Priya P and Sujatha L. B.
Department of Zoology, Pachaiyappa’s College, Chennai-600030. India.
Corresponding Author E-mail: priyaponmudi79@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1341
Abstract
The present investigation intended to evaluate the independent and combined toxicological effects of synthetic fungicide Morpholine derivative of dithiocarbamate (MMDTC) and two metallic compounds viz. copper sulphate and zinc sulphate on the biochemical constituents like total proteins, total sugars and total lipids in the muscle, liver, brain and kidney of Catla catla. Biochemical studies were carried out in both the control (untreated) healthy fish and the fish exposed to sub-lethal concentrations of MMDTC, CuSO4, ZnSO4, MMDTC +CuSO4,, MMDTC + ZnSO4 and CuSO4+ ZnSO4 on 4th, 7th, 14th, 21st, and 28th day of exposure.
Keywords
Catla Catla MMDTC; CuSO4; ZnSO4; Synergistic Toxicity;
Download this article as:| Copy the following to cite this article: Priya P, Sujatha L. B. Toxic Effects of Morpholineum-4-Morpholine Dithiocarbamate and Heavy Metals (Cu and Zn) on the Indian Major Carp Catla Catla: A Biochemical Analysis. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Priya P, Sujatha L. B. Toxic Effects of Morpholineum-4-Morpholine Dithiocarbamate and Heavy Metals (Cu and Zn) on the Indian Major Carp Catla Catla: A Biochemical Analysis. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=18305 |
Introduction
General health of the fish is influenced by the physiological activities occurring in the body. The metabolic activities in the liver are crucial in determining the physiological state of the organism. Any toxic substance reaches soon the liver and causes deleterious effect resulting in disturbed physiological activities. The total carbohydrate, protein and lipid contents of the tissues are crucial for the normal health of the organism because there appears a balanced distribution of these three biochemical constituents; a reduction in one should be compensated by others. If not, the entire metabolic processes become abnormal showing its effects on various physiological activities and may even lead to death. Due to this reason, studies on tissue biochemistry have formed basic necessity to evaluate the health profiles of the organisms under stress.
Dithiocarbamate fungicides are used generally as seed dressing and protection of fruit, vegetable and ornamental crops from a variety of fungal diseases.1 In an aquatic system pesticide concentration increases as it moves up the food chain because of biomagnifications and the animals accumulate non-biodegradable pesticides from the corresponding environment.2 Pesticides acting as stress factor affecting the fish has been well documented.3
Copper and Zinc are essential trace elements required for the body, but at higher level toxic in nature. Exposure to copper can induce stress responses such as changes in the fish’s ion regulation, olfaction and swimming performance.4,5 A significant reduction in three biochemical constituents viz. protein,lipid and sugars of Tilapia under the influence of zinc has also been reported.6 Several investigators have reported the toxicological effect of zinc in decreasing the protein content of common carp.7,8 Elevated levels of heavy metals can cause death and mutation in animal populations.
In an aquatic environment the pollutants reach easily as runoff from pesticide residues and heavy metals like copper, mercury, nickel, lead and zinc pollute the aquatic system through industrial and municipal wastes. In such a scenario, the metal compounds react with the chemical pesticides to form complexes that may have entirely different properties than the original one.
The toxic kinetics of the pollutant may inflict some pathogenic effect on the organism depending on the rate of metabolic transformation and elimination. There is paucity of information on how the fish reacts to cumulative toxic synergism.
Hence an attempt has been made in the present study to know the effects of a synthetic fungicide, Morpholineum-4-Morpholine Dithiocarbamate (MMDTC), and two heavy metal compounds, Copper sulphate (CuSO4.5H2O) and Zinc sulphate (ZnSO4) acting independently and combined on the important biochemical constituents of one of the commercially important Indian Major Carp, Catla catla.
Materials and Methods
Maintenance of Fish Stock and Feeding
Catla fingerlings of the same size (10-12 cm in length and 6-8 g in weight ) were procured from culture ponds of Tamil Nadu Fish Seed Farm, Poondi, Thiruvallur district and brought to laboratory in oxygen packs. The fish were acclimatized and maintained in ferro-cement tanks (3’L× 2’W×2’H) filled with bore water. The stock fish were fed with pelleted feed prepared with rice bran, groundnut oil cake, tapioca powder and mineral mixture.9 The fish were fed daily with pelleted feed at 5% body weight in two split doses, in the morning and evening. Feeding was started one day after the fish were stocked and stopped 24 hr prior to experiment.
Selection of Experimental Fish
Healthy fish without any observable pathological symptoms were chosen for the experiments and were maintained in disinfected glass aquarium tanks (2’L x 1’W x 1’H) filled with water at rate of 2 litres per fish. During the period of experimentation, the room temperature fluctuated from 30-320C. The water used for the experiment had dissolved oxygen content of 4.4 – 4.8 ml/l and salinity of 0.82 – 0.84 ppm. The pH of water was in the range of 7.2 – 7.4.
Procurement of Toxicants
Morpholineum-4-Morpholine Dithiocarbamate (MMDTC) has insecticidal and fungicidal properties and used as a pesticide. It was prepared by mixing Morpholine and methyl alcohol in 1:2 proportions. Keeping the mixture in ice bath (<100C), carbon disulphide is added drop by drop with continuous agitation. Later the mixture is evaporated in room temperature to get amorphous powder of MMDTC.
