Afiat Berbudi1 , Ulfah Hasna Hasibah2
, Ulfah Hasna Hasibah2 , Ahmad Faried3
, Ahmad Faried3 , Almahitta C. Putri4
, Almahitta C. Putri4 and M. Ersyad Hamda1
and M. Ersyad Hamda1
1Department of Microbiology and Parasitology, Padjadjaran University, Bandung, Indonesia.
2Faculty of Medicine, Padjadjaran University, Bandung, Indonesia.
3Department of Neurosurgery, Faculty of Medicine, Padjadjaran University/Hasan Sadikin General Hospital, Bandung, Indonesia.
4Department of Surgery, Faculty of Medicine, Padjadjaran University, Indonesia.
Corresponding Author E-mail: a.berbudi@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1392
Abstract
The aim of this study was to know whether the administration of turmeric extract gel topically can accelerate diabetic wound healing. This studywas an experimental analytical research in vivo laboratory using quantitative method. The subjects of this study were alloxan-induced diabetic mice model. The skin excision wounds were made on the back of the mice. The subjects were divided into 3, i.e. the group that given 1) blank gel (control) 2) Curcuma longa extract gel 3) nanocurcumin gel, topically on wound once daily for 24 days. There was a tendency of nanocurcumin gel to accelerate wound closure on day 3 and 9 of observation (p=0.459 and p=0.317). Whereas, Curcuma longa gel had tendency to accelerate wound closure on day 6 and 12 (p=0.429 and p=0.485). On day 15 until 24, there was no tendency of nanocurcumin and Curcuma longa gels to accelerate wound closure. However, overall there was no differences in the rate of wound closure in all three groups as analyzed by ANOVA, thus represented by the area under curve (AUC) (p=0.521). Despite there is a tendency of nanocurcumin and Curcuma longa gels to accelerate the wound closure in the early stages of wound healing, administration of Curcuma longa and nanocurcumin gels topically do not accelerate skin excision wound closure in alloxan-induced diabetic mice model.
Keywords
Curcuma Longa Extract Diabetes Diabetic Wound;Gel;Mice Model; Nanocurcumin Gel; The Rate of Wound Closure
Download this article as:| Copy the following to cite this article: Berbudi A, Hasibah U. H, Faried A, Putri A. C, Hamda M. E. Administration of Curcuma longa Extract Topically does not Accelerate Skin Excision Wound Closure in Alloxan-induced Diabetic Mice Model. Biomed Pharmacol J 2018;11(1). |
| Copy the following to cite this URL: Berbudi A, Hasibah U. H, Faried A, Putri A. C, Hamda M. E. Administration of Curcuma longa Extract Topically does not Accelerate Skin Excision Wound Closure in Alloxan-induced Diabetic Mice Model. Biomed Pharmacol J 2018;11(1). Available from: http://biomedpharmajournal.org/?p=18959 |
Introduction
Wound due to injury is the most common case in human. Wound can decrease the productivity and be an economical burden to the patient. Every year, more than 1 billion USD are spent in order to care wound in the world.1 The high number of injuries and the high cost required to care wound encourages efforts to develop appropriate way of wound care in order to accelerate wound healing process. The development of science and wound care in Indonesia is very important given the high number of people with the diseases that are potentially followed by chronic wound, such as diabetes, whose numbers are increasing in recent times.2
Loss of tissue integrity due to injury impact in various negative effects, such as damage of the barrier to the external environment, thus increasing the possibility of exposure of exogen substances including microorganisms. As the result, the wound become infected and will be more difficult to cure. Furthermore, the wound could lead to decrease organ function, reduce the quality of life and productivity of the patients. Moreover, the extension of wound healing time in chronic wound due to pathological abnormalities leads to increase wound care cost burden of the patient.3 Therefore, the favorable wound healing is desirable to prevent worsening of wound complication.
