outhoff K, Greeff O. B. W. An In-Vivo Investigation of the Cardio-Protective Potential of Aspirin, β-oestradiol and Calcipotriol for Trastuzumab Treatment of Her-2 Positive Breast Cancer. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=19237 |
Introduction
Breast cancers that overexpress transmembranous human epidermal growth factor receptor type 2 (Her-2) account for 15-25% of all breast cancers.1 These tumours are poorly differentiated and aggressive, relatively resistant to standard chemotherapy, and are associated with poorer patient outcomes than oestrogen receptor positive (ER+) breast cancer subtypes. This is unsurprising given the sheer distorting weight of these constitutively activated upregulated Her-2 receptors,2 compounded by their overzealous signalling and overabundant dimerising opportunities with three ligand-activated mitogenic tyrosine kinase family members (Her-1, Her-3, Her-4). Extensive crosstalk with diverse pathways promotes additional signalling layers that ultimately change gene transcription, causing increased cellular growth and proliferation, division, differentiation, migration, adhesion and survival.3
Trastuzumab is a therapeutic monoclonal antibody that specifically targets extracellular domains of overexpressed Her-2 receptors and downregulates Her-2 expression and function. This leads to inhibition of intracellular downstream pro-survival Ras/mitogen-activated protein kinase (Ras/MAPK) and cell death-inhibiting phosphatidylinositol 3’ kinase (PI3/Akt) and mammalian target of the rapamycin (mTOR) signalling cascades, and ultimately via G1 cycle arrest, to reduced cellular survival and tumour regression. Its other potential therapeutic mechanisms include recruitment of host immune responses, inhibition of angiogenesis and of DNA repair, altered cross-talk with other signalling pathways, potentiation of chemotherapy and inhibition of Her-2 receptor extracellular domain cleavage and shedding.4
Although adjuvant trastuzumab has significantly improved the prognosis of women with early and metastatic Her-2 positive breast cancer, the antibody is constrained by sub-optimal efficacy and clinically relevant cardio-toxicity.5-7 Approximately 15% of patients with early breast cancer eventually develop metastatic disease, and objective response rates in Her-2 positive metastatic breast cancer range from 12-34% for a median duration of 9 months.8 Current strategies to overcome this challenge in advanced breast cancer include additional Her-2 targeted treatment with lapatinib (a dual Her-1/Her-2 kinase inhibitor), pertuzumab (Her-2/Her-3 dimerisation inhibitor) or trastuzumab-DM1 (potent micro-tubule polymerization inhibitor) antibody-drug conjugate.9
Trastuzumab may cause cardiac dysfunction in up to a quarter of women treated for Her-2 positive breast cancer.7,10 The antibody shows a proclivity for inducing left ventricular dysfunction, which may lead to progressive heart failure. This is especially evident in patients who receive prior or concurrent anthracyclines, which cause cumulative dose-dependent cardiotoxicity, possibly via inhibition of cardiomyocyte topoisomerase IIβ.11 The subsequent cardiac stress is thought to induce cardiomyocyte Her-2 expression, inadvertently allowing trastuzumab to target this physiologically upregulated repair and survival pathway.12 Her-2 signalling pathways appear essential to regulate a number of important cardiac processes including cell growth, survival and protection from apoptosis, sarcomere synthesis, organization and stability, myocyte-matrix coupling, cellular adaptations to oxidative or metabolic stress in the myocardium, angiogenesis and the counteraction of undue sympathetic tone.13 Trastuzumab may also cause significant cardiac dysfunction in the absence of pre-existing cardiovascular disease, and off-target mechanisms possibly involve direct alterations of cardiomyocyte gene expression, Notch and NF-kB pathways or even immunologic cell death.14,15
Cardiac function is assessed prior to trastuzumab treatment to determine patient eligibility, and monitored at three monthly intervals, irrespective of previous or concurrent treatment, and stopping criteria are based on clinically relevant declines in left ventricular ejection fraction (EF) from baseline. Softer recommendations include assessing earlier predictive markers of cardiac dysfunction including troponin and global longitudinal strain detectable by high resolution transthoracic tissue Doppler imaging or speckle tracking echocardiography.11 Up to 15% of women lose the therapeutic benefit of completing 52 weeks of trastuzumab treatment.16 No cardio-protective agent has been identified for this group of patients, who, depending on tumour stage and age of patient, may be more at risk of potentially fatal heart disease than cancer relapse.17
This project therefore aimed to investigate the potential of several agents with disparate mechanisms of action to attenuate the cardio-toxic effects of trastuzumab.
Two hormones (β-oestradiol and a vitamin D analogue, calcipotriol), two growth factors [epidermal growth factor (EGF) and heregulin (HRG)] and the irreversible cyclo-oxygenase (COX)-inhibitor, aspirin, were identified as reasonable candidates from the literature. Initially, a set of in-vitro experiments, using doxorubicin and geldanamycin as positive controls, established which of these agents complemented trastuzumab’s cytotoxic efficacy in a Her-2 overexpressing (SK-Br-3) breast adenocarcinoma model. These results are published elsewhere (18-21). Briefly, trastuzumab (100 µg/ml) significantly reduced SK-Br-3 cell viability, which was accompanied by G1 cell cycle arrest and dramatic reductions in relative surface Her-2 receptor expression. High concentration aspirin (1 mg/ml; 5.5 mM), both as a single agent, and when combined with trastuzumab, caused significantly greater reductions in cell survival compared to the antibody alone, with similarly significant G1 phase cell accumulation. Although calcipotriol (1 µg/ml; 2.4 µM) was cytotoxic to ER-overexpressing MCF-7 cells, and caused significant G1 arrest in SK-Br-3 cells, this did not translate to discernible reductions in Her-2 positive cellular survival. Similarly, pharmacologically relevant concentrations of β-oestradiol (0.1 µg/ml; 0.36 µM) exerted negligible effects in SK-Br-3 cells, and importantly, did not compromise trastuzumab’s efficacy. Only doxorubicin (0.1 µg/ml; 0.17 µM) demonstrated additive cytotoxic effects when used concurrently with trastuzumab in-vitro. Its cardio-toxic effects are however, well documented.
Based on these results, commercially available calcipotriol, β-oestradiol and aspirin, were identified as suitable candidates for further investigation of their potential cardio-protective effects. There is no published evidence for these agents in a trastuzumab-induced cardio-toxicity setting. There is, however, some evidence of their benefits in existing cardiovascular disease.
Vitamin D deficiency, associated with hypertension, coronary artery disease as well as heart failure,22-27 is now considered an independent risk factor for cardiovascular disease.28 In heart failure patients, vitamin D supplementation improves inflammatory markers29 and is associated with reduced mortality.27 A 30 ng/ml threshold for plasma vitamin D sufficiency has been associated with a reduced risk of left ventricular (LV) concentric remodelling, which is a desirable cardiac outcome.30 Among the elderly, higher circulating vitamin D concentrations have been positively associated with superior LV systolic function (increased fractional shortening, increased ejection fraction) and measures of cardiac geometry.31 Calcipotriol is a nontoxic synthetic analogue of the most active form of vitamin D (calcitriol), currently licensed for use in psoriasis.
