Bayu Putra Danan Jaya, Endang Linirin Widiastuti , Endang Nurcahyani
, Endang Nurcahyani and Sutyarso
and Sutyarso
Department of Biology, Faculty of Mathematic and Sciences, Universitas Lampung, Jalan Soemantri Brojonegoro No. 1, Gedung Meneng, Bandar Lampung, Lampung 35145, Indonesia.
Corresponding Author E-mail: elwidi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1320
Abstract
Paraquat is a toxic substance that can cause oxidative damage through increased ROS production. Oxidative damage can be prevented by supplementing with antioxidants, such as taurine and oyster mushrooms. This study aims to determine the ability of taurine and oyster mushrooms in preventing oxidative damage of the liver of mice induced by paraquat. This study uses a completely randomized design. A total of 30 DDY mice were divided into five treatment groups for 3 weeks, namely: 1) control, 2) the oyster mushroom (6.25% in feed and 2.5 g/L in drinking water), 3) paraquat (20 mg/kg, IP), 4) paraquat and taurine (15.6 g/kg) and 5) paraquat and oyster mushrooms. Parameters measured were MDA, glutathione, SOD enzyme levels and histopathological changes in liver. The results showed paraquat increases in liver MDA levels significantly but decreases in liver glutathione levels significantly compared to controls, while taurine and oyster mushrooms reduce the levels of MDA (p<0.05) and increase glutahion levels (p<0.05). Paraquat also increases the levels of SOD (p<0.05), while taurine and oyster mushroom are able to inhibit the increased levels of SOD although they do not show significant (p> 0.05). Paraquat induces liver histopathology change which is characterized by congestion, hydropic degeneration and cloudy swelling. In conclusion, paraquat causes oxidative damage to the liver, while taurine and oyster mushrooms can prevent the damage.
Keywords
Antioxidants; Oxidative Damage; Oyster Mushrooms; ROS and Taurine
Download this article as:| Copy the following to cite this article: Jaya B. P. D, Widiastuti E. L, Nurcahyani E, Sutyarso S. Taurine and Oyster Mushroom (Pleurotus Ostreatus) Prevents Oxidative Damage in Liver of Mice Induced by Paraquat. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Jaya B. P. D, Widiastuti E. L, Nurcahyani E, Sutyarso S. Taurine and Oyster Mushroom (Pleurotus Ostreatus) Prevents Oxidative Damage in Liver of Mice Induced by Paraquat. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17731 |
Introduction
Paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride), is a highly toxic compound for animals and humans. Large quantities of paraquat exposure cause death, while exposure in small quantities over long periods, causing permanent damage to various organs, such as lungs, brain, liver and kidneys (Ortiz et al., 2016; Awadalla, 2010).
Paraquat toxic mechanism, based on its ability to increase free radicals, such as superoxide anions. This causes the proliferation of ROS (Reaxtive Oxygen Species) molecules and the oxidation of NADPH (Nicotinamide Adenine Dinucleotide Phosphate) which is required for redox reactions in metabolic processes (Suntres, 2002; Oliviera et al., 2008; Franco et al., 2009). The ROS formed will bind to polyunsaturated fatty acids are abundant in cell membrane, causing oxidative damage to various organs. Liver is one of the most affected organs due to paraquat exposure (Ortiz et al., 2016).
Based on that fact, many researchers try to use antioxidants to prevent the damage caused by paraquat. Antioxidants are compounds that can protect the body’s biological system from damage. Several studies have shown that antioxidants, such as vitamin C, vitamin E, N-acetylcysteine and melatonin are able to prevent the damage caused by paraquats (Mood et al., 2011; Moon and Chun, 2010; Awadalla, 2010; Hong et al., 2003).
Taurine, one of the amino acids containing sulfur groups, performs various functions in body, such as osmoregulator, neuromodulation and detoxification (Shim et al., 2009; Ripps and Shen, 2012). These compounds are found in high concentrations in liver tissue (Batista et al., 2012). Taurine has been known have the ability to protect liver from chemical compounds that cause hepatotoxicity (Heidari et al., 2013; Liao et al., 2008; Tabassum et al., 2006). Taurine is known to play a role as an antioxidant by inhibiting ROS production and binding to ROS in cells (Ripps and Shen, 2012; Ozden et al., 2012; Yildrim and Killic, 2011). In addition, taurine is also capable to increasing activity of antioxidant enzymes (Tasci et al., 2008; Zhang et al., 2014).
