Manuscript accepted on :September 13, 2017
Published online on: --
Samah Ahmed Kadhum
University of Babylon, College of Pharmacy, Head of Department of Clinical Laboratory Sciences.
Corresponding Author E-mail: yakthanalsaqer@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1282
Abstract
In this study, we measured the antimicrobial activity of two types of nano-particles ZnO & SiO2 against different types of (G+ & G-) bacteria. We collected 90 samples from four sites of infections (burn, wound, urine & sputum) to isolate the following bacteria from hospitalized patients: P. aeruginosa, K. pneumoniae & E. coli as gram-negative bacteria (about 15 swabs for each one) and the other were S. aureus, S. epidermidis & S. pneumoniae as gram-positive bacteria (about 15 swabs for each one). After collecting the samples we diagnosed these bacteria by culturing and biochemical tests and we prepared ZnO & SiO2 nano-particles by chemical method through surface modification in the structure and morphological properties. The antibacterial activity of these nano-particles against different types of bacteria were measured by agar diffusion method, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). It was concluded that the best inhibition zone was (33 mm) at a concentration of 10 µg/ml of nano-ZnO in P. aeruginosa growth while the lower inhibition zone was (8 mm) at the concentration of 2 µg/ml at the same nano particle in S. epidermidis . It was also concluded that the best inhibition zone was (20 mm) at a concentration of 10 µg/ml of nano-SiO2 in P. aeruginosa while the lower inhibition zone was (2 mm) at the concentration of 4 µg/ml at the same nano-particle in S. epidermidis with no inhibition zone at the concentration of 2 µg/ml observed in the same bacteria. We had noted that all tested bacteria were completely inhibited at the concentration of 1.25 µg/ml of nano-ZnO (MIC) with no significant antibacterial activity less than this concentration. The (MBC) was the same as (MIC) as compared with data which showed that all tested bacteria were completely inhibited at the concentration of 0.625 µg/ml of nano-SiO2 and the (MBC) was the same as (MIC) for all tested isolates. So the two nano-particles (ZnO & SiO2) had antibacterial activity, but nano- ZnO was better than nano-SiO2 and could inhibit most of the important pathogenic bacteria at the tested concentrations isolated from hospitalized patients.
Keywords
Antimicrobial; Aeruginosa; bacteria Diagnosed;
Download this article as:| Copy the following to cite this article: Kadhum S. A. The Effect of two Types of Nano-Particles (ZnO and SiO2) on Different Types of Bacterial Growth. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Kadhum S. A. The Effect of two Types of Nano-Particles (ZnO and SiO2) on Different Types of Bacterial Growth. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17386 |
Introduction
New researches have interested in studying new compounds with unique features at the atomic, molecular and macromolecular levels in order to reduce the microbial infections.1 Nowadays, most biologists have been focused on the use of this material in their researches because of their effectiveness in inhibition of resistant pathogenic strain due to their chemical, physical stability with less toxicity in addition to their anticancer effect in human2&3
The increasing in the bacterial infections among the world and the appearance of new pathogenic strain with increasing in the bacterial antibiotic resistant lead to studying the features of this new compounds and their effected factors on pathogenic bacterial growth like: size, concentrations, morphology, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).4
Nano-particles have shown the antibacterial activity when interact with bacterial surface and then entering inside the cell causing its destruction. Sometimes, these materials may have bactericidal effects that are important in several antimicrobial applications just like in food industry.5 The use of nano-particles ZnO & SiO2 with exact concentrations are also important in medical applications like: therapeutics, diagnostics and surgical devices.2&3
The toxic effect of this material is appeared due to the damaging of the cell wall and membrane of bacterial cells which happen through the oxidative stress (OS) that damages lipids, carbohydrates, proteins and DNA. Many researches are discovered that the highest concentrations and the larger surface area of the nano-particles have better antibacterial activity.6&7 They have also shown the structure and conditions of different culture means and their effect on the physicochemical and biological properties of nano-particles and their toxicity at variable pH and temperature.8
The study of the effect of nano-particles on different microbial growth are important for the biologists according to their distinguished antimicrobial and distinct activity in biological sciences, especially in their nano-scale towards a wide range of micro-organisms including bacteria, fungi, fish, algae and plants.9
The pathogenic bacteria are causing chronic infections that are difficult to be treated due to their ability to damage host tissues which causing resistance to antibiotics and lead to death.10 As a result, Researchers have discovered new strategies to be used in the next generation of drugs or agents to control the bacterial infections from natural or inorganic substances and use these materials to reduce the toxicity of pathogenic bacteria as a modern approach in biological applications because they contain environmentally safe mineral elements essential to humans.11
Objectives
The aim of this study is to determine the antibacterial activity of two types of nano-particles (ZnO & SiO2) towards P. aeruginosa, K. pneumoniae and E. coli as gram-negative bacteria and S. aureus, S. epidermidis and S. pneumoniae as gram-positive bacteria at laboratory conditions.
