M. Ricky Ramadhian1 and Khairil Pahmi2
and Khairil Pahmi2
1Department of Microbiology and Parasitology, Faculty of Medicine, Universitas Lampung, Lampung, Indonesia.
2Department of Pharmacy, Diploma of Pharmacy, Faculty of Health Sciences, Universitas Nahdlatul Wathan, Mataram, Indonesia.
DOI : https://dx.doi.org/10.13005/bpj/1303
Abstract
Non-communicable disease have became pandemic disease in developing countries, such as cardiovascular diseases (CVD), diabetes, chronic obstructive pulmonary diseases (COPD) and cancers. Epidemiological studies have confirmed a positive correlation between salt intake and elevated blood pressure in up to half of patients with hypertension. The fibrotic process is associated with renin-angiotensin-aldosteron system, transforming growth factor-β (TGF-β), connective tissue growth factor (CTGF), matrix metalloproteinases and peroxisome proliferator-activated receptor gamma (PPARγ). Telmisartan is antihypertensive drug that not only blocks angiotensin receptor but also leads to the decrease of blood pressure, activates peroxisome proliferator activated receptor gamma (PPAR-γ) and presumeably inhibits expression of transforming growth factor beta-1 (TGF-β1). Does telmisartan inhibit thickness of aortawall in excessive NaCl-induced Wistar rats are studied in this experiment. Twenty five male Wistar 2,5-3 months of age and 100 – 150 g BW rats were used in this experiment. They were grouped into 5, each contain of rats. Group I (G I) as placebo did not receive NaCl 8% nor telmisartan. G II received NaCl 8% only. G III, IV and V received NaCl 8% and telmisartan 3, 6 and 12 mg/ kg BW. The treatments were given every day for 8 weeks. At the day of 56 all rats were sacrificed by mean of neck dislocation and operated to take aorta. The thickness aortic wall were analyzed with imageJ 1.49c. Data were expressed as mean ± standard deviation. They were analyzed by parametric test (ANOVA) that followed by Post Hoc test for comparison of multiple groups or nonparametric test (Kruskal-Wallis). A value of p<0.05 was considered statistically significant. Ratio of aortawall thickness to lumen diameter in rat decreased significantly on telmisartan-given rat groups comparing NaCl 8% only (p<0,05). In conclusion, ratio of aortawall thickness ratio to lumen diameter were higher in aorta of NaCl 8% only male Wistar rats comparing to telmisartan-given male Wistar rats.
Keywords
NaCl; Telmisartan; Thickness Aorta
Download this article as:| Copy the following to cite this article: Ramadhian M. R, Pahmi K. The Effects of Telmisartan to Thickness of Aortic Tunica Media in 8% Sodium Chloride-Induced Wistar Rats (Rattus Norvegicus). Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Ramadhian M. R, Pahmi K. The Effects of Telmisartan to Thickness of Aortic Tunica Media in 8% Sodium Chloride-Induced Wistar Rats (Rattus Norvegicus). Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17298 |
Introduction
Non-communicable disease have became pandemic disease in developing countries, such as cardiovascular diseases (CVD), diabetes, chronic obstructive pulmonary diseases (COPD) and cancers.1 Epidemiological studies have confirmed a positive correlation between salt intake and elevated blood pressure in up to half of patients with hypertension.2
Hypertension is the most common cardiovascular disease and is a major public health problem in developed countries and developing countries. Although generally easily diagnosed, hypertension can cause death due to complications of untreated. Hypertension caused 6% of deaths in adults in the world.3 Based on the World Health Organization (WHO) and the International Society of Hypertension (ISH) in 2003, there were 600 million people with hypertension worldwide, and three million of them die every year.4-5
Increased blood pressure in the long term will cause a variety of complications may include stroke, renal arteriosclerosis, heart failure, disorders of the blood vessels in the retina and others.6-10 Fibrosis is one of the complications of hypertension.11-13 The process of fibrosis associated with the renin-angiotensin-aldosterone system, transforming growth factor-β (TGF-β), connective tissue growth factor (CTGF), matrix metalloproteinases, and peroxisome proliferator-activated receptor gamma (PPARγ).14 Peroxisome proliferator-activated receptor gamma is a nuclear receptor that has three subunits, ie subunits α, β and γ. Some derivatives of lipids, especially unsaturated fatty acids are ligands of this receptor.15 Activation of PPARγ will prevent bonding between p-300 proteins with Smad proteins leading to decreased production of TGF-β1 and decrease the incidence of fibrosis.16 In addition, PPARγ would prevent bonding between Smad3 protein (R-Smad) with response elements and Co-Smad protein that will inhibit the production of TGF-β1 and decrease the incidence of fibrosis.17