Copper sulphate (CuSO4. 5H2O) ‘AR’ grade supplied by Sarabhai Merck and Zinc sulphate ‘AR’ Grade supplied by Qualigen were used for experiment.
Experimental Design
Fish were divided into groups of ten each and exposed to the different toxicants viz. MMDTC, CuSO4 and ZnSO4 independently and synergistically. In the synergistic toxicity experiment, the fish were exposed to two toxicants together (combined synergism).
Experimental Groups
Fingerlings of Catla catla were treated with pesticide and heavy metals individually and synergistically to determine 96 hr LC50 values for the test toxicants. Groups of fish were maintained in separate tanks and considered as experimental groups which were categorized as follows:
Group I: Independent Toxicity
MMDTC (DTC)
Copper sulphate (CuSO4)
Zinc sulphate (ZnSO4)
Group II: Combined Synergism
DTC + CuSO4
DTC + ZnSO4
CuSO4 + ZnSO4
Determination of LC50
To determine LC50 for the different groups based on the cumulative percentage mortality at the end of 96 h of experimentation the standard graphic method was followed. One fourth of LC50 values obtained from the above experiments was taken as the sub-lethal concentration (SLC). Apparently normal and healthy catla fingerlings were exposed to respective SLC of MMDTC (DTC), CuSO4, ZnSO4 for independent toxic analysis, DTC + CuSO4 ,DTC + ZnSO4. CuSO4 + ZnSO4 for combined toxic analyses. Suitable controls were maintained in normal bore well water for all the experimental groups, without dissolving the chemicals.
Biochemical Analyses
Biochemical parameters like total carbohydrates, total proteins and total lipids were analyzed on zero day control and on 4th, 7th, 14th, 21st and 28th day after exposing the fish with the toxicants in the experimental groups of independent toxic exposure and combined synergistic toxic exposure.
For the third group involving successive synergistic toxic exposure, these analyses were done on zero day and 7th day post exposure.
Four different tissues Muscle, Liver, Brain and Kidney were dissected out carefully and weighed using K-Roy Single pan electrical balance for biochemical investigation. The dissected tissues were kept in an ice box till taken out for homogenization.
Colorimetric method was followed for the biochemical analyses using Spectronic-21 (Bausche and Lomb) spectrophotometer. Total sugars was estimated by anthrone method10 and the total protein content in the tissues was done by folin phenol method11 while for the estimation of lipids the method of Bligh and Dyer 12 was followed.
Statistical Analysis
The data obtained were subjected to statistical analysis to arrive at Arithmetic Mean, Standard Deviation and standard error of mean using statistical package software SPSS version 16. To test the significance of differences observed between control and experimental groups, the data was subjected to students ‘t” test.
Results
The analysis of 96 h LC50 by arithmetic graphic method, showed 16 ppm (Fig. 1), 4.8 ppm (Fig.2) and 160 ppm (Fig.3) concentrations of MMDTC, CuSO4 and ZnSO4 respectively. Further, the SLC (1/4th of LC50 value) for MMDTC, CuSO4 and ZnSO4 were calculated as 4 ppm, 1.2 ppm and 40 ppm respectively for independent toxicity.
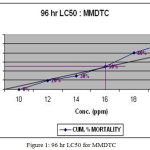 |
Figure 1: 96 hr LC50 for MMDTC
|
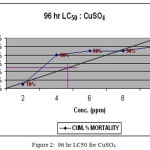 |
Figure 2: 96 hr LC50 for CuSO4
|
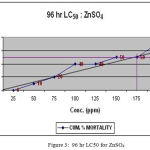 |
Figure 3: 96 hr LC50 for ZnSO4
|
The LC50 value for synergistic toxicity were calculated as 10ppm of MMDTC with 4.4ppm of CuSO4 (Fig.4), 5ppm of MMDTC and 24ppm of ZnSO4 (Fig.5) and 2.6 ppm of CuSO4 with 10ppm of ZnSO4 (Fig. 6). ¼ th these values were calculated as SLC.
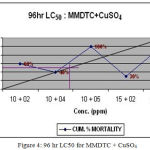 |
Figure 4: 96 hr LC50 forMMDTC + CuSO4
|
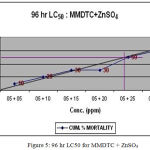 |
Figure 5: 96 hr LC50 for MMDTC + ZnSO4
|
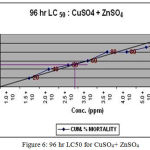 |
Figure 6: 96 hr LC50 for CuSO4+ ZnSO4
|
The total sugar (Table 1), protein (Table 2) and lipid (Table 3) content in the muscle, liver, brain and kidney tissues of C. catla healthy untreated and treated with MMDTC, CuSO4 and ZnSO4 for independent toxic effects and with MMDTC and CuSO4, MMDTC and ZnSO4 and CuSO4 with ZnSO4 for syngergistic toxic effects were recorded at 4th, 7th, 14th, 21stand 28th days.
