Manuscript accepted on :October 31, 2017
Published online on: --
Dewa Putu Gede Purwa Samatra1,2 , Mahadewa Tjokorda G. B2
, Mahadewa Tjokorda G. B2 , I Dewa Made Sukrama2,3
, I Dewa Made Sukrama2,3 , Ni Wayan Sucindra Dewi4
, Ni Wayan Sucindra Dewi4 , Rian Ka Praja5, Dian Nurmansyah5 and I. Putu Eka Widyadharma2
, Rian Ka Praja5, Dian Nurmansyah5 and I. Putu Eka Widyadharma2
1Neurology Deparment, Faculty of Medicine, Udayana University, Bali, Indonesia.
2Doctoral Program of Biomedical Science, Faculty of Medicine, Udayana University, Bali, Indonesia.
3Clinical Microbiology Department, Faculty of Medicine, Udayana University, Bali, Indonesia.
4Pharmacology Deparment, Faculty of Medicine, Udayana University, Bali, Indonesia.
5Master Degree of Biomedical Science, Udayana University, Bali, Indonesia.
Correspondent Author E-mail: tjokmahadewa@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1290
Abstract
Salmonella typhi infection in typhoid fever can produce oxidative stress. Earthworm, as an herbal medicine, nowadays is widely used to treat cases of typhoid fever. This study aimed to prove the antioxidant effect of earthworm extract (Lumbricus rubellus) in reducing the levels of malondialdehyde (MDA) and 8-hydroxy-deoxyguanosine (8-OHdG) in male wistar rats infected by S. typhi. The research was conducted using posttest-only control group design method with 28 samples divided into 4 treatment groups: the negative control group (not S. typhi infected), positive control (only S. typhi infected), treatment group 1 (infected with S. typhi on day 1 and giving L. rubellus earthworm extract on the next day until day 18) and treatment group 2 (giving L. rubellus earthworm extract in the first week then infected with S. typhi on day 8, followed by extract until day 18th). On the 18th day, blood samples were taken for further measurement of MDA and 8-OHdG levels. Based on the results of statistical analysis, treatment group 1 was not able to significantly decrease MDA levels (p> 0.05) with mean of 4.38 ± 0.38 nmol/mL, while the treatment group 2 significantly decreased MDA levels (p < 0.05) with a mean of 3.54 ± 0.19 nmol/mL. In addition, treatment 1 and 2 significantly decreased levels of 8-OHdG (p <0.05) with mean treatment rates of 0.86 ± 0.08 ng / mL and mean treatment of 2 0,52 ± 0.07 ng / mL. Based on the results, it can be concluded that the earthworm extract (Lumbricus rubellus) can reduce levels of MDA and 8-OHdG in male wistar rats infected by S. typhi.
Keywords
Earthworm; Lumbricus rubellus; malondialdehyde; 8-hydroxy-deoxyguanosine; Salmonella typhi
Download this article as:| Copy the following to cite this article: Samatra D. P. G. P, Tjokorda G. B. M, Sukrama D. M. I, Dewi N. Y. S, Praja R. K, Nurmansyah D, Widyadharma I. P. E. Extract of Earthworms (Lumbricus Rubellus) Reduced Malondialdehyde and 8-hydroxy-deoxyguanosine Level in Male Wistar Rats Infected by Salmonella typhi. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Samatra D. P. G. P, Tjokorda G. B. M, Sukrama D. M. I, Dewi N. Y. S, Praja R. K, Nurmansyah D, Widyadharma I. P. E. Extract of Earthworms (Lumbricus Rubellus) Reduced Malondialdehyde and 8-hydroxy-deoxyguanosine Level in Male Wistar Rats Infected by Salmonella typhi. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17537 |
Introduction
Typhoid fever is an infectious disease caused by Salmonella typhi and is very common in developing countries. A study of population-based typhoid fever involving 13 countries on various continents, reported that during the year 2000 there were 21,650,974 cases of typhoid fever with a 10% mortality rate.1 Indonesia is one of the countries with high incidence of typhoid fever, with reported cases of 180.3/100.000 population in the 5-15 years age group.2
Salmonella typhi infections may result in lipid peroxidase and subsequently causes tissue damage caused by ROS (Reactive Oxygen Species).3 Syphilis-infected rats show elevated lipid peroxidase characterized by increased MDA and decreased GSH (Glutation). This suggests that Salmonella infections may induce oxidative stress in infected hosts