Oyster mushrooms are a edible mushrooms that rich in phenolic compounds, such as polyphenols, which is known to have high antioxidant activity (Iwakolun et al., 2007; Neelam and Singh, 2013). This fungus also contains β glucan compounds that have high antioxidant activity (Patel et al., 2012). Administration of this mushrooms, proven to protect the liver from damage caused by acetaminophen poisoning (Naguib et al., 2014). This mushrooms is also known to increase the activity of important antioxidant enzymes, such as SOD, catalase and peroxidase (Patel et al., 2012).
Given the enormous potential of taurine and oyster mushroom as an antioxidant, it is interesting to examine its ability to prevent oxidative damage arising from the exposure of paraquates to the liver. The parameters of oxidative damage measured were MDA, glutathione and SOD enzyme levels and liver histopathologic changes. The results of this study are expected to provide information on alternative treatments for oxidative damage to the liver caused by paraquat exposure.
Methods
Experiments Animal
A total of 30 DDY strains of mice, weight ranging from 30 to 40 grams, kept in separate cages in room temperature and light cycle 12 hours of light and 12 hours of dark. All animal experiments were fed and drunk in ad libitum.
Mushroom Preparation
Oyster mushrooms are bought from traditional markets. The fruit body is then cut into small pieces and dried using an hot air oven at a temperature of 30-35°C. The dried mushrooms are then crushed using a blender until smooth. Mushroom flour then put in a closed bottle and stored at room temperature. Oyster mushroom extract is obtained by boiling 2.5 grams of mushroom flour in 1 liter of boiling aquadest for 15 minutes. The extract is then filtered and the filter product is stored at -40°C.
Experimental Method
Mice were randomly divided into 5 groups, ie:
Control (K): mice were fed with standard feed without paraquat, taurine and oyster mushrooms.
P1 group: mice given oyster mushroom in feed with dose 62,5 g/kg of feed and drinking water with dose 2,5 g/lt.
P2 group: mice were fed with standard feed and induced with paraquat at doses of 20 mg/kgBW intraperitoneal, 2 times weekly for 3 weeks.
P3 group: mice were fed with standard feed, induced with paraquat as in group P2 and given taurine with a dose of 15,6 gr/kg BW.
P4 group: mice were given with oyster mushroom as in group P1 and induced paraquat as in group P2.
After 3 weeks, the mice were sacrificed. As many as 100 mg of liver, then made homogenate using Tissue Lyser in 1 ml PBS 0.1 M pH 7.4. Homogenate is then centrifuged at 5,000 rpm for 10 min. The supernatant was transferred to another tube and stored at -20°C.
Oxidative Damage Analysis
MDA levels were measured using a modified test method of tiobarbituric acid (TBA) based on Zainuri and Wanandi (2012). Glutathione levels were examined using a glutathione examination kit based on Syafrudin and Subandrate (2015). SOD enzyme activity was examined using a RanSOD inspection kit from Randox in a manner consistent with the manufacturer’s recommendations.
Histopathological Examination
After surgery, the liver was fixed using a 10% formalin buffer, then histopathologic preparations were made with Mayer Hematoxilin stain. The degree of histopathologic changes of the liver was assessed using the Manja Roenigk method with criteria: 0 = normal; 1 = if there is parenchymatous degeneration; 2 = if there is hydropic degeneration and 3 = if there is necrosis.
Data Analysis
The results of oxidative damage test were tested using one way Anova test followed by Least Significant Difference (LSD). Histopathologic test results were tested using Kruskal Wallis test followed by Mann Whitney test. All tests were performed at a 95% confidence level.
Results
Taurine and Oyster Mushrooms Decrease Peroxidation of fat in Liver
The results showed induction of paraquat increased of lipid peroxidation, characterized by elevated liver MDA levels significantly (p<0.05). Administration of taurine and oyster mushrooms, significantly reduce liver MDA levels (Figure 1).
Taurine and Oyster Mushroom Increases Glutathione Levels
As expected, induction of paraquat, significant decrease in hepatic glutathione levels (p<0.05). Administration of taurine and oyster mushrooms, able to increase glutathione levels significantly (Figure 1).