Because morbidity and incidence of hypertension is still high, various types of drugs have been recommended for the control of hypertension.18 For many years conducted research on hypertension therapy, ehich targets the management of hypertension is not only limited to the control of blood pressure but also intended to prevent the complications of hypertension, where the prevention of organ damage are important in the achievement of antihypertensive drug treatment.19-20
Telmisartan is an angiotensin receptor blocker (ARB) in addition to the unique properties inhibit the angiotensin II type 1 receptor (AT1), also serves as a partial PPARγ agonist.21
The aim of this research is to know the effect of telmisartan to prevention of fibrosis aorta of Wistar rats (Rattus norvegicus) that are induced by 8% NaCl.
Materials and Method
Animal Model Maintenance and Treatment
Twenty five male Wistar 2,5-3 months of age and 100-150 gram body weight rats were used in this experiment. They were maintained in individual pen and given feed pellet and drinking water adequately. Placed in room temperature 20-24°C, dark-bright cycle for 12 hours. Before its treatment, animal model was acclimatized for maximal seven days. They were grouped into 5, each contain of rats. Group I (G I) as negative control or vehicle which did not receive NaCl 8% nor telmisartan. G II received NaCl 8% only. Group III, IV and V received NaCl 8% and telmisartan 3, 6 and 12 mg/ kg BW. The treatments were given every day for 8 weeks. At the day of 56 all rats were sacrificed by mean of neck dislocation and operated to take the aorta.22-23
Telmisartan Suspension
Fourty or eighty miligram telmisartan was crushed by mortal, and then add water until 40 mL for 40 mg telmisartan and until 80 mL for 80 mg telmisartan. Telmisartan suspension was taken by orogastric gavage suitable to rats dosage that have been determined to be entered directly to rat stomach.
Histological Analysis
Histological picture of aorta stained by picrosirius red. Aortawall collagen fraction volume and ratio of thickness aortawall to lumen diameter was determined with ImageJ software.24
Histopathology Preparation
Tissue of aorta was fixed by 10% buffer formalin for 24-48 hours at room temperature, and then dehydration was committed respectively on 75%, 80% and 95% alcohol each 45 minute followed by 100% each for 1 hour with 3× repletion. Furthermore clearing process was executed by xylene each for 1 hour with 2× repetition and then tissue was infiltrated by paraffin each 1 hour with 3× repetition. The next process, tissue was blocked in paraffin block. Tissue that had been blocked then sectioned with 4-8 µm thickness. Tissue that had been sectioned then transferred to usual object glass for picrosirius red staining. Object glass was allowed to dry for a night and keep object glass until ready to be used.
Picrosirius Red Staining Procedure
Aorta sections were deparafinized then continued to hydration process with water. Aorta sections were entered into A reagen (picric red sirius) for 1 hour. Furthermore, aorta sections were entered to absolute ethyl alcohol 3×, and then entered to xylene for 15 minutes. Aorta sections were washed and then mounted by Canada balsam.
Statictical Analysis
Data are expressed as mean ± standard deviation. They are analyzed by parametric test (one way ANOVA) that followed by post hoc test for comparison of multiple groups or nonparametric test (Kruskal-Wallis). A value of p<0.05 was considered statistically significant.
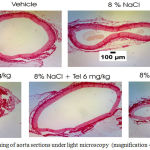 |
Figure 1: Picrosirius red staining of aorta sections under light microscopy (magnification 40×)
|
Results
Effect 8% sodium chloride and telmisartan to ratio of aortawall thickness to lumen diameter in rat aorta. All group aorta sections have been stained by picrosirius red. Aortawall thickness to lumen diameter ratio in aortic rats were quantified with ImageJ software. Aortawall thickness to lumen diameter ratio in aortic rat measurement results in Figure 2 and Table 1.
