Minaxi Parmar1, Neepa Pandhi1 and Prabhudas Patel2
1Department of Microbiology, Shree M and N Virani Science College, Yogidham, Rajkot, Gujarat, India.
2Department of Cancer Biology, The Gujarat Cancer Research Institute, Ahmedabad, India.
Corresponding Author E-mail: minaxip@vsc.edu.in
DOI : https://dx.doi.org/10.13005/bpj/1324
Abstract
Sialic acid plays a significant role in cancer due to increased sialylation and sialyltransferase activity. Patients with cancer have been reported to have significant elevations of serum Total Sialic Acid (TSA) and Lipid Bound Sialic Acid (LBSA) levels as compared to control persons. The present study was carried out to evaluate sialic acid levels in control, non-cancer smokers or tobacco chewers and Head and Neck Squamous cell carcinoma (HNSCC) cancer patients. Blood samples were obtained from the histopathologically diagnosed HNSCC patients, healthy controls and those persons who were either smokers or tobacco chewers with no oral cancer. Serum TSA and LBSA were measured spectrophotometrically. Serum TSA and LBSA levels were significantly elevated in HNSCC patients compared to healthy control with P<0.0001. These levels were also significantly increased in individuals who were smokers or tobacco chewers with no cancer compared to healthy control (P<0.0001). Our results found significant elevation of serum sialic acid levels in HNSCC patients and in smokers and tobacco chewers with no cancer as compared to control individuals. These findings suggested role of tobacco in biochemical changes during the malignant transformation. These results also indicate that these parameters can be utilized in diagnosis of the HNSCC.
Keywords
Sialic acid; Head and neck squamous cell carcinoma; spectrophotometer
Download this article as:| Copy the following to cite this article: Parmar M, Pandhi N, Patel P. Clinical Evaluation of Sialic Acid In Head and Neck Squamous Cell Carcinoma Patients and Tobacco Chewers or Smokers with No Cancer. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Parmar M, Pandhi N, Patel P. Clinical Evaluation of Sialic Acid In Head and Neck Squamous Cell Carcinoma Patients and Tobacco Chewers or Smokers with No Cancer. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17985 |
Introduction
In recent years, the field of glycobiology has emerged due to its relevant discoveries in biological as well as medical field with special reference to cancer.1 Glycans have gained importance due to its role in fundamental mechanisms of malignant transformation of the cells and various cellular mechanisms that includes cell signaling, tumor cell invasion and metastasis and immune modulation.2
They are ubiquitously present in all biological systems on the cell surface as a complex array of sugar chains which are mainly conjugated to proteins and lipids. Sialic acid is one class of sugar which is located at the outermost end of glycan chains of all cell types.3 Sialoglycans are involved in regulation of glycoprotein and glycolipid structure, stability, trafficking and they are mediators of wide variety of physiological and pathological processes. Sialic acid plays a significant role in cancer due to increased sialylation and sialyltransferase activity found in some cancer cell.4
Aberrant glycosylation in cell membrane occurs due to newly activated glycosyl transferases during tumorigenesis. Malignant transformation of cell shows the alteration in structural components and glycoprotiens causing the increase in sialic acid levels on the cell surface. Glycoprotein and glycolipid bound sialic acid together makes total sialic acid (TSA) and glycolipid bound sialic acid is termed as lipid bound sialic acid (LBSA). These glycoconjugates enters the circulation due to increased production, secretion and/or shedding from malignant cells resulting in increased levels of sialic acid in blood. 4,5,6,7 Various studies have shown the alteration in sialic acid levels in different cancers. Patients with oral cancer were reported to have significant elevations of serum levels of TSA and LBSA compared to control persons.8,9
India harbors about 57.5% of global head and neck cancer cases, for both sexes.10 The generous use of tobacco in various forms is the most common risk factor for HNSCC, with oral cancer being the most common in Indian population. Various carcinogens present in tobacco may have a role in certain biochemical and molecular changes during malignant transformation. If these alterations are detected well before the appearance of any physical changes associated with cancer, the chances of prevention of the cancer can increase.
The current investigation was aimed at determining the biochemical alterations in serum TSA and LBSA levels in non cancer smoker or tobacco chewers and its comparison with oral cancer patients and healthy controls.