The process of tissue repair on wound healing is divided into 3 phases, i.e. the inflammatory phase, the proliferation phase, and the maturation phase.4 These three phases occur synergistic sequentially and overlapped each other. Disturbance of one or more of these phases will extend the wound healing time as occurs in chronic wound.5 The wound healing process begins with the release of various cytokines and growth factors, such as Transforming Growth Factor-β (TGF-β) and Platelet-derived Growth Factor (PDGF) by platelets that induce neutrophil chemotaxis into wound tissue in the first 24 hours, and monocytes in the next 24 hours.6
The migrating monocytes will then differentiate to macrophages. Futhermore, these macrophages will play an important role in all phases of wound healing. In healing process, macrophages have function to:1 clean debris, neutrophil containing bacteria and residual bacteria by phagocytosis,2 produce proteases and extracellular matrix molecules,3 produce growth factor, such as PDGF, TGF-β, Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor-2 (FGF-2) which subsequently contribute to the proliferation of fibroblast, angiogenesis and reepithelization in the proliferation phase.6–8
Based on the mode of activation and its characteristic, macrophages are distinguished into 2 types, classically activated macrophages (CAM/M1) and alternatively activated macrophages (AAM/M2).8 In the initial response to tissue damage, M1 serves in phagocytosis and pro-inflammatory cytokines production, facilitating congenital immunity and wound debridement.7 In the later stage, M2 will produce VEGF, TGF-β, dan Interleukin 10 (IL-10) which helps in suppressing inflammation, and stimulates tissue formation.8 Inhibition of macrophage activation has been known in leading to elongation of healing time.7 In addition, persistent M1 in wound tissue and inhibition of M2 polarization will also result in disturbance of healing process, especially in certain diseases, such as diabetes.9 Therefore, things that support macrophage migration in the early phase and M2 polarization in the later phase of wound healing need to be further investigated to optimize wound healing process.
Turmeric (Curcuma longa) is common plant as cooking spices in Indonesia. This plant also frequently used by the society to treat some diseases, such as stomach diseases, diarrhea, fever, and itchy.10 In India, Curcuma longa has been used to treat open wound.11
Turmeric has been known containing curcumin which was identified to have anti-inflammatory effect. Curcumin in turmeric can modulate inflammatory response by reducing cyclooxygenase-2 (COX-2) activity, lypooxygenase, and inducible nitric oxide synthase (iNOS) enzymes; inhibit pro-inflammatory cytokines production, such as tumor necrosis factor-alpha (TNF-α), IL-1, -2, -6, -8, and -12, monocyte chemo-attractant protein (MCP), and migration inhibitory protein (MIP); and also reduce mitogen and Janus kinase.12 In addition, a study using macrophage cell line (Raw264.7) has also shown that curcumin can induce M2 polarization of Raw264.7 as indicated by upregulation of macrophage manose receptor (mmr), arginase1 and pparg gene expression, which are markers of M2.13
Therefore, further investigation of the effect of turmeric extract administration topically on chronic wound, such as diabetic wound, and immunologic mechanism on the affected wound tissue repair should be investigated.
Materials and Methods
Research Subject and Design
The research design is in vivo laboratory experimental analytic research by quantitative method. Subject of this research was animal experiment using 14 male mice model diabetes strain Balb/C with age 10 – 12 weeks and weight 22 – 40 grams according to inclusion and exclusion criterias, obtained from Pharmacology Laboratory, Faculty of Medicine, Universitas Padjadjaran. Prior to the experiment, mice were adapted to reduce stress after mobilization to the laboratory. Mice were housed in proper cage and were given food and drink ad libtium. Type 1 diabetic mice model were obtained by alloxan induction intraperitoneally.
This experiment was conducted in Pharmacology and Biochemistry Laboratories, Faculty of Medicine, Padjadjaran University in May until July 2017.
All of the experiments were performed according to 3R principle. This studyhas obtained an ethical approval from health research ethics committee, Faculty of Medicine, Padjadjaran University (No. 711/UN6.C.10/PN/2017).
