Mohammed Sami Kadhim
Department of Basic Science, College of Dentistry, University of Baghdad, Iraq.
Corresponding Author E-mail: dr.alkarkhi@gmail.comDOI : https://dx.doi.org/10.13005/bpj/1326
Abstract
Polycystic ovary syndrome (PCOS) is one of the most intricacy endocrine defect that affecting approximate 10 percent of female in the procreation age. This study aimed to estimate the concentration value of ghrelin in non-obese polycystic ovary syndrome females during menstrual cycle day 2. This study was conducted at Baghdad teaching hospital, from April 2015 until October 2016. Serum ghrelin, LH, FSH concentration were measured in 40 non-obese PCOS women and 40 healthy women during menstrual cycle day 2. The results indicates high significant increases (P ˂ 0.001) in mean of Ghrelin, LH and FSH in non-obese PCOS female group when compared with healthy female during day 2 menstrual cycle. It was concluded that ghrelin is a potential biomarker for diagnosis of PCOS.
Keywords
Serum ghrelin; LH; and FSH concentrations; non-obese PCOS women
Download this article as:| Copy the following to cite this article: Kadhim M. S. Serum Ghrelin, LH and FSH Concentrations During Menstrual Cycle in Non-Obese PCOS Women Compared to Healthy Women. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Kadhim M. S. Serum Ghrelin, LH and FSH Concentrations During Menstrual Cycle in Non-Obese PCOS Women Compared to Healthy Women. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=18034 |
Introduction
Ghrelin, an acylated 28-amino acid peptide hormone with a unique octanoyle adjustment of hydroxy group on serine at the position three, that is substantial for its role, latterly synthesis by the gastric oxyntic gland human stomach, is the long-searched natural ligand for the GH secretagogue receptor.1,2 It is regarding with energy equilibrium obesity, IR, and gonadal role. Hypothalamic areas are the essential inding sites of ghrelin.3 The obtainable datum on ghrelin level in women with PCOS is rather inconsistent: some studies reported a decreased,4 some opposite reported elevation5 of concentrations and some others reported no significant differences between women with PCOS and normal cycling women.6
Polycystic ovary syndrome (PCOS) is one of the generality usually endocrine disorder and a very comples endocrine disorder influence almost 10 percent of women in the profileration age. 7,8 Polycystic ovary syndrome (PCOS) is an endocrine and metabolic disproportionate disorder, with a likely genetic origin, affected by environmental agents such as nutrition and physical activity. The main clinical signs of PCOS are related to hyperandrogenism, such as hirsutism, acne and menstrual abnormal.9,10
The exact pathogenic mechanism of PCOS is not discovered yet. There are many hypothesis concerning reasons of PCOS evolution and the rival coexistence of many interdependent defect is also likely. Most notice is paid to the hypersecretion of LH and insulin resistance as well hyperinsulinemia. The ancient theory emphasized the relation between thecal cells encouragement with LH and the consequent androgen high releasing.11,12 Lutropin is the best known androgen synthesis stimulator. However last contract brought new hypothesis emphasizing the role of insulin and insulin-like growth factor I (IGF-I) system.13,14
LH and FSH are the hormones that encourage ovulation. Both LH and FSH are secreted from the brain by the pituitary gland. At the beginning of the cycle, LH and FSH levels usually range between about 5-20 mlU/ml. Most women have about equal amounts of LH and FSH during the early part of their cycle. However, there is a LH surge in which the amount of LH increases to about 25-40 mlU/ml 24 hours before ovulation occurs. Once the ova are released by the ovarian, the LH levels back down. 15
Materials and Methods
Forty healthy normally cycling females, aged (32.72 ±0.62) years with BMI (20.67±0.22) Kg/m2 and forty abnormal cycling PCOS females aged (31.7 ±0.6) years with BMI (22.05 ±0.24) those two groups who were referred from the department of Gynecology\ Baghdad teaching hospital, from April 2015 until October 2016 were included in current study. All females were having normal body mass index.16 The exclusion criteria were any female with medication, alcohol, smoke usage or hormonal treatment for the last 3 months.
Blood samples were collected in the morning following an overnight fast during early day 2 menstrual cycle for healthy and patients women. The blood samples were centrifuged and then serum aliquots were frozen at -20C0 until assayed. Serum ghrelin, LH, and FSH was measured for all subjected by ELISA technique (mybiosource, USA).17 The results data were analyzed by using computer program SPSS package 16.0 for Windows XP and were expressed as mean ± standard error of mean (mean ± SEM).