Materials and Methods
Patients
We collected 90 different samples (burn, wound, urine and sputum) from patients who were suffering from skin, urinary & respiratory tract infections from both sexes of different age groups which ranged between (≥20 to 61≤) years old. The sample of patients were taken from the Medical Marjan city in Al-Hilla province through the period from March to June 2016. Forty five clinical swabs were positive for P. aeruginosa, K. pneumoniae & E. coli as gram-negative bacteria (about 15 swabs for each one) and the other were positive for S. aureus, S. epidermidis & S. pneumoniae as gram-positive bacteria (about 15 swabs for each one).
Collection of the Specimens
Sterile swab samples were collected from different sources of infections (burn, wound, urine and sputum)12&13 then the swabs were inoculated on blood agar used for the growth of different types of bacteria and identified by using different biochemical tests.14&15
Preparation of Nano-ZnO and SiO2 Concentrations
A white powder of nano- ZnO was prepared previously and SiO2 nano particles (about 0.02 gm) were dissolved in (10 ml) of dimethyl sulfoxide (DMSO) to prepare a stock solution of (1 mg/ml) then (1 ml) of this solution was diluted to (10 ml) of DMSO again giving a solution of (100 µg/ml) concentration. The tested concentrations were prepared from this solution that include 2 – 4 – 6 – 8 & 10 µg/ml for agar diffusion method and dilutions: 10 – 5 – 2.5 – 1.25 – 0.625 & 0.312 had been prepared for the determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).16
Bacterial Samples
Sterile loop was used for transference of different bacterial growth samples in nutrient agar & nutrient broth media and then they were incubated at 37° for 24 hrs. After that we had transferred 0.1 ml of each sample into sterilized test tubes containing 0.9% NaCl solution as shown in the figure below.
 |
Figure 1: The different bacterial growth samples in nutrient agar and nutrient broth media
|
Antibacterial Activity Against Nano- ZnO and Nano- SiO2
We used the agar diffusion method to determine the antimicrobial activity of nano-ZnO & nano-SiO2 particles in vitro against different types of bacterial growth as follows:
After solidification of nutrient agar in petri dish plates, hollows of wells about 5 mm diameter were cut into the agar by cork borer, then the different types of bacterial isolates were tested on this agar. 0.1 ml of nano-ZnO or nano-SiO2 solutions dissolved in (DMSO) prepared from different concentrations: 2 – 4 – 6 – 8 &10 µg/ml of two nano-particles were applied in these wells. The plates were incubated at 5-8° C for 2-4 h to permit good diffusion and then incubated for 24 h at 37°C. After that we measured the diameter of the inhibition zone (mm) formed around the well compared with the measured activity of (DMSO) alone without nano-ZnO or nano-SiO2 on different bacterial isolates and it had been found that there was no effect on these bacteria.16&17
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) against nano-ZnO& nano-SiO2
The minimum inhibitory concentrations were measured in nano- ZnO & Nano-SiO2 and toward the different bacterial isolates according to.12&18 The MIC was recorded as the lowest concentration which prevent the bacteria under test to grow. In this study 1 ml of media ( nutrient broth ) was taken in a test tube to it 1 ml of test solution was added, then 0.1 ml of bacterial isolates that prepared in 0.9 % of NaCl were added to the test tube containing the media and test solution. Serial dilution was done six times giving concentrations of (10 – 5 – 2.5 – 1.25 – 0.625 & 0.312) µg/ml for each nano-particles. All test tubes were incubated for 24 h at 37°C. The positive result measured by the presence of turbidity and compared with a sample of 0.5 McFarland standards. The control test was contained inoculated broth samples with only DMSO at the same dilutions used in this research and we found that the solvent had no effect on bacterial growth. The MIC values were taken as the lowest concentration required to keep the bacterial growth after incubation (no turbidity) while the minimum bactericidal concentration (MBC) was determined by sub culturing 50µg/ml from each test group which showed that there was no clear growth. If there was no growth, this concentration would be considered as MBC.