Table 1: Independent and combined toxic effects of MMDTC, CuSO4, ZnSO4 , MMDTC+ CuSO4 , MMDTC + ZnSO4 CuSO4 + ZnSO4on total sugar (Mg/G wet Wt) of Catla catla
| Days of exposure | MMDTC (SLC-4ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 0.97 ± 0.11 | 26.25 ± 1.1 | 1.13 ± 0.15 | 2.86 ± 0.4 | ||
| 4th day | 0.55 ± 0.13* | 5.77 ± 2.13* | 1.56 ± 0.1* | 4.67 ±1.16* | ||
| 7th day | 2.18 ± 0.72* | 26 ± 1.6a | 2.38 ± 0.37* | 1.87 ± 0.23* | ||
| 14th day | 0.45 ± 0.02* | 26.35 ± 1.12a | 3.05 ± 0.34* | 6.65 ± 0.44* | ||
| 21st day | 0.57 ± 0.13* | 31 ± 1.1* | 2.26 ± 0.44* | 4.22 ± 0.37* | ||
| 28th day | 1.07 ± 0.15a | 21.55 ± 0.56* | 3.91 ± 0.45* | 3.21 ±0.3d | ||
| Days of exposure | CuSO4 (SLC-1.2ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 0.97± 0.11 | 26.25 ± 1.1 | 1.13 ± 0.15 | 2.86 ± 0.4 | ||
| 4th day | 3.05 ± 1.25* | 12.3 ± 3.01* | 11.28 ± 2.55* | 27.94 ± 2.7* | ||
| 7th day | 1.47 ± 0.28* | 32.11 ± 2.45* | 1.62 ± 0.23* | 13.66 ± 0.88* | ||
| 14th day | 0.44 ± 0.02* | 31.71 ± 1.25* | 1.46 ± 0.3b | 1.06 ± 0.09* | ||
| 21st day | 0.44 ± 0.03* | 15.28 ± 0.48* | 0.84 ± 0.03* | 0.63 ± 0.03* | ||
| 28th day | 1.28 ± 0.27b | 15.21 ± 0.52* | 2.25 ± 0.2* | 10.62 ± 0.8* | ||
| Days of exposure | ZnSO4 (SLC-40ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 0.97± 0.11 | 26.25 ± 1.1 | 1.13 ± 0.15 | 2.86 ± 0.4 | ||
| 4th day | 0.88 ± 0.11a | 9 ± 0.49* | 2.46 ± 0.63* | 3.16 ± 0.93a | ||
| 7th day | 1.09 ± 0.18a | 32.98 ±1.9* | 0.41 ± 0.02* | 4.43 ± 0.56* | ||
| 14th day | 0.48 ± 0.08* | 2.03 ± 0.42* | 0.83 ± 0.05* | 1.12 ± 0.1* | ||
| 21st day | 0.44 ± 0.02* | 4.33 ± 0.52* | 4.1 ±0.31* | 2.05 ± 0.32* | ||
| 28th day | 0.51 ± 0.02* | 3 ± 0.22* | 0.44 ± 0.04* | 1.02 ± 0.1* | ||
| Days of exposure | MMDTC+ CuSO4 (SLC-2.5ppm+1.1ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 0.97± 0.11 | 26.25 ± 1.1 | 1.13 ± 0.15 | 2.86 ± 0.4 | ||
| 4th day | 0.65 ± 0.09* | 3.55 ± 0.42* | 3.09 ± 0.6* | 4.53 ± 0.5* | ||
| 7th day | 0.46 ± 0.04* | 5.96 ± 0.6* | 0.47 ± 0.05* | 0.76 ± 0.1* | ||
| 14th day | 0.45 ± 0.04* | 34.32 ± 3.33* | 1.15 ± 0.34a | 1.15 ±0.36* | ||
| 21st day | 2.7 ± 0.6* | 55.69 ± 2.3* | 14.63 ± 2.3* | 24.66 ± 1.97* | ||
| 28th day | 6.54 ± 1.75* | 42.56 ± 5.81* | 20.77 ± 4.03* | 24.82 ± 4.78* | ||
| Days of exposure | MMDTC+ZnSO4 (SLC-1.25ppm+6ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 0.97± 0.11 | 26.25 ± 1.1 | 1.13 ± 0.15 | 2.86 ± 0.4 | ||
| 4th day | 0.42 ± 0.05* | 2.65 ± 0.6* | 1.85 ± 0.59b | 2.68 ± 0.49a | ||
| 7th day | 0.46 ± 0.05* | 1.87 ± 0.47* | 5.21 ± 0.4* | 8.07 ± 0.64* | ||
| 14th day | 0.85 ± 0.08c | 25.75 ± 2.5a | 3.34 ± 0.51* | 3.2 ± 0.51a | ||
| 21st day | 1.18 ± 0.52a | 4.09 ± 0.54* | 2.9 ± 0.5* | 1.28 ± 0.28* | ||
| 28th day | 3.41 ± 0.66* | 79.97 ± 1.95* | 7.73 ± 1.4* | 4.73 ± 1.04b | ||
| Days of exposure | CuSO4+ ZnSO4 (SLC-0.65ppm+2.5ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 0.97± 0.11 | 26.25 ± 1.1 | 1.13 ± 0.15 | 2.86 ± 0.4 | ||
| 4th day | 0.44 ± 0.07* | 2.86 ± 0.34* | 3.8 ± 0.36* | 15.65 ± 0.75* | ||
| 7th day | 0.44 ± 0.03* | 23.4 ±1.6* | 4.15 ± 1.21* | 6.65 ±0.87* | ||
| 14th day | 5.83 ± 1.8* | 36.27 ±3.64* | 8.38 ± 1.9* | 9.72 ± 1.85* | ||
| 21st day | 0.49 ± 0.08* | 1.07 ±0.437* | 0.5 ± 0.04* | 0.76 ± 0.04* | ||
| 28th day | 3.07 ± 0.79* | 9.9 ± 2.131* | 2.13 ± 0.56* | 1.01 ± 0.26* | ||
Student t -Test * P<0.001; a P N.S; b P<0.01; c P<0.02; d p<0.05
Table 2: Independent and combined toxic effects of MMDTC, CuSO4, ZnSO4, MMDTC+ CuSO4, MMDTC + ZnSO4, CuSO4 + ZnSO4 on total protein (Mg/G wet Wt) of Catla catla
| Days of exposure | MMDTC (SLC-4ppm) | ||||||
| Muscle | Liver | Brain | Kidney | ||||