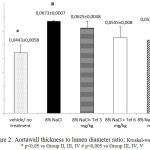 |
Figure 2: Aortawall thickness to lumen diameter ratio; Kruskal-walis p < 0,05 * p<0,05 vs Group II, III, IV # p<0,005 vs Group III, IV, V
|
Table 1: Aortawall thickness to lumen diameter ratio in aortic rats (Group I= vehicle/negative control/no treatment, Group II= positive control/8% NaCl, Group III, IV and V= 8% NaCl+ telmisartan 3, 6 and 12 mg/kg)
| Group | Mean±SD (µm) | p* |
| I | 0,0443±0,0058 | 0,00 |
| II | 0,0673±0,00076 | |
| III | 0,0625±0,0048 | |
| IV | 0,0555±0,008 | |
| V | 0,0538±0,0121 |
* Kruskal-Wallis test
Kruskal-Wallis test showed significant difference (p <0.05). Analysis Mann-Whitney test showed the group II had ratio of aortawall thickness to diameter lumen more thicker than the group III, IV, and V with significance p<0.05.
Discussion
Excessive salt intake can increase reactive oxygen species (ROS) in tissue.25-26 Xu and Liu (2013) showed that telmisartan can lower systolic arterial pressure by blocking the angiotensin II receptor I, activate P13K/Akt/eNOS and AMPK pathways, increase the release of NO and decrease oxidative stress in SHR rats.27
Ying and Sanders (1999) showed that endothelial cells and the production of TGF-β1 glomeruli in vivo is modulated by dietary salt which aortic endothelial in rats feeding with saline containing 8% causes increase in the expression of TGF-β1.28 Nataatmadja et al. (2013) showed that the administration of losartan, an angiotensin receptor blocker drugs, can decrease the expression of TGF-β on intravesikular and ekstravesikular in cultured vascular smooth muscle of aortic aneurysm.29 Wu et al. (1997) showed that the mice that carried the subtotal nephrectomy increased TGF-β1 gene transcription in the glomerulus and tubulointersisial, where the provision of valsartan and ramipril can reduce the expression of TGF-β1.30 In this research showed that the collagen volume fraction were not different in all groups. Zhu et al. (2004) showed that the administration of sodium reduces renin and angiotensin II in contrary receptor of angiotensin I increased.31 In normotensive Wistar and WKY rats administration of excess salt can cause an increase in the mass of the left heart ventricle, remodeling and fibrosis in the heart and kidney.32 Telmisartan blocks the angiotensin receptor, but also can serve as a PPARγ partial agonist.33 Angiotensin II is known to stimulate the production of TGF-β and plasminogen activator inhibitor-1, which causes an increase in the accumulation of matrix.34 Zhang et al. (2014) showed that telmisartan can inhibit collagen synthesis and metabolic imbalance of collagen.35 In this study it was found that the ratio of aortawall thickness to lumen diameter more thicker in group II than group III, IV, and V (p<0.05), thus telmisartan can inhibit aortic wall thickness. Shang et al. (2012) showed that telmisartan can inhibit the increase in the ratio of the thickness of the tunica media with the lumen (p <0.05).36 Increase of thickness of the aortic wall due to cell hypertrophy in vascular smooth muscle cells that showed by Gu et al. (1998) that administration of high concentrations of sodium can cause hypertrophy in both cells in myocardial cells and vascular smooth muscle cells.37 Cell hypertrophy suspected of protein synthesis through increased influx of sodium leads to exchange of Na+/H+, so that the cell becomes alkaline, where the pH is high increase in cell growth in several cell types.38
Acknowledgments
We gratefully acknowledge the excellent support from Prof. Dr. Mustofa., M.Kes., Apt., Dr. Muhammad Ghufron, dr. Dwi AA Nugrahaningsih, Ph.D, Prof. dr.Sofia Mubarika, M.Med.Sc., Ph.D, and Faculty of Medicine, University of Lampung.