Oestrogen plays an important role in cardiovascular protection,32 which is generally ascribed to its beneficial effects on lipid profiles and vascular endothelium.33 The cardiovascular benefits of oestradiol supplementation in the peri-menopausal period are documented34 and yet oestrogen reintroduced to heavily postmenopausal women is associated with no benefit for coronary heart disease, and an increased risk of stroke.35 The discrepant epidemiological accounts underscore the critical timing of hormone replacement therapy (HRT). While animal cardiac ischaemia reperfusion injury models suggest that the hormone is cardio-protective,36 β-oestradiol’s potential to protect the heart from left ventricular contractile dysfunction in pre- or peri-menopausal women, or in those at risk of trastuzumab-induced heart failure, have not been assessed.
Aspirin, a non-steroidal anti-inflammatory drug (NSAID), irreversibly inhibits the two cyclo-oxygenase (COX) isoforms, preventing synthesis of pro-thrombotic platelet thromboxanes (COX-1) and pro-inflammatory prostaglandins (COX-2). Aspirin’s chemo-prophylactic and therapeutic properties in breast cancer37,38 are attributed to its inhibition of inducible COX-2,39 overexpression of which is linked to critical components of carcinogenesis including stimulation of cellular proliferation, inhibition of apoptosis, increased invasiveness, increased production of vascular endothelial growth factor and angiogenesis, and increased aromatase-induced oestrogen biosynthesis.37,40 COX-2 is overexpressed in Her-2 positive breast cancer cells, driven perhaps by Her-2 induction of the COX-2 promoter,40 and aspirin has been shown to inhibit proliferation21 and induce apoptosis in these cells.41 In addition, aspirin increases serum nitric-oxide which inhibits breast cancer cell growth in-vitro.42 Nitric oxide is also a vasodilator which may confer vascular benefits, especially in the thrombo-embolic disease setting. However it is aspirin’s antiplatelet properties that are highly desirable and effective in preventing ischaemic events such as myocardial infarction, stroke or transient cerebral ischaemia.43
Aspirin use for atrial fibrillation associated with existing heart failure is controversial as this NSAID may activate counterproductive neurohormonal mechanisms that exacerbate salt and fluid retention and, compared to the anticoagulant, warfarin, may significantly increase the incidence of hospitalisation for worsening heart failure.44 There is currently no evidence on aspirin’s role in the primary prevention of cardiac dysfunction, whether trastuzumab-induced or not.
Materials and methods
This longitudinal study explored the potential of calcipotriol, β-oestradiol or aspirin to attenuate trastuzumab’s cardio-toxicity in adult female mice, using serial echocardiographic parameters as primary endpoints.
Ethics
All animal experiments conformed to the guidelines of the South African National Standard for “The care and use of animals for scientific purposes” (SAN 10386-2008) and the University of Pretoria Committee for Research Ethics and Integrity: Policy and Procedures for Responsible Research and the Code of Ethics for Research. University of Pretoria Animal Ethics Committee (AEC: S4283-15; h004-15) as well as The University of Texas Institutional Animal Care and Use Committee (IACUC: 00001272-RN00) approvals were obtained prior to commencing mouse experiments.
Mice
A total of 30 female adult Balb/c age-matched mice (8 weeks), strain BALB/c ByJ (Stock number 000651), obtained from The Jackson Laboratory (Bar Harbor, Maine, USA), were housed at an accredited animal care facility (Small Animal Imaging Facility, MD Anderson Centre for Cancer Research, The University of Texas, Houston, USA) in groups of 5, maintained under standard light (12 hour light/dark cycles) at a constant temperature of 20oC-22oC with free access to standard rodent chow and water. General wellbeing was monitored for the first 4 hours post dosing and daily thereafter, and body weights were monitored at baseline and at weekly intervals until the end of the study, following the SANS 10386:2008 guidelines for animal research.
Mice were treated with trastuzumab, either as monotherapy, or with concurrent calcipotriol, β-oestradiol or aspirin, for a maximum period of 22 days. Control mice were left untreated. Serial echocardiography was performed at baseline (Day 0) and after treatment (Day 18). Mice were euthanized humanely by cervical dislocation at the end of the study or before if mice showed signs of distress, advanced cachexia or abnormal behaviour. Significance (P< 0.05) was determined by comparing the mean changes in left ventricular contractile function of the different treatment groups to untreated control and trastuzumab-treated groups. The mean changes between baseline (Day 0) and post treatment (Day 18) were also compared within groups.
Agents
Drug doses were based either on those reported in the literature or on pharmacologically relevant human-equivalent doses.
Trastuzumab, purchased from Genentech Inc (South San Francisco, CA, USA), was solubilised in 0.4 ml phosphate-buffered saline (PBS), diluted in 0.9% sterile saline solution to appropriate concentrations and administered to mice by intra-peritoneal injection at a dose of 100 μg (~4 mg/kg) twice weekly (Days 1, 4, 7, 10, 14, 17) over a period of three weeks, reaching a total cumulative dose of 24 mg/kg, and representing 6 months of patient treatment.45-47 Trastuzumab-induced cardiac dysfunction may only occur after a minimum cumulative dose of 20 mg/kg.48 The intra-peritoneal route was chosen because, despite the bio-distribution being almost identical, there appears to be less mouse-to-mouse variability with this route compared to the intravenous route of administration of the antibody.49
Calcipotriol (Sigma Aldrich, USA: catalogue C4369), 1 mg dissolved in 10 ml ethanol and diluted with 100 ml corn oil to a final concentration of 1 µg/ml, was administered at a dose of 1 µg/kg by intra-peritoneal injection 4 days prior to antibody treatment to allow for its slow onset of action, and then twice weekly to coincide with trastuzumab treatment for the duration of the study.50,51 Rat toxicity studies have shown an increase in skeletal variations at oral doses of 18 mg/kg/day, a dose that far exceeds the one used in these experiments.52
17-β oestradiol (Innovative Research of America, catalogue E-121) 21-day release 0.5 mg pellet was implanted subcutaneously 4 days prior to antibody treatment. This dose has been used previously in rodents,53 reflects the human dose of exogenous oestradiol in hormone replacement therapy and correlates with the concentration that exerted insignificant effects in Her-2 positive breast cancer cells.20
Low dose aspirin (1 mg/kg) (Sigma Aldrich, USA: catalogue A2093) dissolved and diluted in 0.9% sterile saline solution was administered orally by gastric gavage on week days only (Days1-4, 7-11 and 14-17), as logistics precluded weekend dosing. However, aspirin maintains a thromboxane inhibition of over 90% after 72 hours, consistent with platelet turnover,54 and the pharmacodynamics of this irreversible COX-inhibitor were theoretically not compromised by this regimen. Aspirin at a low to medium dose of 75-325 mg (1-5 mg/kg) daily is considered reasonable for chronic administration.38,55
High dose aspirin (15 mg/kg) was administered orally by gastric gavage twice weekly concurrent with trastuzumab dosing. Mice receiving this dose had their food intake monitored due to the potential risk of gastric ulceration. This dose was derived from the high antiproliferative concentrations used in the initial in-vitro experiments,21 and equated to approximately 1 g per oral dose for a woman weighing 65 kg. High dose aspirin has been justified previously in acute migraine (1 g intravenously) and chronic rheumatoid arthritis (5-8 g orally daily).55-57
Drug Delivery Vehicles
Drug delivery vehicles included sterile 0.9% saline solution (S5815), purchased from Teknova (Hollister, CA, USA), and ethanol (E7023), corn oil (C8267) and PBS (D5652), purchased from Sigma Aldrich (St Louis, MO, USA).