Results
The mean ± standard error of mean (SEM) of women age for Normal menstrual cycle (MC) group and Polycystic ovary syndrome(PCOS) group were 32.72 (±0.62) years, 31.7 (±0.6) years, respectively. There were insignificant difference (P<0.05) in the mean of women age in Polycystic ovary syndrome group when compared with Normal menstrual cycle group (Figure 1).
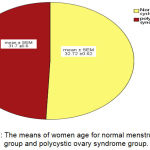 |
Figure 1: The means of women age for normal menstrual cycle group and polycystic ovary syndrome group.
|
The mean ±SEM of BMI for Normal MC group and PCOS group were 20.67 (±0.22) Kg/m2, 22.05 (±0.24) Kg/m2, respectively. There were very high significant increases (P ˂ 0.001) in mean of BMI in PCOS group when compared with Normal MC Group (Figure 2).
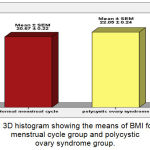 |
Figure 2: 3D histogram showing the means of BMI for normal menstrual cycle group and polycystic ovary syndrome group.
|
The mean ±SEM of serum Ghrelin levels for Normal MC group and PCOS group were 122.65 (±9.62) pg/ml, 564.72 (±38.61) pg/ml, respectively. There were very high significant increases (P ˂ 0.001) in mean of Ghrelin in PCOS group when compared with Normal MC group (Figure 3).
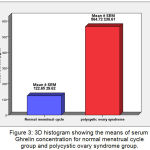 |
Figure 3: 3D histogram showing the means of serum Ghrelin concentration for normal menstrual cycle group and polycystic ovary syndrome group.
|
The mean ±SEM of serum LH levels for Normal MC group and PCOS group were 13.62 (±0.96) mIU/ml, 17.65 (±0.53) mIU/ml, respectively. There were very high significant increases (P ˂ 0.001) in mean of LH in PCOS group when compared with Normal MC Group (Figure 4).
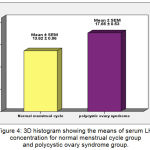 |
Figure 4: 3D histogram showing the means of serum LH concentration for normal menstrual cycle group and polycystic ovary syndrome group.
|
The mean ±SEM of serum FSH levels for Normal MC group and PCOS group were 12.44 (±0.95) mIU/ml, 6.23 (±0.11) mIU/ml, respectively. There were very high significant increases (P ˂ 0.001) in mean of FSH in Normal MC Group when compared with PCOS group (Figure 5).
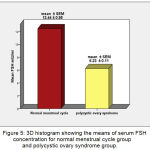 |
Figure 5: 3D histogram showing the means of serum FSH concentration for normal menstrual cycle group and polycystic ovary syndrome group.
|
The mean ±SEM of LH/FSH ratio for Normal MC group and PCOS group were 1.77(±0.34), 2.83 (±0.08), respectively. There were high significant increases (P ˂ 0.01) in LH/FSH ratio in Normal MC group when compared with PCOS group (Figure 6).
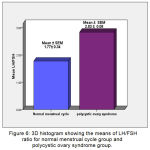 |
Figure 6: 3D histogram showing the means of LH/FSH ratio for normal menstrual cycle group and polycystic ovary syndrome group.
|
In the PCOS group, there were highly significant correlation of FSH (P˂0.001) with LH (r = 0.6) as shown in (Figure7).
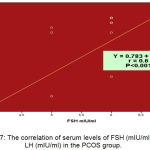 |
Figure 7: The correlation of serum levels of FSH (mIU/ml) with LH (mIU/ml) in the PCOS group.
|
Discussion
This study represents the first attempt to find the association between ghrelin level and non-obese PCOS during day 2 menstrual cycle. The results of our study demonstrate of fasting ghrelin levels with PCOS patients and comparable to control women matched for age and BMI.
Fasting ghrelin concentration was found highly significant elevation in non-obese PCOS in day 2 cycling than healthy women during day 2 cycling (p<0.001). These findings in line with Wasko et al. 5 have reported elevated levels of serum ghrelin in PCOS females compared to healthy controls. Other studies revealed serum ghrelin was lower in the PCOS group than healthy controls.18-20 While study conducted on lean and obese PCOS group and compared to control group, ghrelin level were found to be similar between both groups.21 This discrepancy of results may be explained by confounding factors, such as body weight, fat mass, age, hormonal status and severity of disease.