Results and Discussion
Nanoparticles SEM Study
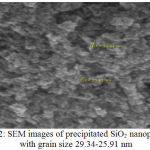 |
Figure 2: SEM images of precipitated SiO2 nanoparticles with grain size 29.34-25.91 nm
|
Table 1: Shows EDS analysis of SiO2
| Elament | App | Intensity | Weight% | Weight% | Atomic% |
| Conc. | Corrn. | sigma | |||
| O Korbital | 97.40 | 0.9676 | 55.86 | 0.67 | 68.08 |
| Si Korbital | 83.10 | 0.9797 | 44.14 | 0.67 | 31.92 |
| Totals | 100.00 |
Bacteriological Study
Isolation of Bacteria from Patients
Different types of bacterial isolates considered to be the main causes of infections which are associated with urinary, respiratory and gastrointestinal tract, burn, wound, eyes, ears, as well as with other parts of the body.19&20 The following table (2) shows the distribution of these isolates according to their location of infection.
Table 2: Distribution of different bacterial isolates according to the source of infection.
| Bacterial Isolates | Source of Isolates n(%) | Total | |||
| Sputum | Burn | Wound | Urine | ||
| P.
aeruginosa |
1
(5%) |
7
(31.81%) |
4
(21.05%) |
3
(10.34%) |
15
(16.66%) |
| K.
pneumoniae |
4
(20%) |
4
(18.18%) |
2
(10.52%) |
5
(17.24%) |
15
(16.66%) |
| E.
coli |
1
(5%) |
3
(13.63%) |
3
(15.78%) |
8
(27.58%) |
15
(16.66%) |
| S.
aureus |
3
(15%) |
3
(13.63%) |
3
(15.78%) |
6
(20.68%) |
15
(16.66%) |
| S.
epidermidis |
1
(5%) |
4
(18.18%) |
5
(26.31%) |
5
(17.24%) |
15
(16.66%) |
| S.
pneumoniae |
10
(50%) |
1
(4.54%) |
2
(10.52%) |
2
(6.89%) |
15
(16.66%) |
| Total | 20
(22.22%) |
22
(24.44%) |
19
(21.11%) |
29
(32.22%) |
90
(100%) |
The results showed an increasing in infections in females rather than males as shown in table (3) among different age groups and this may be associated with different reasons like: changes in menstrual hormones concentrations, social, anatomical differences, smoking and swimming in case of otitis, use of catheters in some cases of (UTIs) in addition of less care by hospital and prolonged hospitalization.20,21&22
Table 3: Distribution of isolates according to the age and gender
| Age group | Male | % | Female | % | Total |
| (years) | n(%) | ||||
| ≥ 20 | 4 | 10.25 | 5 | 9.8 | 9(10) |
| 21-30 | 6 | 15.38 | 9 | 17.64 | 15(16.66) |
| 31-40 | 6 | 15.38 | 10 | 19.6 | 16(17.77) |
| 41-50 | 8 | 20.51 | 12 | 23.52 | 20(22.22) |
| 51-60 | 8 | 20.51 | 8 | 15.68 | 16(17.77) |
| 61 ≤ | 7 | 17.94 | 7 | 13.72 | 14(15.55) |
| Total | 39 | 100 | 51 | 100 | 90(100) |
Agar Diffusion Method
The results of this study have been shown the effect of new compounds ( Nano-ZnO & SiO2) as antibacterial activity and this may be important in controlling and reducing the infections that are caused by this bacteria in patients because these compounds act as chemotherapeutic agents for the treatment or prevention of bacterial infections.5 In this study, we tested the effects of two nano-particles compounds on different G+ & G– bacterial growth obtained from hospitalized patients.