Gel Material
Blank gel was made from pure gel without active substances. Curcuma longa extract gel was prepared by mixing Curcuma longa 96 % v/v ethanol extract into gel (0.3%). Both gels were prepared in Pharmacognosy Laboratory, Faculty of Pharmacy, Padjadjaran University. Nanocurcumin gel was prepared by mixing curcumin (0.25% w/v) into a conventional emulsion using GMO (16 w/v) and Chemophor RH40. Curcumin nanoemulsion then was formulated into gel. This nanocurcumin gel was prepared in Semisolid Laboratory, Faculty of Pharmacy, Bandung Institute of Technology (ITB).
Induction of Diabetes
After mice were adapted for 1 week, induction of diabetes was prepared by injecting alloxan (150 mg/kgBB) intraperitoneally. Immediately after alloxan induction, mice were given food necessarily. In the first 48 hours after alloxan induction, mice were given 10% glucose orally to avoid hypoglycemia and to prevent mice death. On day 11 after alloxan induction, mice blood glucose levels were measured by glucometer (GlucoDRTM, Allmedicus, South Korea). Mice with blood glucose >135 mg/dL were included as research subjects in this study.
Induction of Wound
Initially, mice back’s feather was shaved to facilitate wound induction. About 2 – 3 days after shaving procedure, wound induction experiment was started. First of all, isofluran was given by inhalation as anesthesia. Aseptic and antiseptic step was performed at the wound area by alcohol 70%. Wound induction was made on the back of the mice using 6 mm biopsy punch (GlaxoSmithKline GmbH & Co. KG, Germany) in order to homogenize shape, depth and diameter of the wound of each mice.
Gel Administration
Prior to the experiment, the subjects were grouped into 1 control group and 2 treatment groups, i.e. the group that given 1) blank gel (control) 2) Curcuma longa extract gel (treatment I) 3) nanocurcumin gel (treatment II). Immediately after wound induction, topically gel was given on the wound surface in compliance with the group once daily for 24 days.
Wound Measurement
Wound area was measured every 3 days, started from day 3 until day 24 post-wound induction. Wound size was analyzed using image J (open source). Then, percentage of wound closure was calculated by formula below:
![]()
Initial wound closure (in day 0) was defined as 0% wound closure.
The percentage of wound closure is considered as observation data in this research for the next analysis process.
Statistical Analysis
The observation data were analyzed in order to know normality of the data distribution by using Shapiro-Wilk. ANOVA test was used for normal distribution data and followed by multiple comparisons post-hoc Turkey. Whereas Kruskall-Wallis test was used for abnormal distribution data. A p-value of <0.05 was regarded as significant.
Results
The Development of the Percentage of Wound Closure Between Control and Treatment Groups
The development of the percentage of wound closure among those three groups (control, Curcuma longa, and nanocurcumin) on day 3, 6, 9, 12, 15, 18, 21, and 24 was shown in the figure 1.
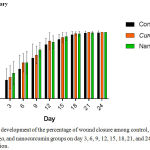 |
Figure 1: The development of the percentage of wound closure among control, Curcuma longa, and nanocurcumin groups on day 3, 6, 9, 12, 15, 18, 21, and 24 after wound induction.
|
As indicated in the figure 1, there was a tendency of nanocurcumin gel to accelerate wound closure on day 3 and 9 (p=0,459 and p=0,317). Whereas, Curcuma longa gel showed a tendency to accelerate wound closure on day 6 and 12 (p=0,429 and p=0,485). On day 15 until 24, the tendency was not shown in both of nanocurcumin and Curcuma longa gels to accelerate wound closure. The progress of wound closure day by day of all groups was indicated in the figure 2. The progress of wound area was not shown any differences among those three groups on day 15 until 24 (Figure 1 and 2). Furthermore, the representative of wound closure progress of three groups was presented in the figure 3.