Two-dimensional B-mode speckle tracking echocardiography (STE)
To acquire micro-ultrasound images, high resolution non-invasive transthoracic echocardiography was performed using a Vevo 2100 ultrasonograph (Fujifilm Visual Sonics Inc, Toronto, ON, Canada) equipped with a high frequency 40 MHz linear array transducer, specially designed for imaging small animals.58 Mice were placed supine on a warm water pad to maintain body temperature at 37oC under light isofluorane anaesthesia (1.5% isofluorane; 98.5% oxygen). Continual ECG trace monitoring was obtained via limb electrodes. Heart rates approximated 450 beats/min for the duration of the study.
B-mode was used to acquire image data of the left ventricle in parasternal long-axis views at a frame rate of 483 Hz. Analyses of left ventricular (LV) dimensions, ejection fraction (EF) and percentage fractional shortening (%FS) were performed using dedicated speckle tracking software which utilizes semi-automated border tracking: A number of tracking points were placed on the endocardial and epicardial borders as a guide for border delineation and subsequent frame-by-frame tracking throughout the cardiac cycle. From these borders and their motion, end-systolic and end-diastolic LV dimensions were calculated using a method of disks technique. Ejection fraction EF% = [(LVEDV – LVESV) / (LVEDV)] x 100, and percentage fractional shortening %FS = [(LVEDD−LVESD) / (LVEDD)] × 100 were calculated automatically. Data was saved as single non-animated images (image frame) and as multiple-frame animations (cine loop).
In order to quantify cardiac wall motion and myocardial mechanical function, cine loops were analysed with STE VevoStrainTM software (Fujifilm Visual Sonics Inc, Toronto, ON, Canada) which automatically divided the LV myocardium into six regional segments (two basal, two mid, and two apical). Speckle tracking algorithms generated parametric data of average radial velocity and displacement, and subsequent peak strain (S) and strain rate (SR), as well as the time to peak strain (time from the onset of the QRS complex to peak strain value).
Statistical Analyses
Allowing for a 0.05% significance level, 80% statistical power, equal sample sizes, a standard deviation of 5.4 and an ejection fraction effect size of 10, the sample size was estimated at 5 mice per treatment group. No allowances were made for dropouts as data was collected for all animals and statistical analyses were conducted on the intent to treat (ITT) population. Continuous response variables were presented as mean ± SEM. The statistical significance of mean differences within and between treatment groups was determined using GraphPad Prism version 5.0 for Windows (GraphPad Software; San Diego, California, USA). The two-tailed unpaired t-test was used to compare the mean values at baseline (Day 0) and post treatment (Day 18) with Welch’s correction, which does not assume equal variance, used for post hoc analyses. The repeated measures (mixed model) ANOVA was used to compare the mean values of the various treatment groups to both control and trastuzumab-treatment groups, with Bonferroni’s multiple comparisons test used for post hoc analyses. Significance was set at p<0.05. Intra-observer variability was not assessed.
Results
Left ventricular (LV) echocardiographic imaging is used for quantifying cardiac dimensions and contractility, allowing for the detection of abnormal LV chamber enlargement or increased wall thickness from which measures of cardiac function, including ejection fraction and percentage fractional shortening are calculated. These quantifiable markers are useful for monitoring changes in global left ventricular performance. In this study, the mean trends in ejection fraction (EF) and fractional shortening (%FS) declined in mice treated with trastuzumab and in those that remained untreated, although these differences were statistically insignificant. (Figure 1; Table I) Conversely, these traditional echocardiographic assessments of left ventricular function were either preserved or increased in mice treated with trastuzumab plus sequential or concurrent calcipotriol, β-oestradiol, high or low dose aspirin. Significance was not reached within or between treatment arms. (Figure 1; Table I)
Two-dimensional B-mode speckle tracking echocardiography (long and short axis) is now the preferred method for analysing regional wall motion and deformation that occurs in 6 pre-defined segments of the left ventricular myocardium. In contrast to ejection fraction and haemodynamic tissue Doppler imaging measurements, it reveals the contractile function of the heart directly. As contractile dysfunction occurs before ejection fraction drops, it can be used as an early marker of systolic dysfunction, even detecting sub-clinical disease. This echocardiographic modality is particularly suited to rodents that have small rapidly beating hearts, and is considered superior to tissue Doppler imaging as problems with transducer angles of insonation are eliminated. Strain is the myocardial deformation that occurs, whether lengthening or shortening, normalised to the original shape, whereas strain rate is the speed at which this deformation takes place.
Global radial endocardial strain assessing myocardial deformation was derived automatically from averages obtained from six discrete segments of the myocardial curve. Whereas untreated control mice retained left ventricular contractile function, global strain deteriorated significantly in trastuzumab treated mice over the treatment period of 17 days. (Figure 2; Table I) Calcipotriol-trastuzumab treatment resulted in indifferent responses. Conversely statistically significant increases in global strain were noted in mice treated with trastuzumab plus β-oestradiol or high dose aspirin, resulting in significance of P<0.001 and P<0.05 respectively, between these and antibody-treated groups post treatment. In addition, β-oestradiol co-treatment improved myocardial motion significantly compared to untreated controls, and furthermore, like high dose aspirin, increased global strain rate significantly from baseline, accruing significant differences compared to trastuzumab-only and control arms. (Figure 3; Table I) Mice treated with trastuzumab and low dose aspirin displayed non-significant increases in global strain and strain rate, yet compared to trastuzumab-treated mice, these differences were sufficient to confer significant cardio-protection against antibody-induced left ventricular dysfunction. (Figures 2 and 3; Table I)
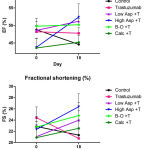 |
Figure 1: Mean (±SEM) ejection fraction (EF) (top) and fractional shortening (FS) (bottom)
|
In mice treated with trastuzumab (100 μg; twice-weekly) compared to mice treated with trastuzumab plus calcipotriol (1 µg/kg; twice-weekly), β-oestradiol (21-day release 0.5 mg pellet), high dose aspirin (15 mg/kg; twice-weekly), or low dose aspirin (1 mg/kg; Monday – Fridays), and untreated controls. T: trastuzumab; Asp: Aspirin; B-O: β-oestradiol; Calc: Calcipotriol. n=5 per treatment group
Table 1: Echocardiography results and within group comparisons of control, trastuzumab, and trastuzumab plus calcipotriol, β-oestradiol or aspirin treated mice, expressed as means ± SEM
| Day 0 | Day 18 | Significance | |
| Control | |||
| EF (%) | 48.8% ± 2.27 | 44.6% ± 3.08 | ns |
| FS (%) | 22.86% ± 0.92 | 21.32% ± 2.85 | ns |