Table (4) shows the best inhibition zone which is (33 mm) at a concentration of 10 µg/ml of nano-ZnO in P. aeruginosa growth while the lower inhibition zone is (8 mm) at the concentration of 2 µg/ml at the same nano particle in S. epidermidis. The antibacterial activity of this particle has occurred because this nano-ZnO is smaller than bacterial cells and this facilitate its adherence to the bacterial cell wall which leads to the destruction of wall and death of bacterial cells.11
In the other hand we have found the best inhibition zone which is (20 mm) at a concentration of 10 µg/ml of nano-SiO2 in P. aeruginosa while the lower inhibition zone is (2 mm) at the concentration of 4 µg/ml at the same nano-particle in S. epidermidis with no inhibition zone at the concentration of 2 µg/ml observed in the same bacteria. This result agrees with,11&23 because of Silica has many properties like: biocompatible, biodegradable, and nontoxic natures, which make it safe to use in reducing the infections in human as different drug carriers due to their low surface energy, heat stability, low surface tension, hydrophobicity, good electric properties, low fire hazard and limited solubility in organics coupled with water insolubility.24&25
These results explain that the growth inhibition for the gram-negative or positive bacteria clearly occurs at higher nano-ZnO or nano-SiO2 concentrations and the inhibition may occur due to the variations in cell physiology, cell wall constitution, and cell metabolism,2&5 further that the constitute of culture media and cultivation conditions.8
Table 4: Inhibition zone diameters (mm) of two nano particles (ZnO & SiO2) against different bacterial growth
| Bacterial Isolates | Mean of inhibition zone(mm) | |||||||||
| Nano-ZnO Concentrations µg/ml | Nano -SiO2 Concentrations µg/ml | |||||||||
| 2 | 4 | 6 | 8 | 10 | 2 | 4 | 6 | 8 | 10 | |
| P. aeruginosa | 12 | 15 | 19 | 24 | 33 | 8 | 10 | 14 | 18 | 20 |
| K. pneumoniae | 10 | 12 | 16 | 20 | 23 | 6 | 8 | 12 | 15 | 18 |
| E. coli | 12 | 13 | 18 | 22 | 28 | 6 | 9 | 13 | 16 | 17 |
| S. aureus | 10 | 12 | 14 | 18 | 20 | 4 | 8 | 12 | 14 | 16 |
| S. epidermidis | 8 | 10 | 14 | 16 | 19 | 0 | 2 | 4 | 9 | 12 |
| S. pneumoniae | 11 | 14 | 16 | 18 | 22 | 6 | 8 | 10 | 11 | 14 |
The both nano-particles have antibacterial effect on G+ & G–bacteria) but they have better effect on G– bacteria because of the presence of a layer of lipopolysaccharides at the exterior of their cell wall that are composed of lipids and polysaccharides which lack strength and rigidity. Negative charges on the lipopolysaccharides are attracted towards the positive charges available on nano-particles, so when these nano-particles interact with bacterial cell wall, they will lead to inhibition of the cell wall synthesis, damage the cytoplasmic membrane, inhibition of nucleic acid and protein synthesis which causes cell death.26
In G+ bacteria the cell wall is composed of thick layer of peptide polyglycogen containing pores which allow foreign molecules to enter the cell without difficulty allowing these nano-particles to enter the bacterial cells and lead to damage their particles.27&30
From all results above, we have discovered that the increasing in both nano-particles concentrations leads to increasing in the inhibition zones of all tested bacterial growth and this results are in agreement with the results of other researches.6;7;10&11 We also find that some variations in the results of the inhibition zone between different tested bacterial strain and these differences are occurred due to the variation in genetic composition, enzyme role in metabolism of bacteria, antibiotic resistance mechanisms of bacterial strain when isolated from hospitalized patients.4
Measurement of MIC and MBC as Antibacterial Activity of Nano-ZnO and Nano-SiO2
Our results have explained the antibacterial activity of nano-ZnO & nano-SiO2 suspensions against different types of pathogenic G+ & G– bacteria. We use six nano-ZnO & nano-SiO2 suspensions with different concentrations that are tested of (10 – 5 – 2.5 – 1.25 – 0.625 & 0.312 µg/ml) as in table (5) the data show all tested bacteria are completely inhibited at the concentration of 1.25 µg/ml of nano-ZnO (MIC) with no significant antibacterial activity less than this concentration. We also show that the (MBC) is the same as (MIC) (1.25 µg/ml) for all bacterial isolates. As compared with data that show all tested bacteria are completely inhibited at the concentration of 0.625 µg/ml of nano-SiO2 (MIC) without any significant antibacterial activity less than this concentration, the (MBC) is the same as (MIC) (1.25µg/ml) for all bacterial isolates for nano-SiO2 .This is happened because these nano-particles have the ability to penetrate the bacteria cell walls and cause damage to its cytoplasm which leads to bacteria inhibition.28