| Control | 85.38 ± 0.75 | 72.31 ± 6.8 | 53.71 ± 6.63 | 66.84 ± 4.28 | |||
| 4th day | 80. 05 ± 3.79 | 106.21 ± 7.82 | 59.41 ± 6.22 | 131.46 ± 0.79 | |||
| 7th day | 172.63 ± 2.74 | 154.23 ± 2.36 | 136.42 ± 1.84 | 194.73 ± 3.17 | |||
| 14th day | 91.75 ±1.05 | 76.41 ± 1.33 | 60.25 ± 1.04 | 109.36 ±1.24 | |||
| 21st day | 120.56 ± 1.59 | 117.84 ± 0.93 | 123.86 ± 1.09 | 203.17 ± 1.49 | |||
| 28th day | 82.32 ±1.56 | 83.52 ± 1.92 | 94.85 ± 1.22 | 114.23 ±1.90 | |||
| Days of exposure | CuSO4 (SLC-1.2ppm) | ||||||
| Muscle | Liver | Brain | Kidney | ||||
| Control | 85.38 ± 0.75 | 72.31 ± 6.8 | 53.71 ± 6.63 | 66.84 ± 4.28 | |||
| 4th day | 82.21 ± 2.018 | 38.21 ±1.41 | 27.52 ± 2.05 | 48.25 ± 1.18 | |||
| 7th day | 92.08 ± 2.70 | 104.91 ± 2.61 | 87.04 ± 0.91 | 161.27 ± 1.29 | |||
| 14th day | 96.88 ± 1.38 | 87.31 ±1.2 | 71.56 ± 0.91 | 121.39 ± 3.46 | |||
| 21st day | 61.01 ± 1.27 | 55.95 ±1.5 | 50.17 ± 1.25 | 94.34 ± 2.89 | |||
| 28th day | 118.79 ± 1.72 | 89.33 ±1.68 | 88.7 ± 2.17 | 166.31 ± 1.92 | |||
| Days of exposure | ZnSO4 (SLC-40ppm) | ||||||
| Muscle | Liver | Brain | Kidney | ||||
| Control | 85.38 ± 0.75 | 72.31 ± 6.8 | 53.71 ± 6.63 | 66.84 ± 4.28 | |||
| 4th day | 99.78 ± 1.86 | 35.24 ±1.18 | 19.93 ± 1.54 | 8.61 ± 0.92 | |||
| 7th day | 101.67 ± 0.58 | 112. 3 ± 1.37 | 65.93 ± 1.93 | 197.32 ± 1.2 | |||
| 14th day | 30.44 ± 1.02 | 29.89 ± 0.84 | 64.51 ± 2.55 | 35.87 ± 0.6 | |||
| 21st day | 65.69 ± 1.42 | 106.11 ± 2.25 | 102.28 ± 1.44 | 107.4 ± 0.99 | |||
| 28th day | 72.01 ± 2.42 | 65.6 ± 2.01 | 75.12 ± 2.6 | 104.45 ± 2.8 | |||
| Days of exposure | MMDTC+ CuSO4 (SLC-2.5ppm+1.1ppm) | ||||||
| Muscle | Liver | Brain | Kidney | ||||
| Control | 85.38 ± 0.75 | 72.31 ± 6.8 | 53.71 ± 6.63 | 66.84 ± 4.28 | |||
| 4th day | 30.79 ± 0.68 | 46.65 ± 1.2 | 32.01 ± 0.99 | 61.11 ± 0.82 | |||
| 7th day | 77.64 ± 3.42 | 96.13 ± 2.5 | 93.52 ± 3.85 | 124.26 ± 4.19 | |||
| 14th day | 107.32 ± 4.32 | 76.09 ± 5.13 | 83.81 ± 5.07 | 144.53 ± 5.25 | |||
| 21st day | 152.95 ± 2.63 | 72.59 ± 4.3 | 60.83 ± 3.63 | 72.74 ± 2.83 | |||
| 28th day | 144 ± 3.87 | 113.69 ±3.67 | 144.18 ± 7.62 | 144.08 ± 3.44 | |||
| Days of exposure | MMDTC+ZnSO4 (SLC-1.25ppm+6ppm) | ||||||
| Muscle | Liver | Brain | Kidney | ||||
| Control | 85.38 ± 0.75 | 72.31 ± 6.8 | 53.71 ± 6.63 | 66.84 ± 4.28 | |||
| 4th day | 25.38 ± 0.66 | 71.67 ± 0.74 | 37.75 ± 1.25 | 117.15 ± 3.55 | |||
| 7th day | 97.44 ± 3.21 | 128.22 ± 4.83 | 124.06 ± 4.58 | 125.65 ± 9.44 | |||
| 14th day | 124.59 ± 2.84 | 87.21 ± 2.9 | 84.39 ± 3.16 | 114.36 ± 2.75 | |||
| 21st day | 168.35 ± 3.2 | 125.23 ± 6.67 | 153.25 ± 4.71 | 112.84 ± 4.26 | |||
| 28th day | 67.33 ± 1.343 | 90.15 ± 3.69 | 40.39 ± 2.88 | 100.06 ± 3.77 | |||
| Days of exposure | CuSO4+ ZnSO4 (SLC-0.65ppm+2.5ppm) | ||||||
| Muscle | Liver | Brain | Kidney | ||||
| Control | 85.38 ± 0.75 | 72.31 ± 6.8 | 53.71 ± 6.63 | 66.84 ± 4.28 | |||
| 4th day | 102.84 ± 4.44 | 77.31 ± 1.05 | 48.59 ± 1.13 | 101.29 ± 0.74 | |||
| 7th day | 108.62 ± 1.64 | 102.89 ± 1.56 | 82.39 ± 1.98 | 133.35 ± 3.68 | |||
| 14th day | 120.08 ± 3.52 | 108.65 ± 4.26 | 108.48 ± 3.34 | 228.89 ± 5.46 | |||
| 21st day | 174.88 ± 4.99 | 152.01 ± 6.69 | 161.79 ± 5.01 | 180.23 ± 5.12 | |||
| 28th day | 112.35 ± 3.83 | 113.12 ± 3.58 | 101.5 ±2.28 | 96.3 ± 3.99 | |||
Table 3: Independent and combined toxic effects of MMDTC, CuSO4, ZnSO4 , MMDTC+ CuSO4 , MMDTC + ZnSO4 CuSO4 + ZnSO4on total lipid (Mg/G wet Wt) of Catla catla
| Days of exposure | MMDTC(SLC-4ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 31.62 ± 0.72 | 55.37 ± 1.81 | 42.11 ± 0.91 | 23.97 ± 1.21 | ||
| 4th day | 8.63 ± 1.85 * | 8.38 ± 1.08* | 7.55 ± 1.39* | 4.76 ± 0.55* | ||