References
- Islam S. M.S, Purnat T. D, Phuong N.T.A, Mwingira U, Schacht K & Fröschl G. Non Communicable Diseases (NCDs) in developing countries: a symposium report. Globalization and Health. 2014;10:81.
CrossRef - Orlov S.N & Mongin A. A. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2007;293:2039–2053. Cooper R, Tayo B. Chapter B74 Geographic Patterns of Hypertension: A Global Perspective. In J. Izzo, D. Sica, & H. Black, eds. Hypertension Primer. Lippincott (2008)
- WHO-ISH Hypertension Guideline Committee. Guidelines of the management of hypertension. J Hypertens. 2003;21(11):1983-92.
- Chobanian A.V, Bakris G.L, Black H.R, Cushman W.C, Green L,Izzo J.L, Jones D.W, Materson B.J, Oparil S, Wright J.T, Roccella E.J. Seventh Report of The Joint National Committee on Prevention, Detection, Evaluation, anf Treatment of High Blood Pressure. Hypertension. 2003;42:1206-52.
CrossRef - Kokkinos P, Pittaras A, Narayan P, Faselis C, Singh S, Manolis A. Exercise capacity and blood pressure associations with left ventricular mass in prehypertensive individuals. Hypertension. 2007;49(1):55-61.
CrossRef - Sipahi I, Tuzcu E.M, Schoenhagen P, Wolski K.E, Nicholls S.J, Balog C, Crowe T.D, Nissen S.E. Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J. Am Coll Cardiol. 2006;48(4):833-8.
CrossRef - Kshirsagar A, Carpenter M, Bang H, Wyatt S.B, Colindres R.E. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006;119(2):133-41.
CrossRef - Nguyen T.T, Jinwang J, Wong T.Y. Retinal Vascular Changes in Pre-Diabetes and Prehypertension: New findings and their research and clinical implications. Diabetes Care. 2007;30(10):2708-15.
CrossRef - Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Tsuruya K, Sueishi K, Tsuneyoshi M, Iida M, Kiyohara Y. Prehypertension increases the risk for renal arteriosclerosis in autopsies: the Hisayama Study. J. Am Soc Nephrol. 2007;18(7):2135-42.
CrossRef - Díez J. Mechanisms of Cardiac Fibrosis in Hypertension. J Clin Hypertens. 2007;9(7):546-50.
CrossRef - Fang , Xu S, Wang P, Tang F, Zhou S, Gao J, Chen J, Huang H, Liu P. Tanshinone II-A attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine. 2010;18(1):58-64.
CrossRef - Lan T, Huang X, Tan H. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 2013;22(5):401-7.
CrossRef - Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(6 March):879-87.
- Ghosh A.K, Bhattacharyya S, Wei J, Kim S, Barak Y, Mori Y, Varga J. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004;50(4):1305-18.
CrossRef - 1 Han C, Demetris A.J, Liu Y, Shelhamer J.H, Wu T. Transforming growth factor-beta (TGF-beta) activates cytosolic phospholipase A2alpha (cPLA2alpha)-mediated prostaglandin E2 (PGE)2/EP1 and peroxisome proliferator-activated receptor-gamma (PPAR-gamma)/Smad signaling pathways in human liver cancer cells. J. Biol Chem. 2004;279(22 October):44344-54.
- Badyal D.K, Lata H, Dadhich A.P. Animal Models of Hypertension and Effect of Drugs. Indian J Pharmacol. 2003;35:349-62.
- Beckett N.S, Peters R, Fletcher A.E, Staessen J.A, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen R.L, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt C.J. Treatment of Hypertension in Patients 80 Years of Age or Older. N Engl J Med. 2008;358(18):1887-98.
CrossRef - Zanchetti A. Vascular Complications in Hypertension: The VHAS Study. Cardiovasc Drugs Ther. 1995;9:529-31.
CrossRef - Towfighi A, Ovbiagele, B. Partial Peroxisome Proliferator-Activated Receptor Agonist Angiotensin Receptor. Cerebrovasc Dis. 2008;26:106-12.