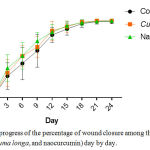 |
Figure 2: The progress of the percentage of wound closure among three groups (control, Curcuma longa, and naocurcumin) day by day.Click here to View figure |
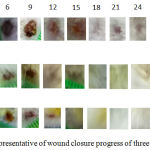 |
Figure 3: The representative of wound closure progress of three groups.
|
Although there was the tendency of nanocurcumin dan Curcuma longa groups to accelerate wound closure on day 3, 6, 9, and 12, wholly, there was no significant differences of wound closure rate of all groups. It was shown by area under the curve (AUC) analysis in the figure 4 which revealed that there was no statictical differences of AUC among all three groups (p=0,521).
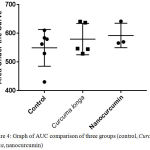 |
Figure 4: Graph of AUC comparison of three groups (control, Curcuma longa, nanocurcumin)
|
Discussion
Based on observation of the excisional wound closure rate of diabetic type 1 mice model, there was no significant differences among control and treatment groups on day 3, 6, 9, 12, 15, 18, 21, dan 24. This phenomenon can be explained by understanding mechanism of diabetic wound healing and curcumin intervention to the wound healing process.
There are commonly 3 phases of wound healing that occur synergistic consecutively, and overlapped. All of those three phases are inflammatory, proliferation, and remodeling phases along 3-14 days.6,14 The inflammatory phase, begin with hemostasis. Afterwards, neutrophil and macrophage M1 migrate to the wound tissue to phagocyte bacteria and debris, release pro-inflammatory cytokine and growth factors, such as FGF-2, IL-1, TGF-β, and TNF-α, and also cause inflammation. In the proliferation phase, M2 macrophage release anti-inflammatory cytokines and growth factors which lead to epitelization, angiogenesis, and formation of tissue granulation. The last phase of wound healing involves the balance between degradation of old ECM and synthesis of new ECM to promote remodelling process. Among all cells that play roles in this phase, fibroblast is the most likely important component. This cell synthesizes matrix molecules and tissue inhibitors of matrix metalloproteinases (TIMPs) that regulates matrix metalloproteinases (MMPs) activity. This phase requires several months to form mature scar.6
In diabetic wound, there is an abnormality in wound healing process. Basement membrane thickening in arteriole causes abnormality in wound healing. High levels of advanced glycation end-products (AGEs) in diabetes probably induce inflammatory molecules, such as TNF-α and IL-1, and intrude collagen synthesis.15 In addition, abnormality of immune response in diabetes results in lowering of chemotaxis, phagocytosis, and ability to kill bacteria by immune cells.16 Both of these could probably cause the inflammatory phase becomes longer.
Proliferation phase is also inhibited in diabetic wound due to disfunction of fibroblast and keratinocyte.17 VEGF is a kind of growth factor that play role to induce mobilization of endothelial progenitor cell (EPC) in angiogenesis. In diabetic condition, level of VEGF are probably decrease.18,19 This condition is possible to decrease activation and phosphorylation of endothelial Nitric Oxide Synthases (eNOS) in bone marrow, inhibit mobilization of EPC from the bone marrow to circulation directly.18,20 Level of chemokine stromal cell-derived factor-1α (SDF-1α) that is important to induce homing of EPC to the wound area, reduce in diabetic condition as well. Both of these would probably impede angiogenesis, thus the proliferation phase becomes longer.