| 7th day | 19.76 ± 1.17 * | 102.56 ± 1.61* | 85.24 ± 2.06* | 143.19 ± 2.31* | ||
| 14th day | 61.61 ± 1.14 * | 147.66 ± 1.39* | 151.96 ± 1.23* | 294. 81 ± 2.04* | ||
| 21st day | 118.4 ± 0.91 * | 141.78 ± 1.59* | 175.76 ± 1.79* | 181.03 ± 1.26* | ||
| 28th day | 56.99 ±1.01 * | 139.34 ± 1.38* | 143.28 ± 1.81* | 141.76 ± 1.36* | ||
| Days of exposure | CuSO4 (SLC-1.2ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 31.62 ± 0.72 | 55.37 ± 1.81 | 42.11 ± 0.91 | 23.97 ± 1.21 | ||
| 4th day | 3.71 ± 0.24 * | 33.75 ± 4.71* | 10.3 ± 3.04* | 2.7 ± 0.5* | ||
| 7th day | 114.67 ± 0.92 * | 202.8 ± 1.77* | 144.03 ± 2.17* | 244.7 ± 4.71* | ||
| 14th day | 71.42 ± 0.85* | 138.37 ± 0.85* | 184.25 ± 5.72* | 143.43 ± 1.59* | ||
| 21st day | 47.19 ± 0.84* | 184.37 ± 1.67* | 242.39 ± 1.62* | 196.69 ± 2.41* | ||
| 28th day | 41.01 ±1.64 * | 83.7 ± 2.67* | 117.08 ± 1.53* | 170.42 ± 1.34* | ||
| Days of exposure | ZnSO4 (SLC-40ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 31.62 ±0.72 | 55.37 ± 1.81 | 42.11 ± 0.91 | 23.97 ± 1.21 | ||
| 4th day | 1.41 ± 0.25* | 20.87 ± 0.86* | 7.01 ± 0.13* | 1.91 ± 0.29* | ||
| 7th day | 31.84 ± 0.72 a | 110.92 ± 1.13* | 181.44 ± 0.89* | 85.44 ± 1.16* | ||
| 14th day | 47.48 ± 0.66 * | 113.89 ± 1.78* | 137.6 ± 1.25* | 103.83 ± 1.34* | ||
| 21st day | 77.01 ± 1.21 * | 131.23 ± 0.9* | 242.16 ± 2.13* | 191.13 ± 2.2* | ||
| 28th day | 76.13 ± 2.78* | 99.93 ± 2.12 * | 156.33 ± 3.16* | 340.14 ± 1.31* | ||
| Days of exposure | MMDTC+ CuSO4 (SLC-2.5ppm+1.1ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 31.62 ±0.72 | 55.37 ± 1.81* | 42.11 ± 0.91 | 23.97 ± 1.21 | ||
| 4th day | 6.07 ± 0.12 * | 27.84 ± 0.76* | 5.13 ± 0.19* | 3.11 ± 0.638* | ||
| 7th day | 27.18 ± 2.79 * | 66.67 ± 2.06* | 70.81 ± 1.95* | 53.17 ± 2.63* | ||
| 14th day | 45.63 ±2.52 * | 83.67 ± 1.82* | 88.65 ± 3.58* | 69.01 ± 2.67* | ||
| 21st day | 76.92 ± 3.29* | 126.84 ± 5.47* | 61.32 ± 4.71* | 97.48 ± 3.41* | ||
| 28th day | 48.51 ± 4.49 * | 96.24 ± 3.9* | 172.52 ± 2.98* | 100.73 ± 4.81* | ||
| Days of exposure | MMDTC+ZnSO4 (SLC-1.25ppm+6ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 31.62 ±0.72 | 55.37 ± 1.81 | 42.11 ± 0.91 | 23.97 ± 1.21 | ||
| 4th day | 5.91 ± 0.45* | 23.64 ± 0.72* | 4.65 ± 0.44* | 2.81 ± 0.41* | ||
| 7th day | 28.6 ± 3.47c | 65.67 ± 4.59* | 82.6 ± 5.44* | 53.46 ± 3.75* | ||
| 14th day | 49.09 ± 4.07 * | 96.44 ± 1.95* | 134.31 ± 3.21* | 93.48 ± 5.26* | ||
| 21st day | 81.88 ± 7.47 * | 67.31 ± 3.75* | 244.31 ± 4.02* | 96.28 ± 3.80* | ||
| 28th day | 143.87 ± 3.09* | 164.54 ± 2.35* | 211.89 ± 2.14* | 301.95 ± 5.78* | ||
| Days of exposure | CuSO4+ ZnSO4 (SLC-0.65ppm+2.5ppm) | |||||
| Muscle | Liver | Brain | Kidney | |||
| Control | 31.62 ±0.72 | 55.37 ± 1.81 | 42.11 ± 0.91 | 23.97 ± 1.21 | ||
| 4th day | 4.62 ± 0.46 * | 22.85 ± 0.79* | 3.84 ± 0.5* | 2.06 ± 0.33* | ||
| 7th day | 53.27 ± 3.53 * | 75.68 ± 2.78* | 94.56 ± 1.96* | 108.02 ± 3.56* | ||
| 14th day | 57.51 ± 3.13 * | 158.26 ± 2.85* | 167.08 ± 3.69* | 6.41 ± 1.07* | ||
| 21st day | 48.46 ± 3.36 * | 53.84 ± 4.06a | 115.72 ± 5.11* | 86.5 ± 3.85* | ||
| 28th day | 38.93 ±1.87 * | 42.89 ± 1.89* | 66.95 ± 4.03* | 38.24 ± 6.65* | ||
Student t -Test * P<0.001; a P N.S; b P<0.01; c P<0.02; d p<0.05