Oxidative stress is a state of imbalance between free radicals and antioxidant, where the amount of free radicals is more than antioxidants.5 Free radicals are molecules that do not have electron pairs in their outer parts. The increase in oxidative stress has a negative impact on the cell membrane structure components, i.e., damage to membrane lipids, malondialdehyde formation (MDA), protein, carbohydrate and DNA damage which eventually result in hemolysis and cellular destruction. Free radicals are quite numerous but the most widely present in the body’s biological systems are the oxygen-derived free radicals or reactive oxygen species.6 ROS formation is caused, among others, by NSAIDs, ischemia-reperfusion, inflammation, UV radiation, alcohol, cigarettes, and infections.7
Malondialdehyde (MDA) is the final product of lipid peroxidation and indicator of the presence of free radicals in the body. Unsaturated fatty acids will become peroxidated producing MDA products and these products can be measured indirectly as products of oxidative damage.8 MDA is a reactive molecule that has a molecular formula C3H4O2 and is known as lipid peroxidation marker.9 Deoxiguanosine (dG) is one of the basic constituents of DNA and as a product of peroxidation by ROS, it will transform into 8-hydroxy-2′-deoxyguanosine (8-OHdG). The mechanism of DNA damage due to radical ROS reactions as a normal metabolic response or response to other environmental factors is due to the radical *OH attached to 2’dG (2’deoxiguanosin) creating 8-OHdG, the form of which is DNA damage marker.10
Antioxidants are compounds that can absorb or neutralize a free radicals and prevent damage caused by free radicals to normal cells, proteins, and fats. Thiese compounds have a molecular structures that can give its electrons to free radical molecules without disturbance to its function and can break the free radical chain reaction.11
Chloramphenicol, ampicillin, and cotrimoxazole are the first-line antibiotics that have been used for decades to treat typhoid fever until eventually a resistance called multidrug resistant Salmonella typhi (MDRST) emerged.12 The irrational use of antibiotics and the presence of intrinsic changes in microbes play important roles in the emergence of this resistance.13 These antimicrobial resistance issues have caused researchers to seek treatment alternatives, one of which is natural ingredients that are ubiquitous in Indonesia. Natural materials used as natural remedies can come from plants and animals. One of the medicines that come from nature is the earthworm (Lumbricus rubellus).
Several types of earthworms have been reported to have bioactive compounds and are shown to inhibit pathogenic bacteria. These active substances include, among others, G-90 glycoproteins and fetidin from Eisenia foetida worms (Annelida, Lumbricidae),14 lysozyme from E. fetida Andrei,15 histidine from Dendrobaena veneta earthworms16 and Nereis diversicolor worms.17 In addition to the inhibition of pathogenic bacteria, earthworm flour (L. rubellus) has a fairly high protein content of 63.06% of its dry matter.18
Earthworm extract contains fibrinolytic enzymes, polyphenols, and G-90 glycoproteins consisting of serine proteases, insulin like growth factor, epidermal growth factor, and immunoglobulin like growth factor, with the ingredients of the worm extract have benefits including anti-apoptosis, anti-thrombosis, anti-coagulation, anti-ischemia, tissue regeneration and wound healing, anti-inflammatory, and antioxidants.19 Earthworm extract (Lumbricus rubellus) has a total content of 247.3 mg/L of penolic content dissolved in 80% ethanol.20 Of the many contents possessed by earthworms, polyphenols are substances that have antioxidant properties.19
Based on the above description, the researchers wanted to know the effect of extract of earthworm (Lumbricus rubellus) in decreasing MDA and 8-OHdG levels in male wistar rats infected by S. typhi.
Material and Methods
This research is a laboratory experimental study using posttest-only control group design.