Taurine and Oyster Mushrooms Reduce Enhancement of SOD Enzyme Levels
Paraquat induction, significantly increased SOD enzyme levels (p<0.05), whereas taurine and oyster mushrooms reduced the elevated enzyme levels, although not significant (p> 0.05) (Figure 1).
Taurine and Oyster Mushrooms Reduce Histopathology Liver Damage
Paraquat induced hepatocyte cells exhibit extensive cloudly swelling and hydrophic degeneration, but no necrosis. In addition, there was also a congestion and dilation of the liver sinusoid. Mean of histopathologic liver damage score, presented in Table 1. Administration of taurine and oyster mushrooms, significantly reducing the mean histopathologic damage score (p<0.05).
Discussion
Paraquat is a pesticide that if swallowed can be fatal to humans. Paraquat can accumulate in liver, inducing ROS molecules and causing damage, known as oxidative damage (Choi et al., 2013; Franco et al., 2009).
Increased production of ROS that causes oxidative stress, plays an important role in the process of liver damage due to paraquat induction (Ortiz et al., 2016). The results of this study showed an increase in MDA levels, which is a marker of lipid peroxidation, in paraquat induced mice. This increase is followed by a decrease in glutathione levels and an excessive increase in SOD enzyme levels. These results indicate an increase in oxidative damage to the liver. The presence of oxidative damage, causing cellular dysfunction. The histopathological features of the liver in the paraquat-induced group support this fact.
Taurine, a free β amino acid group compound, is known to prevent oxidative damage to various organs (Wang et al., 2013). This compound, proved to have hepatoprotective ability due to its antioxidant activity (Abbasoglu et al., 2001).
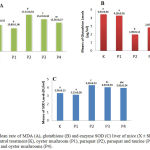 |
Figure 1: Mean rate of MDA (A), glutathione (B) and enzyme SOD (C) liver of mice (X ± SEM nmol / ml) with control treatment (K), oyster mushroom (P1), paraquat (P2), paraquat and taurine (P3 ) as well as paraquat and oyster mushrooms (P4).
|
The mean values followed by the same letter are not significantly different based on the BNT test with α = 5%.
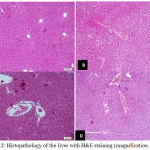 |
Figure 2: Histopathology of the liver with H&E staining (magnification 100 x).
|
(A) Liver controls and fungi without changes in histopathological features. (B) Paraquat induction (20 mg kgBW) appears extensive cloudly swelling and hydrophic degeneration and congestion. (C) Paraquat and taurine induction and (D) Induction of paraquat and oyster mushrooms. There appears to be a decrease in cloudy swollen degeneration in hepatocyte cells, but there is still a congestion.
Tabel 1: Mean score of liver damage
| Control | JT | PQ | PQ + T | PQ + JT | p value | |
| Damage Score | 0,50 ± 0,50a | 0,33 ± 0,21a | 10,50 ± 0,67b | 7,83 ± 0,40c | 7,67 ± 0,33c | 0,0001* |
The value represents the mean ± SEM. JT: oyster mushrooms; PQ: paraquat; T: taurine. * Based on Kruskal Wallis test. Average values followed by different letters indicate a significant difference based on the Mann Whitney test at α 5%.
The results of this study, support the concept. Taurine is known to reduce the score of liver damage induced by paraquat induction significantly. Furthermore, taurine is known to decrease lipid peroxidation and increase antioxidant enzyme levels (Zhang et al., 2014).
Consistent with its ability as an antioxidant, the results of this study also showed taurine was able to lower levels of MDA. This result is consistent with previous studies (Guz et al., 2007). This is related to the ability of taurine to stabilize the electron transport chain and inhibit ROS (Ripps and Shen, 2012) and the direct binding of ROS molecules (Ozden et al., 2012; Yildrim and Killic, 2011), so that circulating of ROS molecules will decrease. As a result, there is a decrease in lipid peroxidation activity characterized by decreased of MDA levels.