The mean value of the carbohydrate content in muscle of the fishes treated with MMDTC was significantly higher on 7th day, when compared to control group. In liver the elevated level of the sugars was noticed on 21st day (p<0.001). A significant increase in levels of sugar content were observed in brain and kidney tissues on 14th, 21st and 28th day. The heavy metal CuSO4 exposed fish recorded a significant increase of the sugar content in muscle on 4th and 7th day. In liver a higher significant value of the average was recorded on 7th and 14th day. A significant increase over the values of control fish on 4th, 7th and 28th day was observed in the sugar content of brain and kidney. When the fishes were exposed to ZnSO4 the sugar content of muscle and liver was greatly reduced on all the exposure groups except on 7th day, where the significant increase was observed. However, a significant increase were recorded on 4th and 21st day in the brain tissues and 4th and 7th day in the kidney tissues. MMDTC when combined with CuSO4, and ZnSO4 revealed the reduction of the sugar content in the muscle tissues on 4th, 7th and 14th day. However, in liver tissues a significant increase in the average of the sugar content was observed on 14th, 21st and 28th day in fishes exposed to MMDTC with CuSO4, while MMDTC with ZnSO4 brought about a significant decline on 4th, 7th and 21st day. In brain and kidney tissues the synergistic effect of MMDTC and CuSO4 revealed significantly elevated level of sugars on 4th, 21st and 28th day,while MMDTC with ZnSO4 significantly decreased the sugar content in kidney tissues on 21st day. Exposure of CuSO4 in addition to ZnSO4 resulted in a significant decrease in the carbohydrate content of the muscle and liver tissue on 4th, 7th and 21st day. The additive effect of these two compounds effected elevated level of carbohydrates from 4th to 14th day in the brain and kidney.
The Protein content in the muscle of catla fingerlings exposed to MMDTC was significantly decreased on 4th day and 28th day while in the liver, brain and kidney tissues of treated fish showed a significant increase in the protein content on all the exposure periods. The heavy metal CuSO4 treated fish revealed significant depletion of protein on 4th and 21st day in the muscle, liver, brain tissues and on 4th day in the kidney tissues. A reduction in the protein content was recorded in the muscle and liver tissues of ZnSO4 exposed fish on 14th and 28th days, while in brain and kidney tissues an increase was observed on 7th. 21st and 28th day.
The protein content in the muscle and liver tissues of combined toxic synergistic group MMDTC and metals viz. CuSO4 and ZnSO4 reduced on 4th day and continued to rise on subsequent days up to 21st day and again dropped on the 28th day. In brain and kidney tissues the protein content of MMDTC+CuSO4 exposed group showed a significant decrease on the 4th day and MMDTC with ZnSO4 group showed a significant increase from 7thto 21st days. When the two metallic compounds were combined together the fish recorded progressively increased protein content in all the tissues viz. muscle, liver , brain and kidney compared to the control.
Exposure to the toxicants MMDTC, CuSO4 and ZnSO4 independently and in combination with one another had effected a very significant reduction in the lipid content in all the tissues on the early period of exposure (4th day). However, the lipid content of the muscle on the 7th day decreased in the fish exposed to MMDTC independently and in combination with CUSO4 and ZnSO4 while an increased trends was observed in groups of fish treated with the heavy metals both independently and combined together. Contrastingly,the lipid content of liver showed a decreasing trend in the fish exposed to CuSO4 and ZnSO4 together on 21st and 28th day. The lipid content of brain and kidney tissues showed significant elevation in the lipid content in all the treated groups on all the exposure periods.