Materials and Methods
Patients
The patients who were histologically diagnosed to have HNSCC, specifically oral cancer were included in the study. The study was approved by Ethical Committee of Smt. V. R. Desai Cancer Research centre, Rajkot. The informed consent was sought from the patients. The patient information was obtained through questionnaire that included demographic (age, gender, marital status, education, occupation, geographic location) and clinical data (family history of cancer, tobacco and alcohol consumption, nutritional status). The information of signs and symptoms of their oral lesion, its duration were also taken.
Control
The persons having no history of oral cancer and are otherwise normal were selected for case-control study. They gave informed consent for the study. Sociodemographic information was obtained through questionnaire. The other group of persons were selected who were tobacco chewers or smokers but do not have show any signs of cancer.
Sample
The venous blood samples were collected from the patients as well as both controls in plain vials. The serum was separated and stored at -80oc until use.
Method
Assays
Estimation of total sialic acid
Total sialic acid content was estimated from serum sample using a periodiate–thiobarbituric acid method. 100 µl serum was hydrolyzed with equal amount of 1N H2SO4, at 80oc for 1 hour. This will release bound sialic acid in serum. The proteins were then precipitated with 1.0 ml of 10% trichloroacetic acid. 0.025N periodic acid was mixed with the supernatant and incubated at 37oc for 30 min. By addition of 2% sodium arsenite the reaction was terminated. After adding 6% thiobarbituric acid, the mixture was kept in boiling water bath for 7.5 min. Adding dimethyl sulphoxide increases the stability of the chromophore. The absorbance was read using spectrophotometer against blank at 549 and 532 nm to overcome any interference from 2-deoxy-D-ribose.
Estimation of Lipid bound sialic acid
Estimation of lipid bound sialic acid from serum was estimated using the method suggested by Katopodis et al.11 50µl serum extraction was done with chloroform–methanol (2:1 v/v) at 4oc. The extract was separated with 0.5 ml of distilled water. Phosphotungastic acid was used to precipitate the aqueous layer. The precipitates were then resuspended in 1 ml of distilled water. LBSA in suspension was determined by resorcinol reagent.
The amount of serum TSA and LBSA were calculated using standard curves which was obtained by various known concentration of N-acetyl Neuraminic Acid
The following formula was used for calculation of TSA and LBSA.
TSA/LBSA (mg/dl) = O.D. of sample/ O.D. of std. X Conc. of std. / Volume of testX100
Results
We included 23 healthy controls, 21 tobacco chewers or smokers without any cancer history and 21 oral cancer patients. Table I shows the mean levels of TSA and LBSA among the study groups. The mean concentrations of TSA and LBSA were increased in oral cancer patients in comparison with controls as well as non-cancer tobacco chewers/smokers. The mean levels of serum TSA and LBSA were compared statistically by independent (unpaired) student t test and One Way ANOVA test using Graph Pad prism 7.03 software. The difference between healthy controls and HNSCC patients, HNSCC and non-cancer tobacco chewers/smokers and Healthy controls and non-cancer tobacco chewers/smokers were statistically significant by individual t test with p value <0.0001 for each pair (Table II). The One Way ANONVA was carried out to compare the difference in the levels of TSA and LBSA among the study groups (Table III and Figure I, II) and the significant difference was found between the various study groups (p value <0.0001).