Potent antioxidant taurine is also associated with its ability to increase glutathione levels and antioxidant enzyme activity, such as the SOD enzyme. The results of this study showed that taurine increased levels of glutathione on paraquat induced mice group. Taurine is reported to increase glutathione levels by increasing its synthesis (Hagar, 2004), inhibition of oxidation by reducing lipid peroxidation activity (Miyazaki et al, 2004; Cetiner et al., 2005), as well as guarding glutathione/GSSG ratios (Zhang et al., 2014 ). This study also showed that taurine reduces the activation of excess SOD enzymes. This is likely due to reduced ROS formed and increased antioxidant defense activity as a result of elevated levels of glutathione.
Actually, inside the body there is a certain amount of endogenous taurine that acts as an antioxidant. The increase of ROS molecules due to induction of paraquat, causing reduced endogenous taurine levels in the body. Administration of taurine, will increase taurine levels in tissues, according to Zhang et al. (2014), in the liver tissue that increase reaches 40%. This increase in taurine levels, will restore respiratory activity, thus increasing ATP synthesis and suppressing the production of superoxide anions formed (Jong et al., 2012).
Like taurine, oyster mushrooms are known to have high antioxidant content. Antioxidant activity of various compounds belonging to oyster mushrooms, reported able to capture free radicals with very efficient (Singh et al, 2015), so as to prevent oxidative damage to the liver. Several previous studies have confirmed the hepatoprotective ability of oyster mushrooms in oxidative stress conditions due to poisoning (Nada et al, 2010; Refaie et al., 2010). The results of this study also reinforce the fact. Provision of oyster mushrooms in diet and drinking water, proven to reduce the score of liver damage due to induced paraquat.
Furthermore, the results of this study showed the provision of oyster mushrooms can reduce lipid peroxidation activity in hepatocytes because paraquat induction. These results are similar to previous studies which also reported similar results with different inductors (Naguib et al., 2014; Anandhi et al., 2013). This is related to the antioxidant compounds of oyster mushrooms, such as polyphenols, which can act as hydrogen donors to neutralize ROS and inhibit the formation of O2– and OH– which are the main causes of lipid peroxidation (Lin et al., 2011).
Another effect of reduced lipid peroxidation occurring during ROS reduction is the reduced consumption of glutathione in cells. This results in decreased levels of glutathione in hepatocyte cells. This concept supports the findings of this study, which increases the level of liver glutathione in the induced group of paraquat and oyster mushrooms. In addition to the decrease in ROS, polyphenols contained in oyster mushrooms, can increase the activity of γ-glutamylcysteine synthetase (γ-GCS) enzyme which catalyzes the synthesis of glutathione (Masella et al., 2005). Oyster mushrooms also contain cysteine amino acids (Jaworska and Bernas, 2011). Cysteine is a precursor to the synthesis of glutathione. The consequences of increased γ-GCS enzyme activity, cysteine availability and reduced glutathione levels due to paraquat exposure are increased glutathione synthesis, so that glutathione levels in cells will increase.
The main mechanism of paraquat toxicity is the increased formation of O2–. This results will changes the antioxidant defense system, including the SOD enzyme. In this study, paraquat induction at a dosage of 20 mg/kg BW causes cells to survive from oxidative stress conditions by increasing their antioxidant defense system. This is characterized by a decrease in glutathione levels and the excessive activity of SOD enzymes that increase significantly. Increased activity of this enzyme, required by cells to neutralize the increase in O2– molecules, due to paraquat toxicity (Ray et al., 2007). The decrease of the O2– molecule, as the primary free radical produced by paraquat, also results in a decrease in excessive activation of SOD enzymes. This concept is in accordance with the results of research where there is a decrease in excessive activation of SOD enzyme due to induced paraquat induction. Various antioxidant compounds contained in oyster mushrooms, such as vitamins (B1, B2 and C), polysaccharides, phenolic compounds and glycoproteins, are known to capture the formed O2– molecules, thus reducing the amount of free radicals in circulation (Nada et al, 2010; Refaie Et al., 2010).
Conclusion
This study proves the ability of taurine and oyster mushrooms as antioxidants to prevent oxidative damage to the liver due to induced paraquat. Taurine and oyster mushrooms have been shown to reduce MDA levels, increase glutathione levels, reduce excessive activation of SOD enzymes and reduce liver damage scores. Further studies of other antioxidant enzyme activity and DNA damage caused by induced paraquat need to be performed.