In diabetic wound, there is also unbalance between MMPs and TIMPs causing maturation and wound healing becomes longer.15
Curcumin as an active substance in Curcuma longa has various mechanisms to accelerate wound healing. Based on previous studies, curcumin has proven to reduce stress oxidative and lipid peroxidation, and inhibit AGEs accumulation (Sajithlal et al., 1998). Curcumin also proven to reduce expression of TNF-α, IL-1β, and MMP-9.22 Previous studies have explained that in the proliferation phase, curcumin could increase anti-inflammatory cytokine IL-10, upregulate expression of VEGF, TGF-β1, HIF-1α, SDF-1α, and HO-1, thus increasing new vascular formation.23
Study that conducted by Qi, et al., showed that there is another factor in diabetic wound healing, i.e. pigment epithelium-derived factor (PEDF). PEDF is 50-kDa glycoprotein family serine proteinase inhibitor (Serpins) that has anti-angiogenic activity24 and counterbalance of VEGF (pro-angiogenic).25 Study that conducted by Jenkins, et al. showed that there is an increased of PEDF levels in diabetic tipe 1 and type 2 patients.26,27 In line with this, study by Qi, et al. indicated an increased of PEDF levels in diabetic mice model.24 However, there are no publication that prove the effect of curcumin on lowering PEDF levels. The high levels of PEDF acting as the counterbalance of VEGF probably resulted in slowing of the accelerated wound healing process due to inhibition of the angiogenesis in the proliferation phase. Although curcumin known in increasing VEGF and other growth factors in acute wounds, high PEDF levels in diabetes might be the reason why curcumin is not sufficient to accelerate wound healing. Therefore, further investigation regarding the effect of curcumin on PEDF in wound healing is required.
As mentioned earlier, wound healing is initiated by the inflammatory phase involving chemotaxis, monocyte migration, phagocytosis, and bacterial elimination by macrophages.16 Interestingly, monocyte chemotaxis might be inhibited by curcumin via modulation of growth response-1 transcription factor.28 This may cause a decrease in the number of macrophages in curcumin-treated wounds since the onset of wound induction, whereas macrophages in the wound tissue play an important role in both the inflammatory phase and the proliferation phase of wound healing. The decrease in the number of macrophages perhaps can lead to the expected acceleration of wound healing by administering curcumin does not occur. Although curcumin is known to increase the polarization of essential M2 macrophages in releasing growth factors in wound healing, the expected improvement in wound healing rate by M2 macrophage polarization may not be occurred if the available macrophages in the wound tissue are not sufficient. In addition, curcumin has been known to have anti-inflammatory effects, so it can inhibit the inflammatory phase. Whereas inhibition of one or more wound healing phases, especially in the inflammatory phase may further impede the acceleration of wound healing.29 Therefore, further study is required to investigate the effect of curcumin on the acceleration of wound closure if the administration begins on day 3 or 4, since at that time it is estimated that the monocytes have migrated to the wound tissue and the inflammatory phase is going to over.30 Thus, if curcumin is administered after the end of the inflammatory phase and monocyte migration to the wound tissue, the effect of curcumin in encouraging wound closure in the proliferation phase through M2 macrophages polarization and the resulting growth factors is expected to be more optimal to support tissue repair.
In conclusion, despite there is a tendency of nanocurcumin and Curcuma longa gels to accelerate the wound closure in the early stages of wound healing, administration of Curcuma longa and nanocurcumin gels topically begin with day 0 do not accelerate skin excision wound closure in alloxan-induced diabetic mice model.
Acknowledgements
This research was granted by internal grant for fundamental research, Padjadjaran University.
Conflict of Interest
No conflict of interests
References
- Sen C.K, Gordillo G.M, Roy S, Kirsner R, Lambert L, Hunt T.K, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71.
CrossRef - IDF. Global Guideline for Type 2 Diabetes. 2012.
- Augustin M, Maier K. Psychosomatic Aspects of Chronic Wounds. Dermatol Psychosom. 2003;4(1):5–13.
CrossRef - Vinish M, Cui W, Stafford E, Bae L, Hawkins H, Cox R, et al. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen. 2016;24(1):6–13.
CrossRef - Schonfelder U, Abel M, Wiegand C, Klemm D, Elsner P, Hipler U-C. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005 Nov;26(33):6664–73.
CrossRef - Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003 Jul;83(3):835–70.