| S (%) | 25.98% ± 2.46 | 23.80% ± 2.82 | ns |
| SR (/s) | 5.75/s ± 0.26 | 5.66/s ± 0.44 | ns |
| Trastuzumab | |||
| EF (%) | 48.2% ± 1.85 | 47.8% ± 1.50 | ns |
| FS (%) | 24.42% ± 1.93 | 20.74% ± 1.64 | ns |
| S (%) | 25.09 % ±1.44 | 17.15% ± 1.89 | *P<0.05 |
| SR (/s) | 5.52/s ± 0.50 | 4.85/s ± 0.61 | ns |
| Calcipotriol-trastuzumab | |||
| EF (%) | 43.6% ± 2.68 | 45.2% ± 1.94 | ns |
| FS (%) | 20.88% ± 1.77 | 22.54% ± 1.33 | ns |
| S (%) | 23.72% ± 3.45 | 24.07% ± 3.51 | ns |
| SR (/s) | 5.61/s ± 0.73 | 5.04/s ± 0.62 | ns |
| B-Oestradiol-trastuzumab | |||
| EF (%) | 49.8% ± 2.27 | 50.2% ± 1.83 | ns |
| FS (%) | 22.76% ± 1.05 | 24.8% ± 2.36 | ns |
| S (%) | 24.19% ± 2.59 | 31.83% ± 1.51 | *P<0.05 |
| SR (/s) | 6.10/s ±0.85 | 7.73/s ± 0.42 | ns |
| Aspirin (high)-trastuzumab | |||
| EF (%) | 43.8% ± 2.58 | 52.4% ± 3.78 | ns |
| FS (%) | 22.34% ± 2.28 | 26.38% ± 2.32 | ns |
| S (%) | 16.81% ± 1.53 | 25.73% ± 2.99 | *P<0.05 |
| SR (/s) | 4.42/s ± 0.28 | 5.91/s ± 0.53 | *P<0.05 |
| Aspirin (low)-trastuzumab | |||
| EF (%) | 48.6% ± 3.46 | 51.2% ± 0.8 | ns |
| FS (%) | 21.06% ± 2.14 | 23.98% ±1.67 | ns |
| S (%) | 25.32% ± 2.81 | 25.95% ±1.74 | ns |
| SR (/s) | 6.58/s ± 0.69 | 6.66/s ± 0.45 | ns |
EF: Ejection fraction; FS: Fractional shortening; S: Radial endocardial strain; SR: Radial endocardial strain rate
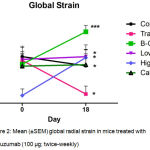 |
Figure 2: Mean (±SEM) global radial strain in mice treated with trastuzumab (100 μg; twice-weekly)
|
Compared to mice treated with trastuzumab plus either calcipotriol (1 µg/kg; twice-weekly), β-oestradiol (21-day release 0.5 mg pellet), high dose aspirin (15 mg/kg; twice-weekly), or low dose aspirin (1 mg/kg; Monday – Fridays), and untreated controls.
Statistically significant (*P<0.05) differences were observed within groups: between Day 0 and Day 18 trastuzumab treatment, between Day 0 and Day 18 β-oestradiol-trastuzumab treatment, and between Day 0 and Day 18 high dose aspirin-trastuzumab treatment.
Highly significant (***P<0.001) differences were observed between Day 18 trastuzumab and Day 18 trastuzumab-β-oestradiol treatments. Significant (*P<0.05) differences were observed between trastuzumab and high dose aspirin-trastuzumab (at Day 0 and Day 18), and low dose aspirin-trastuzumab (at Day 18) treatments.
Significant (*P<0.05) differences were also observed between untreated control and β-oestradiol-trastuzumab (Day 18) and high dose aspirin-trastuzumab (Day 0) treated mice. n=5 per treatment group. T: trastuzumab; Asp: Aspirin; B-O: β-oestradiol; Calc: Calcipotriol.
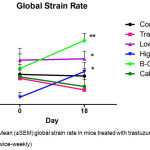 |
Figure 3: Mean (±SEM) global strain rate in mice treated with trastuzumab (100 μg; twice-weekly)
|
Compared to mice treated with trastuzumab plus calcipotriol (1 µg/kg; twice-weekly), β-oestradiol (21-day release 0.5 mg pellet), high dose aspirin (15 mg/kg; twice-weekly), or low dose aspirin (1 mg/kg; Monday – Fridays), and untreated controls. Highly significant (**P<0.01) differences in strain rate were observed between β-oestradiol-trastuzumab and trastuzumab treatments at Day 18. Statistically significant (*P<0.05) differences were observed between low-dose aspirin-trastuzumab and trastuzumab at Day 18, between pre (Day 0) and post (Day 18) high dose aspirin-trastuzumab treatment, and between β-oestradiol-trastuzumab and untreated control groups at Day 18. n=5 per treatment group. T: trastuzumab; Asp: Aspirin; B-O: β-oestradiol; Calc: Calcipotriol.
Discussion
This is the first investigation of commercially available pharmacological strategies to overcome cardio-toxicity associated with trastuzumab treatment.
Trastuzumab
The female adult mouse heart model is attractive because the mouse erbB2 receptor (SwissProt database: P70424) shows high (96%) homology with its human Her-2 (SwissProt database: P04626) counterpart, while providing a relevant intact biological context compared to cultured adult cardiomyocytes. Yet, demonstrating trastuzumab-induced cardio-toxicity in mice has proved challenging; Pre-licensing mouse studies failed to detect cardiac dysfunction or obvious histopathological changes using conventional echocardiography and microscopy. The handful of researchers who investigated the cardio-toxic effects of trastuzumab in mice after the antibody gained FDA approval in 2008, have demonstrated either preserved ejection fraction (EF) and fractional shortening (%FS),48,59,60 delayed reduction in EF and/or %FS,61,62 or reversible LV dysfunction.63 In the latter, trastuzumab was administered to mice for a two week period, reaching a cumulative dose of 10 mg/kg.63 The mice in our study received trastuzumab 4 mg/kg, reaching a much higher cumulative dose of 24 mg/kg after 6 treatments. Similarly, breast cancer patients receive trastuzumab 4 mg/kg initially and 6 mg/kg three-weekly thereafter16 and their cumulative dose after 6 treatments is also 24 mg/kg. Some have found that trastuzumab- induced cardiac dysfunction only occurs after a minimum cumulative dose of 20 mg/kg,48 while others report that trastuzumab-related cardiac dysfunction does not appear to increase with cumulative dose.10 Nonetheless, we were unable to discern cardiac dysfunction using conventional measures.
Although conventional echocardiography is the most commonly used diagnostic method for assessing LV function in clinical cardiology, the measurements may be subjective, semi-quantitative, and relatively insensitive when detecting subtle preclinical abnormalities in contractility, particularly in rodents. In contrast, it has been shown that speckle tracking analyses of myocardial deformation identifies preclinical changes in LV systolic function before conventional measures in EF in women receiving trastuzumab for breast cancer,64-67 including when treated with concurrent anthracyclines.68 An isolated speckle tracking echocardiography (STE) study conducted on trastuzumab-treated mice, showed similar early signs of reduced myocardial strain (despite a preserved EF) after 2 days of trastuzumab treatment (2 mg/kg/day), that occurred prior to reductions in %FS noted after 7 days of treatment.62 There is now some consensus that traditional echocardiographic indices of cardiac function may underestimate subtle changes that occur with trastuzumab and that STE-detected changes may precede these and may therefore predict trastuzumab cardiac dysfunction.69 Our results corroborate accumulating evidence that trastuzumab may in fact ellicit preclinical signs of cardio-toxicity in mice, only detectable by sensitive STE, and independently of anthracycline treatment. The gross structural cardiac abnormalities associated with anthracyclines, are not usually seen in trastuzumab-exposed hearts,7,12 which lends further support to the antibody’s disparate and multiple mechanisms of cardio-toxicity. Although beyond the scope of this paper, routine post-mortem ex-vivo haematoxylin and eosin (H&E) stains of left ventricles revealed varying degrees of epicardial mineralisation in all Balb/c mice, which is considered a background lesion of this strain, as well as Grade 2 (mild, infrequent 10-20%) acute myocardial inflammation in trastuzumab-treated mice, which was also deemed clinically insignificant. (Data not shown) Speckle tracking imaging may thus become the preferred modality for detecting early and subtle LV contractile abnormalities in mice exposed to trastuzumab.