Acknowledgement
We thank to the Ministry of Research –Technology and Higher Education of Republic Indonesia – Directorate of Research and Community Services (DRPM-Kemenristekdikti) for supporting this research through Graduate Program Grants (PPS) 2017.
References
- Abbasoglu D.S, Kanbagli O, Balkan J, Cevikbas U, Aykac T.G, Uysal M. The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum Exp Toxicol. 2001;20(1):23-7.
CrossRef - Anandhi R, Annadurai T, Anitha T.S, Muralidharan A.R, Najmunnisha K, Nachiappan V, dkk. Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in triton WR-1339 induced hypercholesterolemic rats. J Physiol Biochem. 2013;69:313-323.
CrossRef - Awadalla A.E. Efficacy of vitamin C against liver and kidney damage induced by paraquat toxicity. Exp Toxicol Pathol. 2010;64(5):431-4.
CrossRef - Batista T.M, Ribeiro R.A, da Silva P.M.R, Camargo R.L, Lollo P.C. B, Boschero A.C. Taurine suplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Nutr. Food Res. 2012;57(3):423-434.
CrossRef - Cetiner M, Sener G, Sehirli A.O, Demiralp E.E, Ercan F, Gedik N. 2005. Taurine protets against methotrexate induced toxicity and inhibits leukocyte death. Toxicol Appl Pharmacol. 209(1):39-50.
CrossRef - Choi J.S, Jou S.C, Oh M.Y, Kim Y.H, Park M.J, Gil H.Y. The dose of cyclophosphamide for treating paraquat induced rat lung injury. Korean J Intern Med. 2013;28:420-427.
CrossRef - Franco R, Olea R.S, Reyes E.M.R, Panayiotidis M.I. 2009. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutation Research. 2009;674:3-22.
CrossRef - Guz G, Lortlar E.O.N. The effect of taurine on renal ischemia reperfusion injury. Amino Acid. 2007;32:405-411.
CrossRef - Hagar H.H. The protective effect of taurine against cyclosporine A induced oxidative stress and hepatotoxicity in rats. Toxicology Letters. 2004;151:335-343.
CrossRef - Heidari R, Babaei H, Eghbal M.A. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arh Hig Rada Toksikol. 2013;64:201-210.
CrossRef - Hong S.Y, Yang J.O, Lee E.U, Lee Z.W. Effects of N-acetyl-L-cysteine and gluthatione on antioxidant status of human serum and 3T3 fibroblasts. J Korean Med Sci. 2003;18:649-54.
CrossRef - Iwalokun B.A, Usen U.A, Otunba A.A, Olukoya D.K. Comparative phytochemical evaluation, antimicrobial and antioksidant properties of Pleurotus ostreatus. African Journal of Biotechnology. 2007;6(15): 1732-1739.
CrossRef - Jaworska G, Bernas E, Mickowska B. Effect of production process on the amino acid content of frozen and canned Pleurotus ostreatus. Food Chemistry. 2011;125:936-943.
CrossRef - Jong C.J, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acid. 2012;42:2223-2232.
CrossRef - Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacological Research. 2008;57:125-131.
CrossRef - Lin H.H, Chen J.H, Chou F.P, Wang C.J. Protocatechuic acid inhibits cancer cell metastasis involving the downregulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br J Pharmacol. 2011;162(1):237-54.
CrossRef - Masella R, Benedtto R.D, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione related enzymes. Journal of Nutritional Biochemistry. 2005;16:577-586.
CrossRef - Miyazaki T, Matsuzaki Y, Ikegami T, Miyakawa S, Doy M, Bouscarel B. Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acid. 2004;27:291-298.
CrossRef - Mood N.E, Sabzghababee A.M, Yaraghi A, Montazeri K, Golabi M, Sharifian A, Badri S. Effect of antioxidants on the outcome of therapy in paraquat intoxicated patients. Tropical Journal of Pharmaceutical Research. 2011;10(1):27-31.
- Moon J.M, Chun B.J. The efficacy of high doses of vitamin C in patients with paraquat poisoning. Human and Experimental Toxicology. 2010; 30(8):844-850.
CrossRef - Nada S.A, Omara E.A, Salam O.M.E, Zahran H.G. 2010. Mushroom insoluble pollysaccharides prevent carbon tetrachloride induced hepatotoxicity in rat. Food and Chemical Toxicology. 2010;483184-3188.