In the current study there was no significant correlation between ghrelin and BMI, LH and FSH. This result was similar to that obtained by Schofl et al8 showed that ghrelin concentration did not correlate with BMI. While, Daghestani et al 22 showed a significant inverse relationship between ghrelin and BMI in both PCOS and healthy subjects.
Similar to findings of our study, Houjeghani et al 23 showed that not found correlation between ghrelin levels and serum LH, FSH, or LH/FSH ratio in PCOS and control groups. These finding do not support the idea that ghrelin might alter gonadotropin levels.
Study done by Dafopoulos and co-worker 24 demonstrated that serum ghrelin levels remain stable during the normal menstrual cycle. It is suggested that the secretion of these hormone (ghrelin) is not physiologically regulated by ovarian steroids hormone. These findings are line with current study that showed normal levels of ghrelin in control group during menstrual cycle.
Some limitations of the present study were the relative the sample size was low, narrow range of BMI, and measuring ghrelin in fasting only. But to reach better results more study needs to understand the relation between ghrelin and BMI, and ghrelin should measured in fasting and post-prandial states.
References
- Kojima M and Kangawa K. Physio rev. 2005;85(2):495-522.
- Dupont J., Maillard V., Coyral-Castel S., Rame C and Froment P. I nt J Pept. 2010;158102.
- Muccioli G., Tschop M.,Papotti M., Deghenghi R., Heiman M and Ghigo E. Eur J Pharmacol. 2002;440(203):235-254.
CrossRef - Pagotto U., Gambineri A., Vicennati V., Heiman M. L. Tschop M and Pasquali R. J Clin Endocrinol Metab. 2002;87(12):5625-9.
CrossRef - Wasko R., Komarowska H., Warenik-Szymankiewicz A and Sowinski J. Horm Metab Res. 2004;36(3):170-173.
CrossRef - Orio F. Jr.,Lucidi P., Palomba S., Tauchmanova L., Cascella T and Russo T. J Clin Endocrinol Metab. 2003;88(2):942-5.
CrossRef - Scarpitta A. M and Sinagra D. Endocrinol. 2000;14(5):392-5.
- Schofl C.,Horn R., Schill T., Schlosser H. W., Muller M. J and Brabant G. J Clin Endocrinol Metab. 2002;87(10):4607-10.
- Norman R. J., Dewailly D.,Legro R. S and Hickey T. E. Lancet. 2007;370(9588):685-697.
CrossRef - Dasgupta S and Reddy B. M. J Postgard Med. 2008;54(2):115-125.
CrossRef - Erickson G. F. Philadelphia: Lippnicott–Raven. 1996;1143-60.
- Barnes R. B. Curr Ther Endocrinol Metab. 1997;6:256-259.
- Nestler J. E. Semin Reprod Endocrinol. 1997;15:111-22.
CrossRef - Kely C. J.,Speirs A., Gould G. W.,Petvie J. R.,Lyall H and Kelly J. M. J Clin Endocrinol Metab. 2002;87(2):742-746.
- Gulab K.,Neelam N., Monika S, and Nidhi S. Dent.Med Sci. 2015;14(5):64-68.
- National Health and Medical Research Council, Australian Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults. 2003.
- Barbour H. M. J Immunol Methods. 1976;11(1):15–23.
CrossRef - Mitkov M., Pehlivanov B and Orbetzova M. Gynecol Endocrinol. 24(11):625-630.
CrossRef - Glintborg D., Andersen M., Hagen C.,Frystyk J., Hulstrom V and Flyvbjerg A. Eur J Endocrinol. 2006;155(2):337-345.
CrossRef - Kamal M., Mohi A., Fawzy M,and El-Sawah H. Mid E. Fert. Soci. J. 2010;15:91–94.
CrossRef - Welt C. K., Chan J. L., Bullen J. M., Smith P. R and Depaoli A. M. N Engl J Med. 2004:351(10):987-997.
CrossRef - Daghestani M. H., Daghestani M. H and El-Mazny A. Eur J Obstet Gyneocol Reprod Biol. 2011;155(1):65-68.
CrossRef - Houjeghani S., Gargari B and Laya F. J Ferti. Steri. 2012;6(2):117-126.
- Dafopoulos K., Messini C., Anifandis G., Georgulias P., Sourlas D and Messinis I. E. Res. 2016;65:809-814.