The Making Process of Lumbricus Rubellus Extract
The collected earthworms were washed with running water to remove the mucus on its surface. Earthworms were then soaked for 6-8 hours in sterile distilled water to remove the soil from the body and then rinsed again using sterile distilled water. The worm was then dried in the oven at 40° C continuously for 24 hours. The dried worm was then cut into small pieces and grinded until smooth to be put into a glass tube of 80% ethanol solvent for 2 days. The mixture was then evaporated to get the crude extract.
The Making Process of Salmonella typhi Suspension
S. typhi bacterial isolates were obtained from the Microbiology Department, Faculty of Medicine, Udayana University. Bacteria were cultured in Mac Conkey Agar, then incubated for 18-24 hours at 37°C. The preparation of S. typhi suspension used cup count. A total of 3-5 colonies were inserted into a tube containing 10 ml of 0.1% pepton, the S. typhi bacteria suspension was then diluted in multiple series of 106. Each dilution of S. typhi suspension was taken as much as 0.1 ml and planted evenly in Mac Conkey Agar. In addition, 0.1 ml of peptone 0.1% was grown to control the contamination. Bacterial dilution and planting were done in duplicate. Colony counting is performed if the number of colonies grows between 20-200 colonies. To determine the contamination of culture, from each dilution, gram staining was performed and observed under a microscope. The resultant concentration was made of bacterial suspension containing 105 cells/ml.
Treatment Protocol of Rats Experiment
This study used 28 healthy white wistar rats strain and normal tail, 2 months old with body weight 200-250 gram. Rats were then divided into 4 groups, each contained 7 rats. The groups were negative control group (not S. typhi infected), positive control (only S. typhi infected), treatment group 1 (infected by S. typhi on day 1 and giving L. rubellus earthworm extract on the next day until day 18) and treatment group 2 (giving L. rubellus earthworm extract in the first week then infected by S. typhi on day 8, followed by extract until day 18). The amount of mice infected by S. typhi was 105 and dosage of earthworm extract was 200 mg/kg. All rats were fed and given drink in ad libitum way. On the 18th day, blood samples were taken for further measurement of MDA and 8-OHdG levels.
Measurement of MDA and 8-OHdG Levels
Measurements of MDA content used 0.5 μL of blood plasma plus 2.0 mL cold HCl (0.25 N) containing 15% TCA, 0.38% TBA and 0.5% BHT. The mixture was heated in 80oC for one hour. After being cooled, it was centrifuged for 10 minutes. Serum/plasma absorption was measured at 532 nm. As standard solutions, tetraoxypropane solution or standard MDA Assay Kit was used.10
The 8-OHdG level was analyzed using the UV-Vis spectrophotometric instrument by following the 8-OHdG DNA Damage ELISA Kit Cell Biolabs Kit.
Data Analysis
Data on MDA and 8-OHdG levels were described, followed by normality test using Saphiro Wilk, followed by homogenity test using Levene’s test, comparability test using ANOVA, and treatment effect using Least Significance Difference (LSD).
Results
Based on the analysis result in Figure 1, the mean control level of MDA positive control group was 4.69 ± 0.45 nmol/mL, negative control group was 3.73 ± 0.06 nmol/mL, treatment group 1 was 4.38 ± 0.38 nmol/mL and treatment group 2 was 3.54 ± 0.19 nmol/mL. Statistical analysis of LSD test showed that treatment 1 did not been able to significantly decrease MDA levels (p> 0.05) despite the visible decrease of MDA levels when compared with the positive control group. The reason was in the treatment group 1, the administration of earthworm extract was only done after the wistar rats were infected by S. typhi. In contrast, the treatment group 2 significantly decreased MDA levels (p <0.05), because the administration of earthworm extract was given a week before the wistar rats got infected and continued until the 18th day. It was obvious that the continuous supplementation of the earthworm extract before and after the infection would be able to reduce or neutralize ROS in a better fashion, as compared with supplementation after the infection only.