CrossRef - Naguib Y.M, Azmy R.M, Samaka, R.M., Salem, M.F. Pleurotus ostreatus opposes mitochondrial dysfunction and oxidative stress in acetaminophen induced hepato-renal injury. BMC Complementary and Alternative Medicine. 2014;14:494-515.
CrossRef - Neelam S, Singh S. Comparative studies on antioxidant capacity of ethanol extracts of Pleurotus florida and Pleurotus ostreatus. Annals of Biologycal Research. 2013;4(4):77–82.
- Oliviera R.J.D, Duarte J.A, Navarro A.S, Remiao F, Bastos M.L, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features and treatment. Clinical Reviews in Toxicology. 2008;38:13-71.
CrossRef - Ortiz M.S, Forti K.M, Martinez E.B.S, Munoz L.G.M, Husain K, Muniz W.H. Effects of antioksidant N-acetylcysteine against paraquat induced oxidative stress in vital tissues of mice. Int J Sci Basic Appl Res. 2016;26(1):26-46.
- Ozden S, Catalgo B, Oktayoglu S.G, Karatug A, Bolken S, Alpertunga B. Acute effects of methiocarb on oxidative damage and the protective effects of vitamin E and taurine in the liver and kidney of wistar rats. Toxicology and Industrial Health. 2012;29(1):60-71.
CrossRef - Patel Y, Naraian R, Singh V.K. Medicinal properties of Pleurotus spesies (Oyster mushroom): A Review. World Journal of Fungal and Plant Biology. 2012;3(1):01-12.
- Ray S, Sengupta A, Ray A. Effects of paraquat on antioxidant system in rats. Indian Journal of Experimental Biology. 2007;45:432-438.
- Refaie F.M, Esmat A.Y, Daba A.S, Osman W.M, Taha S.M. Hepatoprotective activity of polysaccharopeptides from Pleurotus ostreatus mycelium on thioacetamide intoxicated mice. Micologia Aplicada International. 2010;22(1):1-13.
- Ripps H, Shen W. Review: taurine: a very essential amino acid. Molecular Vision. 2012;18:2673-2686.
- Safrudin and Kadar S. glutathion (GSH) darah karyawan SPBU di kota Palembang. Jurnal Kedokteran dan Kesehatan. 2015;2(3):277-281.
- Shim K. S, Jung H.J, Na C.S, Yoon C, Park G.H. Effect of taurine on lipid metabolism and protein synthesis in poultry and mice. Asian-Aust J Anim Sci. 2009;22(6):865-870.
CrossRef - Singh V, Vyas D, Pandey R, Sheikh I.A. Pleurotus ostreatus produces antioxidant and anti arthritis activity in wistar albino rats. World Journal of Pharmacy and Pharmaceutical Science. 2015;4(05):1230-1246.
- Suntres Z.E. 2002. Role of antioxidants in paraquat toxicity. Toxicology. 2002;18065-77.
CrossRef - Tabassum H, Rehman H, Banerjee B.D, Raisuddin S, Parvez S. Attenuation oftamoxifen induced hepatotoxicity by taurine in mice. Clinica Chimica Acta. 2006;370:129-136.
CrossRef - Tasci I, Mas N, Mas M.R, Tuncen M, Comert B. Ultrastructural changes in hepatocytes after taurine treatment in CCl4 induced liver injury. World .J. Gastroenterol. 2008;14(31):4897-4902.
CrossRef - Wang G.G, Li W, Lu X.H, Zhao X, Xu L. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat Med J. 2013;54:171-179.
CrossRef - Yildrim Z, Kilic N. Effect of taurine and age on cerebellum antioxidant status and oxidative stress. International Journal of Gerontology. 2011;5:166-170.
CrossRef - Zainuri M, Wanandi S. I. Aktifitas spesifik manganese superoxide dismutase (MnSOD) dan katalase pada hati tikus yang diinduksi hipoksia sistemik: hubungannya dengan kerusakan oksidatif. Media Litbang Kesehatan. 2012;22(2):87-92.
- Zhang Z, Liu D, Yi B, Liao Z, Tang L, Yin D, He M. Taurine suplementation reduces oxidative stress and protects the liver in an iron overload murine model. Molecular Medicine Reports. 2014;10:2255-2262.
CrossRef