Calcipotriol
Two studies have demonstrated that vitamin D supplementation is associated with improved cardiac geometry and/or function: Left ventricular mass index reduced (improved) in patients with chronic kidney disease,70 while more pertinently, supplementation resulted in significant increases in ejection fraction in heart failure patients with insufficient or deficient plasma levels of vitamin D.71 It has also been shown in chronic heart failure patients with vitamin D deficiency, that LV end-diastolic and end-systolic diameters are significantly greater, LV end-diastolic and end-systolic volumes are larger (ventricular dilatation) and fractional shortening lower compared to those with normal vitamin D levels.72
The potential cardio-protective mechanisms of vitamin D and its role in the maintenance of cardiac function are not well understood. A single study has investigated how additional vitamin D (in the form of supplementation with the analogue, paricalcitol) may offer protection from heart failure, in mice with surgically induced myocardial infarction.73 Activation of the vitamin D receptor (VDR) pathway may attenuate cardiac dysfunction, cardiac myocyte apoptosis and upregulated pro-inflammatory cytokines; paricalcitol therapy significantly decreases cardiac fibrosis and extracellular matrix remodelling, and attenuates renin-angiotensin system (RAS) gene activation.73 Mice treated with the alternative vitamin D analogue, calcipotriol, maintained left ventricular function in the presence of trastuzumab, although differences in endpoints were statistically insignificant. Large intra- and inter-group biological variability, particularly at baseline, confounds data interpretation and limits opportunities to detect statistical significance especially when numbers are small. Despite these negative findings, it is important to note that vitamin D is critical for optimal cardiac function, and deficiency should be treated to minimize the potential deleterious impact of trastuzumab on the heart. Furthermore, there is a strong positive association between serum levels of vitamin D at diagnosis and breast cancer survival.74 Assessing vitamin D status should therefore form an integral part of primary preventive care.
β-oestradiol
In this experiment, there were no statistically significant differences in mean ejection fraction or fractional shortening within or between treatment groups, despite the trastuzumab group showing a ~3.7% reduction in fractional shortening, and the β-oestradiol-trastuzumab group showing an increase of ~2%.
Substantial and statistically significant differences were detected by B-mode speckle tracking analysis, both within and between groups. Mean global radial strain remained stable in untreated control mice. Yet in mice receiving trastuzumab, strain decreased significantly, indicating diminished segmental myocardial mechanical motion. Conversely, global radial strain increased significantly in the β-oestradiol-trastuzumab treated mice, suggesting that not only does the hormone counteract trastuzumab’s effects, but it may restore regional myocardial contractility to levels greater than both trastuzumab-treated and untreated controls when given concurrently with the antibody. In addition, significant differences in mean global radial strain rate were detected between β-oestradiol-trastuzumab, and both trastuzumab-treated and untreated control mice. Strain rate increased in mice receiving the hormone-antibody combination while strain rate slowed slightly in trastuzumab and control groups. These intra-group differences translated to significant differences between groups. Taken together, this suggests that, as opposed to mice treated with trastuzumab, or left untreated, improved regional myocardial contraction occurs against a backdrop of an increased left ventricular contractile rate in adult female mice that have β-oestrogen added to their trastuzumab treatment. Left ventricular performance was increased significantly by concurrent β-oestradiol and trastuzumab treatment, although the numbers (n=5) were small.
No prior studies have investigated the effects of oestrogen or its complex signalling pathways on cardiac function in women receiving trastuzumab treatment, many of whom are clearly not oestrogen-deficient. Rather, ischaemia reperfusion injury models have been used to demonstrate the hormone’s cardio-protective effects – presumably because the oestrogen-deficient state of late menopause is associated with an increase in thrombotic events – and attribute greater recovery to β-oestradiol increasing coronary flow after reperfusion by various mechanisms.75 In one such model, long-term oestrogen treatment causes up-regulation of protective genes, including anti-apoptotic,76 while in neonatal rat cardiomyocytes, β-oestradiol activated ERβ is a prerequisite for upregulation of nitric oxide synthases.77 Oestrogen upregulates anti-inflammatory cytokines in the recently menopausal, as opposed to pro-inflammatory cytokines in older women.33 Oestrogen may also block NF-κB activation and attenuate pro-inflammatory and apoptotic stress in the myocardium.78 Its protective effects on the vascular endothelium include generating nitric oxide and prostacyclins, which mediate vasodilation and promote endothelial repair and/or regeneration, as well as anti-inflammatory and antioxidant effects.33 Cardiovascular protection by oestrogen may also be partly mediated through modulation of autonomic function by suppressing sympathetic tone79 and nitric oxide production via ER activation plays a key role in this process.75
Cross-talk between sex hormones and vitamin-D may play a pivotal role in regulating vascular functions, particularly in women.80 In addition to the two classic nuclear ER isoforms, ERα and ERβ,81 the novel G-protein coupled ER (GPR30 or GPER),82 localised to either endoplasmic reticulum or cell nucleus, depending on species and cell type, is expressed in cardiomyocytes and plays an acute cardioprotective role via the PI3K/Akt pathway in ischemia reperfusion injury.36 There appears to be a relationship between oestrogen receptors, Her-1 and matrix metalloproteinase in facilitating the cardio-protective effects of this receptor.83 In oestrogen-deficient states, activation of GPER inhibits cardiac fibroblast proliferation leading to a reduction in cardiac collagen deposition, thereby attenuating cardiac remodelling and diastolic dysfunction.83
It is unknown if the protective mechanisms involved in drug-induced cardio-toxicity are similar. Although endocrine therapy is usually given after chemotherapy, one study assessed cardiac function when doxorubicin was administered concurrently with the selective estrogen receptor modulator (SERM), tamoxifen.84 All patients received the combination, making comparisons of cardiac dysfunction impossible. Yet, tamoxifen may confer cardio-protection by improving mitochondrial respiratory function and enhancing their superoxide-scavenging activity.85 Depending on the dose, exogenous β-oestradiol may have effects similar to tamoxifen.21
However, there are real concerns about using exogenous oestrogens, as well as anti-oestrogens (SERMs and aromatase inhibitors) in those at risk for thrombo-embolic events. Aromatase inhibitors come with a warning of potential increased risk for ischaemic cardiovascular events, particularly myocardial infarction, in women with pre-existing ischaemic disease, and are associated with a significant 26% increased risk of cardiovascular disease compared to tamoxifen.86 Tamoxifen is anti-oestrogenic in some tissues, including in the breast, but pro-oestrogenic in others including bones and the endometrium. The incidence of cardiovascular events for tamoxifen is relatively low at 3.4%.87 It is known to have antioxidant and cardio-protective effects.88 Our results suggest that exogenous oestrogen improves cardiac function when added to trastuzumab treatment. Taken together, there is a strong pharmacological rationale for adding β-oestradiol to trastuzumab treatment in Her-2 positive breast cancer, particularly in women who are premenopausal (when oestrogen is opposed by progesterone, thus protecting the uterus) and who do not have risk factors for thrombotic events. However, oestrogen’s pleiotropic nature and cross-talk with other growth promoting pathways make it unpredictable, and future studies should focus on elucidating these finer critical points.