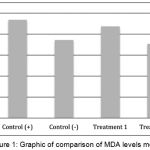 |
Figure 1: Graphic of comparison of MDA levels mean
|
As shown in Figure 2, the mean 8-OHdG levels in the positive control group was 1.11 ± 0.07 ng/mL, the mean negative control group was 0.49 ± 0.04 ng/mL, the treatment group 1 was 0.86 ± 0.08 ng/mL, and the mean of treatment group 2 was 0.52 ± 0.07 ng/mL. Furthermore, statistical tests showed that treatment group 1 and treatment group 2 significantly reduced of 8-OHdG levels (p <0.05).
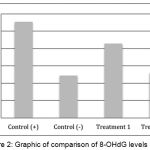 |
Figure 2: Graphic of comparison of 8-OHdG levels mean
|
In this study the mean rate of 8-OHdG treatment group 2 was lower compared with treatment group 1 (p <0.05), and the mean of treatment group 2 was not significantly different when compared with negative control (p >0.05). The reason was in the treatment group 1, the administration of earthworm extract was only done after the wistar rats infected by S. typhi, unlike the case with the treatment group 2, which was given prior to the infection and continued until the 18th day. Provision of earthworm extract before and after infection will certainly be able to increase the capacity of antioxidants higher than the worm extract provided only after infection.
Discussion
In infection, the release of proinflammatory cytokines (IL-2, IL-6, IFN-γ and TNF-α) and anti-inflammatory (IL-4, IL-10) by macrophages results in the release of various secondary mediators such as vasoactive mediators and ROS by monocyte cells, neutrophils and vascular endothelial cells that initiate a series of immunocompromised processes. ROS is the main metabolite produced by the reduction of one electron of oxygen (O2) from the metabolism and chemical reaction in the body, which is a powerful oxidant. Some of the oxidants are free radicals, and their activity can be suppressed by antioxidant compounds. Oxidative stress occurs when the produced ROS is greater than that which can be mitigated by the cell defense mechanism.21
The emergence of ROS in infection has the potential to cause oxidative damage in the form of all cell membranes and tissues containing lipids, including erythrocytes which have two layers composed of two solid molecules of phospholipids. The ROS reactivity results in the molecular structure of the cell membrane composed of cholesterol, phospholipids and glycolipids (both of which contain unsaturated fatty acids) and the DNA very sensitive to the hydroxyl radicals, resulting in cellular damage and the formation of many peroxy-based fatty acid radicals. Lipid peroxidation in erythrocytes result in lysis or commonly known as haemolysis events. This event will result in the release of MDA which in turn will result in the destruction of all cells.22
Malondialdehyde (MDA) is an enzymatic and nonenzymatic product of the breakdown of endoperoxide prostaglandins and the end product of lipid peroxidation. MDA is a reactive molecule that has the molecular formula C3H4O2 and is known as lipid peroxidation marker.9 Deoxyguanosine (dG) is one of the basic constituents of DNA and through oxidation it transforms into 8-hydroxy-deoxyguanosine (8-OHdG). Guanosin can also experiences hydroxylation as a normal metabolic response or due to pollution factors by heavy metals and free radicals.22 The mechanism of DNA damage due to radical ROS reactions as a normal metabolic response or response to other environmental factors is due to the radical *OH attached to 2’dG (2’deoxiguanosin) creating 8-OHdG, the form of which is DNA damage marker.10
The bioactivity of earthworm extract in lowering MDA and 8-OHdG levels is thought to be due to the abundant quantity of antioxidant content of phenolic acid compounds which possesses the ability to neutralizes the ROS reaction resulting from S. typhi infection.21 In addition, earthworm extract also acts as a natural antibiotic that can kills S. thypimurium bacteria so that the ROS resulting from bacterial infection is reduced.24
Previous research has shown that Lumbricus rubellus extract dissolved in 80% methanol contains 228.60 ± 13.07 mg/L phenolic acid, higher than Eudrilus eugenia which contains only 217.00 ± 2.60 mg/L.20 Phenolic compounds are compounds that act as antioxidants.20,25,26 Antioxidant compounds are compounds capable of donating their electrons or 1 molecule of hydrogen to ROS so that ROS becomes stable or in the form of new free radicals that are less reactive.11 Earthworm extract (Lampito mauritii) can act as hepatoprotective and antioxidant indicated by decreased levels of ALP, AST and ALT enzymes and increased GPx, SOD, and CAT in paracetamol-induced wistar rat’s liver tissue.27 Other studies have proven that earthworm extract of other species (Pheretima hawayana and Allolobophora caliginosa) can restore AST and ALT levels to normal and increase GSH, SOD, and CAT in CCL4-induced wistar rat.28 Provision of earthworms (Lampito mauritii) amounted 160 mg/kgBB significantly showed an increase in GSH, GPx, CAT, and SOD and decreased thiobarbituric acid in aspirin-induced Rattus norvegicus.29 GSH, SOD, and CAT are important scavenger for superoxide ions and hydrogen peroxide. These enzymes play a role in protecting cells from free radicals and oxidative damage.