Table 1: Mean levels of TSA and LBSA in study groups
| Study groups | LBSA mg/dl
Mean± SD |
TSA mg/dl
Mean± SD |
| Healthy Control (23) | 23.3±3.58 | 52.15±4.40 |
| Non-cancer Tobacco chewers/smokers (21) | 31.29±2.20 | 61.54±2.77 |
| HNSCC patients (21) | 43.23±3.88 | 80.86±5.25 |
Table 2: Comparison of study groups by unpaired t test
| Test | Group | t-value | p-value |
| LBSA | Healthy control | 8.802 |
P<0.0001
|
| Non Cancer Tobacco chewers/smokers | |||
| Non Cancer Tobacco chewers/smokers | 12.26 | ||
| HNSCC patients | |||
| Healthy control | 17.72 | ||
| HNSCC patients | |||
| TSA | Healthy control | 8.372 | P<0.0001 |
| Non Cancer Tobacco chewers/smokers | |||
| Non Cancer Tobacco chewers/smokers | 14.91 | ||
| HNSCC patients | |||
| Healthy control | 19.72 | ||
| HNSCC patients |
Table 3: Comparison of study groups by One Way ANOVA test
| Test | Study Group | F –value | p-value |
| LBSA | Healthy control | 200.1 | P<0.0001 |
| Non Cancer Tobacco chewers/smokers | |||
| HNSCC patients | |||
| TSA | Healthy control | 255.2 | P<0.0001 |
| Non Cancer Tobacco chewers/smokers | |||
| HNSCC patients |
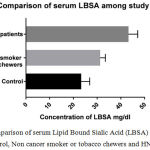 |
Figure 1: Comparison of serum Lipid Bound Sialic Acid (LBSA) levels between Healthy control, Non cancer smoker or tobacco chewers and HNSCC patients. |
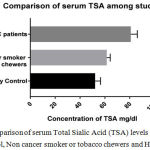 |
Figure 2: Comparison of serum Total Sialic Acid (TSA) levels between Healthy control non cancer smoker or tobacco chewers and HNSCC patients. |
Discussion
The carbohydrate molecules in glycolipids and glycoproteins are often changed during malignant transformation. Altered glycosylation of glycoconjugates plays important role in malignant transformation. Spontaneous discharge of abnormal sialic acid rich glycoproteins and glycolipids is responsible for elevated levels of sialic acid in cancer patients. They have significant diagnostic and prognostic value.1,4,12 The aberrant structure of terminal sialic acid and its up regulation is considered as a hallmark of cancer.13,14,15
Comparison of alterations in sialic acid levels between oral precancer and oral cancer have been done in many studies. Increased levels of LSA and protein bound hexose in oral precancer and high levels of TSA and LBSA were found in oral cancer,16,17,18 while the significant increase in TSA and LBSA levels in both precancer and oral cancer were reported compared to healthy controls.8 A study showed the increase in serum TSA in oral precancer and oral cancer compared to control individuals.19
Significantly increased levels of serum TSA and LBSA were reported in oral precancer patients compared to control group in one study.20,21 The study also found the statistically significant difference in serum TSA and LBSA levels between untreated oral cancer and precancer group. This indicates that serum TSA and LBSA can be used to distinguish between oral cancer and precancer.
Biochemical changes in glycoproteins begin at an early stage of tumorigenesis. Elevated TSA and LSA values are indicative of a premalignant change. Through constant monitoring of these parameters malignancy can be detected at an early stage.22 This surely signifies that cell surface glycoconjugates are altered during early malignant transformation.23
Measurement of serum sialic acid in cancer patients can serve as a diagnostic and prognostic marker in malignant diseases. While comparing the serum TSA and LBSA with other tumor markers like ferritin, carcinoembryonic antigen and neuron specific enolase, it was found that TSA, LBSA and TSA/total proteins are better in monitoring the disease extent and anticancer therapy. Sialic acid can be a useful biomarker for monitoring of response to chemotherapy of those cancers where tumor markers are unavailable.24 Sialic acids promote tumorigenesis and enhance tumor progression at multiple levels by facilitating escape from apoptosis, formation of metastasis, and resistance to therapy.
Most of the previous study compared the serum TSA and LBSA between oral cancer, pre-cancer and healthy control.25,26 The present study included the oral cancer, healthy control and non-cancer smokers or tobacco chewers who have no sign of any cancer in oral cavity. This study reported the significant elevation in serum TSA and LBSA levels in HNSCC patients compared to healthy control. This study is consistent with earlier studies.17,26,27,28,29 Further sialic acid levels were significantly increased in those persons who are either smokers or chewers of tobacco, but do not have cancer yet, compared to healthy individuals. The rise in glycoprotein components in these individuals surely indicates that tobacco usage is responsible for early biochemical changes in sialic acid and altered surface carbohydrate composition resulting into aberrant cell-cell recognition leading to malignant transformation of the cell. The high levels of serum TSA and LBSA in individuals who are habituated to tobacco chewing or smoking without any apparent lesions in mouth can be considered as sign for future neoplastic changes. This group should be closely monitored over a period of time to check any malignant transformation. The serum TSA and LBSA analysis can be done in these individuals if they have refrained from the use of tobacco to ensure the effect of tobacco on serum glycoconjugates.