Nano-particles ZnO & SiO2 have the ability to release free radical like: hydrogen peroxide (H2O2), OH- (hydroxyl radicals), and O2 (peroxide) into the medium after the surface of the dead bacteria is completely covered by nano-particles to prevent any bacterial action, so it shows high bactericidal efficacy.5&11
We have explained that there are many differences between our results and the results of other researches and this has occurred due to several factors like: methods of isolation of clinical bacterial strain that may form effective biofilm, difference in the preparation methods of nano-particles, size and concentrations of tested nano-particles in addition to the types of culture media and other parameters affected the bacterial growth.4,11&29
Table 5: Antibacterial activity MIC/MBC (µg/ml) of nano-particles ZnO & SiO2 on G+ & G–
| Bacterial Isolates | Antibacterial activity | |||
| Nano-ZnO | Nano-SiO2 | |||
| MIC | MBC | MIC | MBC | |
| P. aeruginosa | ≥1.25
µg/ml |
≥1.25
µg/ml |
≥0.625
µg/ml |
≥0.625
µg/ml |
| K. pneumoniae | ||||
| E. coli | ||||
| S. aureus | ||||
| S. epidermidis | ||||
| S. pneumoniae | ||||
Conclusions
Our results have shown that the two nano-particles (ZnO & SiO2) have antibacterial activity but nano- ZnO is better than nano-SiO2 and could inhibit most of the important pathogenic bacteria (G+ & G–) at the tested concentrations that are isolated from hospitalized patients.
Acknowledgements
I’m gratefully acknowledge to Dr. Noor Hadi Aysa at the department of clinical laboratory science in the college of pharmacy at the University of Babylon for her help in the preparation of nano-ZnO & nano- SiO2 with my acknowledgement for all hospitalized patients for their cooperation.
References
- Dušan Z, Vladimir V.S, Maja A.K & Milan N.M. Antimicrobial properties of ZnO nanoparticles incorporated in polyurethane varnish. Processing and Application of Ceramics. 2011;5[1]:41–45.
CrossRef - Zarrindokht E.K & Pegah C. Antibacterial activity of ZnO nanoparticle on gram positive and gram-negative bacteria. African Journal of Microbiology Research. 2011;5(12):1368-1373.
- Rizwan W, Nagendra K.K, Akhilesh K.V, Anurag M, Hwang I.H, You-Bing Y, Hyung-Shik S.h & Young-Soon K. Fabrication and growth mechanism of ZnO nanostructures and their cytotoxic effect on human brain tumor U87, cervical cancer HeLa, and normal HEKcells. J. Biol. Inorg. Chem. 2010;16(3):431-442.
- Aysa N.H & Salman H.D. Antibacterial activity of modified zinc oxid nanoparticles against Pseudomonas aeruginosa isolates of burn infections. WSN. 2016;33:1-4.
- Amna S, Shahrom M, Azman S,Noor H, Mohamad K, Ling C. A, Siti K, Mohd B, Habsah H& Dasmawati M. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242.
CrossRef - Ivanova I.A, Tasheva-Terzieva E, Angelov O, Krusteva L, Andonova I, Papazova K Dimova-Malinovska D & Dushkin C. Effect of ZnO thin films on survival of Pseudomonas cells. J Nanomed Nanotechol. 2012;3(7):2-7.
- Li M, Pokhrel S, Jin X, Mädler L & Damoiseaux R. Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nano particles in aquatic media. Environ Sci Technol. 2011;45:755-761.
CrossRef - Saliani M, Jalal J & Kafshdare E.G. Effects of pH and temperature on antibacterial activity of zinc oxide nano fluidagainst Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J Microbiol. 2015;8(2):e17115.
CrossRef - Govinda R.N, Thripuranthaka M, Dattatray J.L & Sandip S.S. Antimicrobial activity of ZnO nanoparticles against pathogenic bacteria and fungi. JSM Nanotechnol Nanomed. 2015;3(1):1033.
- Sangani M.H, Moghaddam M.N & Forghanifard M.M. Inhibitory effect of zinc oxide nanoparticles on pseudomonas aeruginosa biofilm formation. Nanomedicine Journal. 2015;2(2):121-128.
- Yousef J.M & Danial E.N. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. Journal of Health Sciences. 2012;2(4):38-42.