Based on the results of this study, earthworm extract was able in lowering MDA and 8-OHdG levels, predictably due to its antimicrobial effect that can kills S. typhi so that the severity of infection decreased which can further reduces the occurrence of oxidative stress. In addition, extract of earthworms can increase endogenous antioxidant expression such as GSH, GPx, SOD, and CAT that can resist the occurrence of oxidative stress due to free radicals.
Conclusion
Based on the results of this study, it can be concluded that the provision of earthworms extract (Lumbricus rubellus) can reduce levels of MDA and 8-OHdG in male wistar rats infected by S. typhi.
Acknowledgments
This project was financially supported by a grant from Udayana University, Bali, Indonesia
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Crump J.A, Luby S.P, Mintz E.D. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346-53.
- Retnosari S, Tumbeleka A.R, Akib A.P, Hadinegoro S.R.S. Clinical and Laboratory features of thypoid fever in childhood. Pediatr Indonesia 2001;4:149-54
CrossRef - Rishi P, Kaur H, Tirkey N, Chopra K, Bharrhan S, Chanana V, Koul A. Are the increases in local tumor necrosis factor and lipid peroxidation observed in pre-starved mice infected with Salmonella typhimurium, markers of increased liver damage? Microbes Infect. 2006;8:1695-1701.
CrossRef - Khan K.H. Terminalia Chebula Reduces the Oxidative Stress Induced by Salmonella typhimurium in Mice and May Reduce the Risk of Getting Typhoid. Advances in Biological Research. 2009;3(1-2):01-08.
- Halliwell B. Reactive spesies and antioxidants: Redox biology is a fudamental theme of aerobic life. Plant Physiol. 2006;141:312-322.
CrossRef - Kevin C, Kregel, Hannah J.Z. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2006;292:18-36.
- Bhattacharyya A, Chattopadhyay R, Mitra S.S, Crow S.E. Oxidative stress : an essential factor in the pathogenesis of gastrointestinal mucosal disease. Physiol Rev. 2014;94:329-54.
CrossRef - Auroma O.I. Assessment of potential prooxidant and antioxidant actions. American oil chemists society. 1997;73:1717-1625.
- Tokur B, Korkmaz K And Ayas D. comparison of two tiobarbituric acid (TBA) method for monitoring lipid oxidation in fish. J. Fisheries and aquatic Sci. 2006;23(3-4):331-34.
- Parwata I.M.O. Ekstrak air daun gaharu (Gyrinops versteegii) menurunkan kadar malondialdehid dan 8-hidroksi-deoksiguanosine serta meningkatkan aktifitas superoksida dismutase dan katalase tikus wistar dengan aktivitas fisik maksimal, [Disertasi]. [Denpasar]: Universitas Udayana. 2014.
- Murray R.K, Granner D.K, Rodwell V.W. Biokimia Harper. Ed.27 . 2009. Penerbit Buku Kedokteran EGC, Jakarta.
- Sidabutar S, Satari H.I. Pilihan Terapi Empiris Demam Tifoid pada Anak: Kloramfenikol atau Seftriakson? Departemen Ilmu Kesehatan Anak R.S Dr Cipto. Mangunkusumo. Sari Pediatri. 2010;11(6):434-39.