Aspirin
Both high dose and low dose aspirin appeared cardio-protective when administered as part of a trastuzumab regimen. Yet, the differences between the two dosage forms were striking: high dose aspirin given concurrently with trastuzumab improved regional LV contractility significantly, while low dose chronic administration of aspirin simply but effectively maintained regional LV function. Significant differences at baseline between the two aspirin-trastuzumab treatment groups were lost at Day 18, implying similar ultimate cardio-protection. The low dose, equating to 60 mg for a 60 kg woman, taken 5 days a week, may be associated with easier administration and fewer potential adverse effects such as gastro-intestinal bleeding than high doses.
Interrogation of these results demands caution in their interpretation. In particular, confounding factors include the unusually low and statistically significant different baseline values of the high dose aspirin group, and the low numbers (n=5). These results indicate that further experiments with much larger groups are warranted.
Aspirin’s mechanism of cardio-protection against trastuzumab-induced cardiac dysfunction is unclear. Aspirin is an irreversible inhibitor of both COX-I and COX-2 isoforms; COX-1 usually plays a housekeeping role that includes platelet aggregation and gastric mucosal protection, is expressed in most cells, and is the only isoform found in normal cardio-myocytes.89 However, it is inducible, including in the endothelium in response to shear stress, thrombin and VEGF. It appears to play a role in the resolution, rather than the progression, of inflammation.90 COX-2 is mostly absent from cells but is induced by a number of factors, including the presence of free radicals and hypoxia – The COX-2 gene promoter contains the response elements of an inflammatory or acute-phase gene.91
Aspirin is not usually given to patients receiving cardio-toxic chemotherapy, although it serves as the antithrombotic component of a pegylated liposomal doxorubicin regimen for multiple myeloma.92 It has been shown, however, that doxorubicin induces COX-2 expression in neonatal rat cardio-myocytes, and that up-regulated COX-2 and prostacyclins are cardio-protective in-vitro (93). In an acute in vivo doxorubicin cardiotoxicity model, specific inhibition of COX-2 aggravates doxorubicin-mediated cardiac injury, while inhibition of COX-I does not.91 It therefore appears that COX-2 is important in attenuating anthracycline-induced cardiac injury. Yet, a much older study showed that the NSAID, ibuprofen, increased the survival of doxorubicin treated mice, perhaps by inhibiting neutrophil infiltration, while the other anti-inflammatories, aspirin and sulindac, had no effect.94 Amidst these conflicting reports, it is critical to establish the role of COX-I in the heart as well as to investigate if trastuzumab induces cardiac COX-2 expression, and if inhibition of this isoform is counterbalanced by aspirin’s co-inhibition of COX-1. Simultaneous inhibition of COX/Her-2 may provide an explanation for the significant cardio-protection noted here, but this is yet to be researched.
Conclusion
Calcipotriol, β-oestradiol and aspirin may play a useful role in trastuzumab treatment of Her-2 positive breast cancer. Although small sample sizes preclude sweeping definitive statements, the results of this preliminary study suggest that β-oestradiol may improve left ventricular function of trastuzumab treated mice, while aspirin may provide both significant cardio-protection and improved efficacy, perhaps making it an ideal candidate to prevent trastuzumab-induced cardio-toxicity. Given the confounding factors in interpreting the data, further investigation, including generating dose response curves for aspirin in large groups of mice, is required. Future epidemiological and clinical studies focussing on cardiac function in women taking these agents during trastuzumab treatment may support these initial findings.
Acknowledgement
The authors gratefully acknowledge CANSA, SAMA and the University of Pretoria for their generous funding, Charles Kingsley (Small Animal Imaging Facility, MD Anderson Cancer Center, University of Texas) for his technical expertise in the echocardiography, Mark McArthur (Department of Veterinary Medicine and Surgery, MD Anderson Cancer Center, University of Texas) for performing the histology, Tracey Hurrell (University of Pretoria) for her expertise in conducting the initial in-vitro experiments, and Pieter Meyer (University of Pretoria) for statistical advice.
Conflict of interest
There is no conflict of interest.
Funding source
This study was generously funded by the Cancer Association of South Africa (CANSA), as well as by the South African Medical Association (SAMA), and the University of Pretoria’s Research Development Programme (RDP).
References
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2 neu oncogene. Science. 1987;235:177-82.
CrossRef - Chung I., Reichelt M., Shao L., et al. High cell-surface density of HER2 deforms cell membranes. Nature Communications. 2016;7.
CrossRef - Yarden Y., Sliwkowski M. X. Untangling the Erb B signalling network. Nat Rev Mol Cell Biol. 2001;2:127-37.
CrossRef - Hudis C. A., Dickler M. Increasing Precision in Adjuvant Therapy for Breast Cancer. Mass Medical Soc. 2016.
- Hortobagyi G. N. Trastuzumab in the Treatment of Breast Cancer. N Engl J Med. 2005;353:1734-6.
CrossRef - Nahta R. New developments in the treatment of HER2-positive breast cancer. Breast cancer. targets and therapy. 2012;4:53-64.
CrossRef - Ewer M. S., Lippman S. M. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900-2.
CrossRef - Nahta R., Yu D., Hung M. C., Hortobagyi G. N., Esteva F. J. Mechanisms of Disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269-80.
CrossRef - Salter K. H., Acharya C. R., Walters K. S., et al. An integrated approach to the prediction of chemotherapeutic response in patients with breast cancer. PLoS One. 2008;3:e1908.
CrossRef - Perez E. A. Cardiac toxicity of ErbB2-targeted therapies: what do we know? Clinical breast cancer. 2008;8:114-20.
CrossRef - Yeh E. T., Chang H. M. Oncocardiology—Past Present and Future: A Review. JAMA Cardiology. 2016.
- Ewer M. S., Ewer S. M. The anthracycline–trastuzumab interaction a lesson in not jumping to confusion. Trends in Pharmacological Sciences. 2015;36:321-2.
CrossRef - Pentassuglia L., Sawyer D. B. The role of neuregulin-1β/ErbB signaling in the heart. Experimental Cell Research. 2009;315:627-37.
CrossRef - ElZarrad M. K., Mukhopadhyay P., Mohan N., et al. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PloS One. 2013;8:e79543.
CrossRef - Rochette L., Guenancia C., Gudjoncik A., et al. Anthracyclines trastuzumab new aspects of cardiotoxicity and molecular mechanisms. Trends in Pharmacological Sciences. 2015;36:326-48.
CrossRef - Genentech I. S. S. F. Herceptin (trastuzumab) Highlight of prescribing information. 2009.
CrossRef - Feldman A. M., Lorell B. H., Reis S. E. Trastuzumab in the treatment of metastatic breast cancer . anticancer therapy versus cardiotoxi city. Circulation. 2000;102:272-4.
- Hurrell T., Outhoff K. The in vitro influences of epidermal growth factor and heregulin-β1 on the efficacy of trastuzumab used in Her-2 positive breast adenocarcinoma. Cancer Cell International. 2013;13:1.
CrossRef - Hurrell T., Outhoff K. Human epidermal growth factor receptor 2-positive breast cancer: which cytotoxic agent best complements trastuzumab’s efficacy in vitro? Onco Targets and Therapy. 2013;6:693.