Discussion
Pesticides are mainly used against pests of crop and disease causing vectors, but their improper use in agricultural practice has posed a serious threat to human life and his environment. Any variation in the environment acts as a stress on the organisms. When a pesticide or any pollutant reaches the aquatic ecosystem, the fish are exposed to severe stress and as a natural instinct, the fish tend to adapt themselves by reacting suitably to overcome the stress. When two toxicants act together, the stress shall be more and severe and cause irreparable damage.
In the present investigation, total sugar content in the muscle,liver, brain and kidney revealed a mixed trend in the different experimental groups and different days of the exposure. There was an immediate fall in the sugar content of the 4 th day exposed fish in the muscle and liver, showing a state of recovery on the 7 th day. The recovery trend was normal in DTC exposed fish, and slow in the CuSO4 treated fish. In the synergistic experimental groups, recovery occurred from 21st day onwards. In the brain and kidney of the 4th day experimental groups the sugar content was more compared to control groups, which gradually reduced on 7 th day. The increased or decreased trend was more pronounced in the groups of fish exposed to CuSO4 independently and synergistically with MMDTC and ZnSO4.This shows the severe toxic nature of CuSO4 both independently and in combination with others.
Protein showed a trend of increasing and decreasing activity in the different experimental groups. As far as lipids were concerned there was a reduction in all the tissues of 4 th day experimental group showing trends of recovery on further days of exposure. Increase in the protein content was in accordance with the reduction of sugar content and lipid content suggesting gluconeogenic pathway utilizing lipid and proteins to compensate for the loss of sugars.
Depletion of tissue proteins in fishes exposed to various pesticide toxicants have been reported by many workers. Further, it has been reported that acute or chronic treatment of pesticides cause biochemical alteration in the organs involved in detoxification mechanisms.13,14,15 Decreasing trend in total proteins was reported in the liver,brain and gill tissues of C. catla under sub lethal and lethal concentrations of fenvalerate16. A significant decrease was reported in the protein content in almost all tissues in Ctenophayngodon idellus when exposed to sub lethal and lethal concentrations of both the technical and 20% E.C. formulations offenvalerate.17 Break down and synthesis of protein proceeds simultaneously in all the tissues. But during pesticide stress breakdown of protein occurs which acts as an alternative source of energy. The fish exposed to toxic stress stimulates protein metabolism.18 During protein metabolism the removal of amino group from different amino acids was observed suggesting the elevated levels of amino acids in the fish exposed to pesticide.19
The loss of protein in the tissues of fish exposed to heavy metal stress may be due to excessive proteolysis to overcome the metabolic stress. Heavy metals may alter the protein concentration through impairing the synthesis and metabolism of protein, DNA and RNA as well as by altering the activity of lysosomal enzymes. It is possible that pollutant stress influences the conversion of tissue proteins into soluble fractions reaching the blood and hence, decrease in protein content might have observed in the tissues of liver and muscle. The metal binding protein usually binds the metal ions,preventing them from exerting toxic effects through binding to enzymes or other sensitive sites. However, if the rate of influx of metals into the cell exceeds the rate at which metallothionein or metallothionein-like proteins can be synthesized, there may occur a“spill over” of metals from the metallothionein or metallothionein like proteins due to the displacement of essential metals from metalloenzymes by non-essential metals.20
The changes observed in the protein content in different days of exposure may be due to the influence of exogenous factors like toxic environment. The loss of protein under stress to long period may be attributed to the utilization of amino acid in various catabolic reactions. Another probability is that there might have occurred blocking of the protein synthesis and proteolysis on exposure to chronic period of stress condition. Similar observations of decrease in the protein content were reported in the muscle of Channa punctatus treated with heavy metal,21 in the muscle of Puntius stigma after exposure with pesticides,22 in the muscle, liver and intestine of Cyprinus carpio treated with textile mill effluent,23 in the muscle of Oreochromis mossambicus due to the effect of phenol,24 in Etroplus maculates exposed to Ekalux25 and in Anabas testudineus under the influence of alloxan monohydrate.26
Heavy metals are metabolic inhibitors of animals. They exert toxic effects in the organism at tissue, cellular, sub-cellular and molecular levels. At molecular levels metals interact with protein leading to denaturation, precipitation, allosteric effects or enzyme inhibition. Depletion in total protein could be due to augmented proteolysis and possible utilization of their product for metabolic purpose, decline in protein content may be related to impaired food intake, increased energy cost of homeostasis,tissue repair and detoxification mechanism during stress.27
Conclusions
The study clearly indicates that the toxic effect of pollutants or toxicants will be more when they act together in a synergistic manner. The alterations of biochemical profiles of the test organism as observed in the present study may be used as non–specific biomarkers against anthropogenic stress.
References
- Karchmer J. H. Analytical Chemistry of sulphur and its compounds. Part II, Willey Interscience, New york. 1972.
- Bandyopadhyay B. K. Aquatic pollution with special reference to the effect of pesticide and insecticide on fish and fisheries. Fish-Chimes. 1995;15(3):27-30.
- Bhattacharya S. Stress response to pesticides and heavy metals in fish and other vertebrates. Proc. India Acad. Sci. 2001;673(5):215-246.