CrossRef - Brancato S.K, Albina J.E. Wound Macrophages as Key Regulators of Repair. Am J Pathol. 2011;178(1):19–25.
CrossRef - Ferrante C.J, Leibovich S.J. Regulation of Macrophage Polarization and Wound Healing. Adv Wound Care. 2012;1(1):10–6.
CrossRef - Kraakman M.J, Murphy A.J, Jandeleit-dahm K, Kammoun H.L. Macrophage polarization in obesity and type 2 diabetes : weighing down our understanding of macrophage function? Front Immunol. 2014;5:1–6.
CrossRef - Dhianawaty D, Panigoro R, Martiana A, Surialaga S, Yulianti H, Maliki A.DI.S. Potency of Tumeric Rhizome Decoction on Sperm Infertility of Male Mice to Succeed Family Planning Program in West Java Society. Int J Pharm Pharm Sci. 2016;8:10–3.
- Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee R.K. Turmeric and curcumin : Biological actions and medicinal applications. Curr Sci. 2004;87(1):44–53.
- Jurenka J.S. Anti-inflammatory Properties of Curcumin , a Major Constituent of Curcuma longa : A Review of Preclinical and Clinical Research. Altern Med Rev. 2009;14(2):141–53.
- Gao S, Zhou J, Liu N, Wang L, Gao Q, Wu Y, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015 Aug;85:131–9.
CrossRef - Baum C.L, Arpey C.J. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005 Jun;31(6):674–86; discussion 686.
CrossRef - Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein S.R. Current Aspects in the Pathophysiology and Treatment of Chronic Wounds in Diabetes Mellitus. Biomed Res Int. 2013;2013.
- Sibbald G.R, Woo K.Y. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab Res Rev. 2008;24(1):S25–30.
CrossRef - Guo S, Dipietro L.A. Factors Affecting Wound Healing. J Dent Res. 2010;89(3):219–29.
CrossRef - Gallagher K.A, Liu Z-J, Xiao M, Chen H, Goldstein L.J, Buerk D.G, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007 May;117(5):1249–59.
CrossRef - Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007 May;117(5):1219–22.
CrossRef - Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003 Nov;9(11):1370–6.
CrossRef - Sajithlal G.B, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998 Dec;56(12):1607–14.
CrossRef - Kant V, Gopal A, Pathak N.N, Kumar P, Tandan S.K, Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol [Internet]. 2014;20(2):322–30. Available from: http://dx.doi.org/10.1016/j.intimp.2014.03.009
CrossRef - Kant V, Gopal A, Kumar D, Pathak N.N, Ram M, Jangir B.L, et al. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015 Feb;193(2):978–88.
CrossRef - Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y, et al. High Levels of Pigment Epithelium – Derived Factor in Diabetes Impair Wound Healing Through Suppression of Wnt Signaling. Diabetes. 2015;64(4):1407–19.
CrossRef - Gao G, Li Y, Fant J, Crosson C.E, Becerra S.P, Ma J. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium–derived factor in brown norway and sprague dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002 Apr;51(4):1218–25.
CrossRef - Jenkins A, Zhang S.X, Gosmanova A, Aston C, Dashti A, Baker M.Z, et al. Increased serum pigment epithelium derived factor levels in Type 2 diabetes patients. Diabetes Res Clin .Pract. 2008 Oct;82(1):e5–7.
CrossRef - Jenkins AJ, Zhang SX, Rowley KG, Karschimkus C.S, Nelson C.L, Chung JS, et al. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med a J Br Diabet Assoc. 2007 Dec;24(12):1345–51.
CrossRef - Giri R.K, Rajagopal V, Kalra V.K. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J Neurochem. 2004;91(5):1199–210.
CrossRef - Snyder R.J. Treatment of nonhealing ulcers with allografts. Clin Dermatol. 2005;23(4):388–95.
CrossRef - Park J.E, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004 May;187(5A):11S – 16S.
CrossRef