CrossRef - Outhoff K., Hurrell T. Elucidating the effects of beta-oestradiol, calcipotriol or concurrent trastuzumab in Her-2 overexpressing and oestrogen receptor positive breast cancers. Basic & Clinical Pharmacology & Toxicology Wiley-Blackwell, 111 River St. Hoboken 07030-5774, NJ USA. 2014:307.
- Hurrell T. The effects of selected therapeutic agents on cell cytotoxi city and Her-2 receptor expression using cultured breast adenocarcinoma models. MSc dissertation, 2013, University of Pretoria, Pretoria. https://repository.up.ac.za/handle/2263/33184. Accessed. 2016.
- Shane E., Mancini D., Aaronson K., et al. Bone mass vitamin D deficiency, and hyperparathyroidism in congestive heart failure. The American Journal of Medicine. 1997;103:197-207.
CrossRef - Zittermann A., Schleithoff S. S., Tenderich G., Berthold H. K., Körfer R., Stehle P. Low vitamin D status: a contributing factor in the patho genesis of congestive heart failure? Journal of the American College of Cardiology. 2003;41:105-12.
CrossRef - Holick M. F. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases and some cancers. Southern Medical Journal-Birmingham Alabama. 2005;98:1024.
CrossRef - Pilz S., Marz W., Wellnitz B., et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. The Journal of Clinical Endocrinology & Metabolism. 2008;93:3927-35.
CrossRef - Anderson J. L., May H. T., Horne B. D., et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status and incident events in a general healthcare population. The American Journal of Cardiology. 2010;106:963-8.
CrossRef - Gotsman I., Shauer A., Zwas D. R., et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. European journal of heart failure. 2012;14:357-66.
CrossRef - Chen S., Glenn D. J., Ni W., et al. Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106-12.
CrossRef - Schleithoff S. S., Zittermann A., Tenderich G., Berthold H. K., Stehle P., Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure a double-blind, randomized, placebo-controlled trial. The American Journal of Clinical Nutrition. 2006;83:754-9.
CrossRef - Ameri P., Canepa M., Milaneschi Y., et al. Relationship between vitamin D status and left ventricular geometry in a healthy population: results from the Baltimore Longitudinal Study of Aging. Journal of Internal Medicine. 2013;273:253-62.
CrossRef - Fall T., Shiue I., Geijerstam B. P. et al. Relations of circulating vitamin D concentrations with left ventricular geometry and function. European Journal of Heart Failure. 2012;14:985-91.
CrossRef - Vitale C., Mendelsohn M. E., Rosano G. M. Gender differences in the cardiovascular effect of sex hormones. Nature Reviews Cardiology. 2009;6:532-42.
CrossRef - Chakrabarti S., Morton J. S., Davidge S. T. Mechanisms of estrogen effects on the endothelium: an overview. Canadian Journal of Cardiology. 2014;30:705-12.
CrossRef - Grodstein F. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. MASS MEDICAL SOC 10 SHATTUCK BOSTON, MA. 02115;1996:1406. 1996;335:453 .
- Hendrix S. L., Wassertheil-Smoller S., Johnson K. C., et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425-34.
CrossRef - Deschamps A. M., Murphy E., Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends in Cardiovascular Medicine 2010;20:73-8.
CrossRef - Agrawal A., Fentiman I. NSAIDs and breast cancer: a possible prevention and treatment strategy. International Journal of Clinical Practice. 2008;62:444-9.
CrossRef - Langley R. E., Burdett S., Tierney J. F., Cafferty F., Parmar M. K. B., Venning G. Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Br J Cancer. 2011;105:1107-13.
CrossRef - Simmons D. L., Botting R. M., Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacological Reviews. 2004;56:387-437.
CrossRef - Subbaramaiah K., Norton L., Gerald W., Dannenberg A. J. Cyclooxygenase-2 is over expressed in HER-2 neu-positive breast cancer: evidence for involvement of AP-1 and PEA 3. J Biol Chem. 2002;277:18649-57.
CrossRef - Chattopadhyay M., Kodela R., Nath N., et al. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochemical Pharmacology. 2012;83:715-22.
CrossRef - Nath N., Vassell R., Chattopadhyay M., Kogan M., Kashfi K. Nitro-aspirin inhibits MCF-7 breast cancer cell growth: effects on COX-2 expression and Wnt/beta-catenin/TCF-4 signaling. Biochem Pharmacol. 2009;78:1298-304.
CrossRef - Patrono C. Low-dose aspirin in primary prevention: cardioprotection, chemoprevention, both, or neither? European Heart Journal. 2013:058.
CrossRef - Massie B. M., Collins J. F., Ammon S. E., et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616-24.
CrossRef - Zhang H., Wang Q., Montone K .T., et al. Shared antigenic epitopes and pathobiological functions of anti-p185 her2 neu monoclonal antibodies. Experimental and Molecular Pathology. 1999;67:15-25.
CrossRef - Stagg J., Sharkey J., Pommey S., et al. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proceedings of the National Academy of Sciences. 2008;105:16254-9.
CrossRef - Pentassuglia L., Timolati F., Seifriz F., Abudukadier K., Suter T. M., Zuppinger C. Inhibition of ErbB2 neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytes. Experimental Cell Research. 2007;313:1588-601.
CrossRef - Akolkar G., Bhullar N., Bews H., et al. The role of renin angiotensin system antagonists in the prevention of doxorubicin and trastuzumab induced cardiotoxicity. Cardiovascular Ultrasound. 2015;13:1.
CrossRef - Santes D . K., Slamon D., Anderson S. K., et al. Radiolabeled antibody targeting of the HER-2 neu oncoprotein. Cancer Research. 1992;52:1916-23.
- Zinser G. M., Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004;25:2361-72.
CrossRef - Lee H. J., So J. Y., Castro D. A., et al. Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. J Steroid Biochem Mol Biol. 2010;121:408-12.
CrossRef - Kissmeyer A. M., Binderup L. Calcipotriol (MC 903) pharma cokinetics in rats and biological activities of metabolites. A comparative study with 1, 25 (OH) 2D3. Biochem Pharmacol. 1991;41:1601-6.
CrossRef - Osborne C. K., Hobbs K., Clark G. M. Effect of estro gens and antiestrogens on growth of human breast cancer cells in a thy mic nude mice. Cancer Res. 1985;45:584-90.
- Patrono C., Ciabattoni G., Patrignani P., et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985;72:1177-84.
CrossRef - McCarty M. F., Block K. I. Preadministration of high-dose salicylates suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy. Integr Cancer Ther. 2006;5:252-68.
CrossRef - Adamek A., Hu K., Bayer B., et al. High dose aspirin and left ventricular remodeling after myocardial infarction: aspirin and myocardial infarction. Basic Research in Cardiology. 2007;102:334-40.
CrossRef - Weatherall M. W., Telzerow A. J., Cittadini E., Kaube H., Goadsby P. J. Intravenous aspirin (lysine acetylsalicylate) in the inpatient management of headache. Neurology. 2010;75:1098-103.
CrossRef - Stypmann J. Doppler ultrasound in mice. Echocardiography. 2007;24:97-112.
CrossRef - Jassal D. S., Han S. Y., Hans C., et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and an thracycline mediated cardiomyopathy. Journal of the American Society of Echocardiography. 2009;22:418-24.