- Boeck (De) G., Dewachter B., Vlaeminck A and Blust R. Effect of cortiosol treatment and a sublethal copper exposure on copper uptake and heat shock protein levels in common carp cyprinus carpio. Environ. Toxicol. Chem. 2003;22:1122-1126.
CrossRef - Naskar R., Sen N. S and Firoz M. A. Physiological response of an air breathing teleost, Clarias batrachus (linn.) to sublethal aluminium toxicity in acidic soft water, Proc. Zool. Soc. 2004;57(1):29-34.
- Sheela M., Mallika G and Muniandy S. Impact of zinc on the energetics of feeding and biochemical status of tissues in the fish. Oreochromis mossambicus. Environ. Ecol. 2004;13(2):418-422.
- Kurant V. Z and Arsa O. M. Zinc effect on the content of proteins and nucleic acids in the common carp tissues. Hydrobiological J. 1991;27(6):45-48.
- Radhakrishnaiah K., Venkataramna P., Suresh A and Ramakrishna B. S. Effects of lethal and sublethal concentrations of copper on glycolysis in liver and muscle of the freshwater teleost Labeo rohita(Ham.). J. Environ. Biol. 1992;13(10):63-68.
- Omprakasam M and Manohar L. Experimental infection of some bacterial fish pathogens in the cichlid fish, Oreochromis mossambicus. Indian. J. Fish. 1991;38(2):106-110.
- Carrol W. V., Longley R. W and Roe J. H. The determination of glycogen in the liver and muscle by the use of antrone reagent. J. Biol. Chem. 1956;220:583-593.
- Lowry O. H., Rosebrough N. J., Farr A. L and Randall R. J. Protein measurement with Folin Phenol reagent.J. Biol. Chem. 1951;193:265-275.
- Jayaraman J. (Blight and Dyer method) in Laboratory Manual in Biochemistry Willey eastern Ltd. New Delhi. 1988.
- Maruthi A., Ramakrishna Rao Y. S and Rao M. V. S. Effect of Sugar mill effluents on oxygen consumption of freshwater fish, Channa puctatus using flow through system. J. Nature. Conser. 1999;11(2):227-232.
- Rani A. S., Sudharsan R., Reddy T. N., Reddy P. U M and Raju T. N. Effect of arsenite on certain aspects of protein metabolism in the freshwater teleost,Tilapia mossmabica (Peters). J. Environ. Bio. 2001;22(2):101-104.
- Rao A. P and Rao P. V. V. P. Pollution potential of sago industry: A case study. J. Ecotoxicol. Environ. Monit. 2002;12(1):53-56.
- AnithaSussan T., Veeraiah K and Tilak K. S. A study on the bio-accumulation of fenvalerate, a synthetic pyrethroid, in the whole body tissue of Labeo rohita. Catla catla, Cirrhinus mrigala (Ham.). by gas liquid chromatography
Pollu. Res. 1999;18(1):57-59. - Tilak K. S., Veeraiah K and Yacobu K. Studies of histopathological changes in the gill, liver and kidney of Ctenopharyngodon idellus (valenciennes) exposed to technical fenvalerate and EC 20%. Poll. Res. 2001;20(3):387-393.
- Kabeer A. S. I., Rao K. S J and Rao K. V. R. Effect of Malathion exposure on some physical parameters of whole body and on tissues of Teleost Tilapia mossambica (Peters). J. Biosci. 1981;3(1):17-21.
CrossRef - Tilak K. S., Satyavardhn K and Thathaji P. B. Biochemical changes induced by fenvalerate in the freshwater fish Channa punctatus. J. Ecotoxicol. Environ. Monit. 2003;13(4):261-270.
- Neha B., Vanhekede G. N and Dhande R. R. Heavy metal induced Biochemical alterations in the freshwater fish Labeo rohita. J. Ecotoxicol. Environ. Monit. 2004;14(1):1-7.
- Jana S. R and Bandyopadhyaya N. Effect of heavy metals on some biochemical parameters in the freshwater fish,Channa puntatus. Environ. Ecol. 1987;5(3): 488-493.
- Khillare Y. K and Wagh S. B. Effects of endosulfan, malathion and sevin on biochemical constituents of the fish Puntius stigma. Environ. Ecol. 1989;7(1):66-69.
- Rajan M. R. Sublethal effects of textile mill effluents on protein, carbohydrate and lipid content of different tissue of the fish Cyprinus carpio. Environ. Ecol. 1990;8(1):54-58.
- Ravichandran S., Midhunashanthi K and Indra N. Impact of phenol on protein metabolism in the freshwater fish Oreochromis mossambicus. Ecotoxicol. Environ. Monit. 1994;4(1):033-037.
- Nelson J. D and Sunilkumar G. Effect of Ekalux on biochemical parameters in the freshwater fish Etroplus maculates. J. Ecotoxicol. Environ. Monit. 1996;6(1):065-067.
- Bhaskar G. Effect of alloxan monohydrate administration on the biochemical constituents of the bony fish, Anabas testudineus (Bloch). J. Ecotoxicol. Environ. Monit. 1997;7(1):027-032.
- Saraswathi K., Ramesh M., Maruthappan V and Noortheen A. Influence of a pyrethroid insecticide fenvalarate secondary stress responses in a freshwater teleost fish Cyprinus carpio, Var. Communis. Ind. J. Environ. Toxicol. 2004;14(1):13-18.