CrossRef - Collee J.G, Fraser A.G, Marmiom B.P & Simmon A. Mackie and McCarteny, Practical Medical Microbiology. 4th ed. Churchill Livingstone Inc. USA. 1996.
- Siddiqui A & Bernstein J. Chronic wound infection:Facts and controversies. Clin Dermatol. 2010;28:516-26.
CrossRef - Krieg Noel. Bergey,s Manual of Systemic Bacteriology . Volume1.Baltimore: Williams &Wilkins. 1984.
- Forbes B.A,Sahm D.F & Weissfeld A.S. Bailey and Scotts, Diagnostic Microbiology . 12thed .Elsevier. 2007.
- Perez C, Pauli M & Bazevque P. An antibiotic assay by the agar well diffusion method. Acta Biologiae et Medicine Experimentalis. 1990;15:113-115.
- NCCLS (National Committee for Clinical Laboratory Standards): Methods for dilution antimicrobial susceptibility tests of bacteria that grow aerobically. Approved Standard M Wayne. PA, NCCLS. 2002;100-S12.
- Xi J.H, Yeo S.Y, Lee H.J & Jenog S.H. Preparation of nano composite fibers for permanent antibacterial effect. Journal of Material . Science. 2003;38:2143-2147.
CrossRef - Herfindal E.T & Gourley D.R. Textbook of Therapeutics ,Drug and Disease Management. 7thed . Lippincott Williams & Wilkins, A Wolters Kluwer Co., USA. 2000;1049-1054.
- Fluit A.C, Verhoef J & Schmitz F.J. Frequency of isolation and antimicrobial resistance of gram-negative and gram-positive bacteria from patients in intensive care units of 25 European university hospitals participating in the European arm of the SENTRY antimicrobial surveillance program. 1997-1998. Eur. J.Clin. Microbiol. Infect. Dis. 2001;20:617-625.
CrossRef - Mazzotta P, Lorbstein R. & Koren G. Treating allergic rhinitis in pregnancy : Safety considerations. Drug Saf. 1999;20:361–375.
CrossRef - Anonymous . The cost of antibiotic resistance effect of resistance among Staph. aureus, Kelebsiella pneumoniae , Acinetobacter baumannii , and P. aeruginosa on length of hospital stay . Infect. Control – Hosp – Epidemiol. 2002;23(2):106–8.
CrossRef - Moghaddam M.G & Eslahi H. Synthesis, characterization and antibacterial properties of a novel nanocomposite based on polyaniline/polyvinyl alcohol/Ag. Arabian Journal of Chemistry. 2014;7:(5):846.
CrossRef - Ahmed G.H, Amina L.M, Ahmed A.N & Nabil Y.A. Z. Controlled release of drugs from cellulosic wound bandage using silica microsphere as drug encapsulator module. Journal of Applied Pharmaceutical Science. 2015;5(12):067-073.
- Mohamed A.L, Er-Rafik M & Moller M. Supercritical carbondioxide assisted silicon based finishing of cellulosic fabric: A novelapproach. Carbohydrate Polymers. 2013;98(1):1095–1107.
CrossRef - Karnib M, Holail H, Olama Z, Kabbani A & Hines M. The antibacterial activity of activated carbon, silver, silver impregnated activated carbon and silica Sand nanoparticles against pathogenic E. coli BL21. Int. J. Curr. Microbiol. App. Sci. 2013;2(4):20-30.
- Hassabo A.G,Mendrek A, Popescu C,Keul H &Möller M. Deposition of functionalized polyethylenimine-dye onto cotton and wool fibers. Research Journal of Textile and Apparel. 2014;18(1):36-49.
CrossRef - Aysa N.H, Al-Maamori M.H & Al-Maamori N.A.A. Effect of the unmodified and modified ZnO nanoparticles on the mechanical and antibacterial properties of silicone rubber using in medical applications. Journal of Nanoscience and Nanoengineering. 2015;1(3):119-124.
- Jiang J.O.G, Elder A, Gelein R, Mercer P, Beswas A. Dose nanoparticle activity depend upon size and crystal phase ? Nanotoxicology. 2008;2:33-42 .
CrossRef - Thati V, Roy A.S, Prasad M.V.N.A, Shivannavar C. T &Gaddad S.M. Nanostructured zinc oxide enhances the activity of antibiotics against Staphylococcus aureus. J Biosci Tech. 2010;1(2):64-69.