CrossRef - Walker J. R., Sharma A., Lytwyn M., et al. The cardioprotective role of probucol against anthracycline and trastuzumab-mediated cardiotoxicity. Journal of the American Society of Echocardiography. 2011;24:699-705.
CrossRef - Singh K. K., Shukla P. C., Quan A., et al. Herceptin a recombinant humanized anti-ERBB2 monoclonal antibody, induces cardiomyocyte death. Biochemical and Biophysical Research Communications. 2011;411:421-6.
CrossRef - Fedele C., Riccio G., Coppola C., et al. Comparison of preclinical cardiotoxic effects of different ErbB2 inhibitors. Breast Cancer Research and Treatment. 2012;133:511-21.
CrossRef - Milano G., Raucci A., Scopece A., et al. Doxorubicin and trastuzumab regimen induces biventricular failure in mice. Journal of the American Society of Echocardiography. 2014;27:568-79.
CrossRef - Hare J. L., Brown J. K., Leano R., Jenkins C., Woodward N., Marwick T. H. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. American Heart Journal. 2009;158:294-301.
CrossRef - Fallah-Rad N., Walker J. R., Wassef A., et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II–positive breast cancer treated with adjuvant trastuzumab therapy. Journal of the American College of Cardiology. 2011;57:2263-70.
CrossRef - Sawaya H., Sebag I. A., Plana J. C., et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines taxanes and trastuzumab. Circulation. Cardiovascular Imaging. 2012;5:596-603.
CrossRef - Negishi K., Negishi T., Haluska B. A., Hare J. L., Plana J. C., Marwick T. H. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. European Heart Journal-Cardiovascular Imaging. 2013:159.
- Negishi K., Negishi T., Hare J. L., Haluska B. A., Plana J. C., Marwick T. H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. Journal of the American Society of Echocardiography. 2013;26:493-8.
CrossRef - Tocchetti C. G., Ragone G., Coppola C., et al. Detection, monitoring, and management of trastuzumab‐induced left ventricular dysfunction: an actual challenge. European Journal of Heart Failure. 2012;14:130-7.
CrossRef - Matias P. J., Jorge C., Ferreira C., et al. Cholecalciferol supplementation in hemodialysis patients effects on mineral metabolism inflammation and cardiac dimension parameters. Clinical Journal of the American Society of Nephrology. 2010;5:905-11.
CrossRef - Amin A., Minaee S., Chitsazan M., Naderi N., Taghavi S., Ardeshiri M. Can vitamin D supplementation improve the severity of congestive heart failure? Congestive Heart Failure. 2013;19:E22-E8.
- Ameri P., Ronco D., Casu M., et al. High prevalence of vitamin D deficiency and its association with left ventricular dilation: an echocardiography study in elderly patients with chronic heart failure. Nutrition, Metabolism and Cardiovascular Diseases. 2010;20:633-40.
CrossRef - Bae S., Singh S. S., Yu H., Lee J. Y., Cho B. R., Kang P. M. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. Journal of Applied Physiology. 2013;114:979-87.
CrossRef - Yao S., Kwan M. L., Ergas I. J., et al. Association of serum level of vitamin d at diagnosis with breast cancer survival: A case-cohort analysis in the pathways study. JAMA Oncology. 2016.
- Fukumoto T., Tawa M., Yamashita N., Ohkita M., Matsumura Y. Protective effects of 17beta-estradiol on post-ischemic cardiac dysfunction and norepinephrine overflow through the non-genomic estrogen receptor/nitric oxide-mediated pathway in the rat heart. European Journal of Pharmacology. 2013;699:74-80.
CrossRef - Nikolic I., Liu D., Bell J. A., Collins J., Steenbergen C., Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. Journal of Molecular and Cellular Cardiology. 2007;42:769-80.
CrossRef - Nuedling S., Karas R. H., Mendelsohn M. E., et al. Activation of estrogen receptor β is a prerequisite for estrogen‐dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS letters. 2001;502:103-8.
CrossRef - Sun B., Xiao J., Sun X. B., Wu Y. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice an insight into oestrogen receptor activation and PI3K/Akt signalling. British Journal of Pharmacology. 2013;168:1758-70.
CrossRef - Du X. J., Riemersma R. A., Dart A. M. Cardiovascular protection by oestrogen is partly mediated through modulation of autonomic nervous function. Cardiovascular Research. 1995;30:161-5.
CrossRef - Gangula P., Dong Y. L., Al-Hendy A., et al. Protective cardiovascular and renal actions of vitamin D and estrogen. Frontiers in Bioscience (Scholar edition). 2013;5:134.
CrossRef - Nuedling S., Kahlert S., Loebbert K., et al. 17β-Estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovascular Research. 1999;43:666-74.
CrossRef - Revankar C. M., Mitchell H. D., Field A. S., et al. Synthetic estrogen derivatives demonstrate the functionality of intra cellular GPR30. ACS Chemical Biology. 2007;2:536-44.
CrossRef - Koganti S. Cardioprotective role of G-protein coupled estrogen receptor 1 (GPER1). Molecular Membrane Biology 2015;32:35-8.
CrossRef - Hortobagyi G., Frye D., Buzdar A., et al. Decreased cardiac toxicity of doxorubicin administered by continuous intravenous infusion in combination chemotherapy for metastatic breast carcinoma. Cancer. 1989;63:37-45.
CrossRef - Zhao Y., Wang L. m., Chaiswing L., et al. Tamoxifen protects against acute tumor necrosis factor α-induced cardiac injury via improving mitochondrial functions. Free Radical Biology and Medicine. 2006;40:1234-41.
CrossRef - Amir E., Seruga B., Niraula S., Carlsson L., Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients a systematic review and meta-analysis. Journal of the National Cancer Institute. 2011.
- Laino C. Aromatase inhibitors may raise cardiovascular risk. Oncology Times UK. 2011;8:16.
CrossRef - Daosukho C., Ittarat W., Lin S. m., et al. Induction of manganese super oxide dismutase (MnSOD) mediates cardio protective effect of tamoxifen (TAM). Journal of Molecular and Cellular Cardiology. 2005;39:792-803.
CrossRef - Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins & Other Lipid Mediators. 2002;68:165-75.
CrossRef - Hartner A., Pahl A., Brune K., Goppelt-Struebe M. Upregulation of cyclooxygenase-1 and the PGE2 receptor EP2 in rat and human mesangioproliferative glomerulonephritis. Inflammation Research. 2000;49:345-54.
CrossRef - Dowd N. P., Scully M., Adderley S. R., Cunningham A. J., Fitzgerald D. J. Inhibition of cyclooxygenase-2 aggravates doxorubicin-mediated cardiac injury in vivo. The Journal of Clinical Investigation. 2001;108:585-90.
CrossRef - Hassoun H., Reich L., Klimek V. M., et al. Doxorubicin and Dexamethasone Followed by Thalidomide and Dexamethasone (AD-TD) as Initial Therapy for Symptomatic Patients with Multiple Myeloma. Blood. 2004;104:2409-.
- Adderley S. R., Fitzgerald D. J. Oxi dative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. Journal of Biological Chemistry. 1999;274:5038-46.
CrossRef - Inchiosa Jr M. A., Smith C. M. Effects of ibuprofen on doxorubicin toxicity. Research Communications in Chemical Pathology and Pharmacology. 1990;67:63-78.