- Hadinegoro S.R. Masalah Multi Drug Resistance pada Demam Tifoid Anak. Cermin Dunia Kedokteran. 1999;124:4-8.
- Popovic M, Grdisa M and Hrzenjak T.M. Glycolipoprotein G-90 obtained from theearthworm Eisenia foetida exerts antibacterial activity. Veterinarski Arhiv. 2005;75:119-128.
- Salzet M, Tasiemski A and Cooper E. Innate immunity in Lophotrochozoans: The Annelids. Curr. Pharm. Des. 2006;12:1-8.
CrossRef - Kalac Y, Kimiran A, Ulakoglu G and Cotuk A. The role of opsonin in phagocytosis bycoelomocytes of earthworm Dendrobaena veneta. J. Cell Mol. Biol. 2002;1:7-14.
- Tasiemski A, Schikorski D, Marrec-Croq F L.E, Camp C.P.V, Boidin-Wichlacz C and Sautiere P.E. Hestidin: A novel antimcrobial peptide containing bromotryptophan constitutively expressed in the NK cells-like of the marine annelid, Nereis diversicolor. Dev. Comp. Immunol. 2006;31:749-762.
CrossRef - Istiqomah L, Sofyan A, Damayanti E and Julendra H. Amino acid profile of earthworm and earthworm meal (Lumbricus rubellus) for animal feedstuff. J. Indonesian Trop. Anim. Agric. 2009;34(4):253-257.|
CrossRef - Chang, Yung-Ming et al. Schwann Cell Migration Induced by Earthworm Extract via Activation of PAs and MMP2/9 Mediated through ERK1/2 and p38. Evidence-Based Complementary and Alternative Medicine. 2011. Article ID 395458.
CrossRef - Aldarraji Q.M, Halimoon N, Madjid N.M. Antioxidant activity and total phenolic content of earthworm paste of Lumbricus rubellus (redworm) and Eudrilus eugenia (African night crawler). Journal of Entomology and Nematology. 2013;5(3):33-37.
CrossRef - Oksidan S, dan A, bebas R. Dalam: Ilmu Kedokteran Molekuler. Kapita Selekta. Jakarta: Sagung Seto. 2000;31-46.
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions,and methods for their quantification. The Society of Toxicologic Pathology. 2002;30:620-650
CrossRef - Chabowska S.A, Beck A, Poreba R, Andrerzjack R.J, Antonowicz J. Evaluation of DNA damage in people occupationally expose to arsenik and some heavy metal. Polish J. Of Environ.Study. 2009;18(6):1131-39.
- Septianda I, Debora K, Rochmanti M. Effect of Earthworms (Lumbricus Sp.) Extract Antibacterial Activity Against The Bacteria Salmonella typhii. Folia Medica Indonesiana. 2012;48(3):102-108.
- Piazzon A, Vrhovsek U, Masuero D, Mattivi F, Mandoj F, Nardini M. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric Food Chem. 2012;60(50):12312-23.
CrossRef - Goufo P and Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, coryzanol, and phytic acid. Food Sci Nutr. 2014;2(2):75–104.
CrossRef - Balamurugan M, parthasarathi K, Ranganathan L.S, Cooper E.L. Hypothetical mode of action of earthworm extract with hepatoprotective and antioxidant properties. J. Zhejiang Univ Sci B. 2008;9(2):141-147.
CrossRef - Omar H.M, Ibraheim Z.Z, El-Shimy M.A, Ali R.S. Anti-inflammatory, antipyretic and antioxidant activities of the earthworms extract. Journal of Biology and Earth Sciences. 2012;2(1):1 0-1 7.
- Prakash M, Balamurugan M, Parthasarathi, Gunasekaran G, Cooper E.L, Ranganathan L.S. Anti-ulceral and anti-oxidative properties of “earthworm paste” of Lampito mauritii (Kinberg) on Rattus Norvegicus. European Review for Medical and Pharmacological Sciences. 2007;11:9-15.