CrossRef - Yu H.C.M, Burrell L.M, Black M.J, Wu L.L, Dilley R.J, Cooper,M.E, Johnston C.I. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98(23):2621–8.
CrossRef - Jawi I.M, Yasa I.W.S, Suprapta D.W,Mahendra A.N. Antihypertensive effect and eNOS expressions in nacl- induced hypertensive rats treated with purple sweet potato. Universal Journal of Medicine and Dentistry. 2012;1(9):102-7.
- Hadi A.M, Mouchaers K.T, Schalij I, Grunberg K, Meijer G.A, Vonk-Noordegraaf A, van der Laarse W.J, Beliën J.A. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol. 2001;34(4):343-54.
CrossRef - Banday A.A, Muhammad A.B, Fazili F.R, Lokhandwala M. Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in Sprague-Dawley rats. Hypertension. 2007;49(3):664–71.
CrossRef - Wilcox C.S. Oxidative stress and nitric oxide deficiency in the kidney-a critical link to hypertension? Am J Physiol Regul Integ Comp Physiol. 2005;289(4):913-35.
CrossRef - Bopda S.O.M, Longo F, Bella T, Edzah P.M, Taiwe G, Bilanda D. Antihypertensive activities of the aqueous extract of Kalanchoe pinnata (Crassulaceae) in high salt-loaded rats. J Ethnopharmacol. 2014;153(2):400-7.
CrossRef - Xu L, Liu Y. Administration of telmisartan reduced systolic blood pressure and oxidative stress probably through the activation of PI3K/Akt/eNOS pathway and NO release in spontaneously hypertensive. Physiol Res. 2013;62(4):351-9.
- Ying W, Sanders P.W. Dietary salt increases endothelial nitric oxide synthase and TGF-β1 in rat aortic endothelium. Am J Physiol Heart Circ Physiol. 1999;277:1293-8.
CrossRef - Nataatmadja M, West J, Prabowo S, West. Angiotensin II Receptor Antagonism Reduces Transforming Growth Factor Beta and Smad Signaling in Thoracic Aortic Aneurysm. Ochsner J. 2013;13:42-8.
- Wu L.L, Cox A, Roe C.J, Dziadek M, Cooper M.E, Gilbert R.E. Transforming growth factor beta1 and renal injury following subtotal nephrectomy in the rat: role of the renin-angiotensin system. Kidney Int. 1997;51:1553-67.
CrossRef - Zhu Z, Zhu S, Wu Z, Liu D, Yang Y, Wang X, Zhu J, Tepel M. Effect of sodium on blood pressure, cardiac hypertrophy, and angiotensin receptor expression in rats. Am J Hypertens. 2004;17(1):21-24.
CrossRef - Susic D, Fares H, Frohlich E.D. Salt, arterial pressure, and cardiovascular and renal damage. Ochsner J. 2009;9(4):197-203.
- Shang Q.H, Min X.Q, Liu C, Mao W.H. Effects of high salt diet on arterial remodelling and the intervention of telmisartan in Wistar rats. Heart. 2012;98(Suppl 2):E1-E319.
CrossRef - Benson S, Pershadsingh H, Ho C, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery M, Kurtz T. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43(5):993-1002.
CrossRef - Satoh M, Kashihara N, Yamasaki Y, Maruyama K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K, Makino H. Renal iInterstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;(12):317-25.
- Zhang Y, Zhao N, Wang J, Zhu S, Zhu H, Liu B, Cui Q, Guan G, Tian G. Telmisartan inhibited angiotensin II-induced collagen metabolic imbalance without directly targeting TGFbeta1/Smad signaling pathway in fibroblasts. Exp Clin Cardiol. 2014;20(6):139-53.
- Gu J.W, Anand V, Shek E.W, Moore M.C, Brady A.L, Kelly W.C, Adair T. H. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension. 1998;31(5):1083–87.
CrossRef - Souza R.R.D, Gama E.F, Silva R.D.A, Heimann J.C, Maifrino L.B.M, Liberti E.A. Dietary sodium intake induced myenteric neuron hypertrophy in Wistar rats. Braz J Med Biol Res. 2000;33:847-50.
CrossRef