Limitations of this study are smaller sample size and patients after the treatment were not included in our study. Thus, larger sample size including pre and post treatment patients for close monitoring of sialic acid should be used for considering the usefulness of sialic acid a potential biomarker in oral cancer.
Conclusion
The present study concluded that evaluation of serum TSA and LBSA can differentiate between oral cancer and healthy individual. The study also indicated that tobacco chewing and smoking can lead to abnormal glycosylation in the cell membrane that is responsible for elevated levels of sialic acid. Serum TSA and LBSA can be considered as useful biomarkers for assessing the malignant transformation, its spread and invasiveness. Further large scale study may be carried out to show the association of these factors with the extent of malignancy, metastasis and prognosis of the disease.
Acknowledgement
I am grateful to Dr. Gupta VK, Medical Director of Smt V. R. Desai Cancer Research centre, Rajkot and hospital staff for providing necessary samples.
Conflicts of Interest
No conflict of interest exists.
Reference
- Oliver M. T. P and Läubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26(2):111–128. doi: 10.1093/glycob/cwv097.
CrossRef - Pinho S. S and Reis C. A. Glycosylation in cancer mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540-555.
CrossRef - Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029.
CrossRef - Bull C., Marieke A. S., den Brok M. H and Gosse J. A. Sialic Acids Sweeten a Tumor’s Life. Cancer Res. 2014;74(12):3199-204. doi: 10.1158/0008-5472.
- Bathi R. J., Nandimath K., Kannan N., Shetty P. Evaluation of glycoproteins as prognosticators in head and neck malignancy. Indian J Dental Res. 2001;12:93–99.
- Meany D. L and Chan D. W. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clinical proteomics. 2011;8:7.
CrossRef - Tuccillo F. M., de Laurentiis A., Palmieri C., Fiume G., Bonelli P., Borrelli A., Tassone P., Scala I., Buonaguro F. M., Quinto I and Scala G. Aberrant glycosylation as biomarker for cancer focus on CD43. Biomed Res Int. 2014;2014:742831.
CrossRef - Rajpura K. B., Patel P. S., Chawda J. G., Shah R. M. Clinical significance of total and lipid bound sialic acid levels in oral pre- cancerous conditions and oral cancer. J Oral Pathol Med. 2005;34:263-7.
CrossRef - Patolia K., Shah M. An Assessment of Serum Total Sialic Acid in Oral Leukoplakia and Oral Squamous Cell Carcinoma at Nadiad, Gujarat. International Journal of Medical Pediatrics and Oncology. 2016:2(2):56-59.
CrossRef - Kulkarni M. R. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4:29–35.
CrossRef - Katopodis N., Hirashut Y., Nancy J. G., Stock C. C. Lipid associated sialic acid test for the detection of human cancer. Cancer Res. 1982;42:5370–75.
- de-Freitas-Junior J. C and Morgado-Diaz J. A. The role of N-glycans in colorectal cancer progression Potential biomarkers and therapeutic applications. Oncotarget. 2016;7:19395-413. doi: 10.18632/oncotarget. 6283.
CrossRef - Hakomori S. Glycosylation defining cancer malignancy new wine in an old bottle. PNAS. 2002;99:10231–3.
CrossRef - Amon R., Reuven E. M., Leviatan Ben-Arye S., Padler-Karavani V. Glycans in immune recognition and response. Carbohydr Res. 2014;389:115–122.
CrossRef - Munkleyand J., David J. E. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7(23):35478–35489. doi: 18632/oncotarget.8155.
- Baxi B. R., Patel P. S., Adharyu S. G., Dayal P. K. Usefulness of serum glycoconjugates in precancerous and cancerous diseases of oral cavity. Cancer. 1991;67:135–40.
CrossRef - Kinnari B. R., Prabhudas S. P., Jyoti G. C., Raksha M. S. Clinical significance of total and lipid bound sialic acid levels in oral pre-cancerous conditions and oral cancer. J Oral Pathol Med. 2005;34:263-67.
CrossRef - Hemanth S. C., Anand K. Correlation of serum biomarkers (TSA & LSA) and epithelial dysplasia in early diagnosis of oral precancer and cancer. Cancer Biomarkers. 2011;10:43-49.
CrossRef - Joshi M., Patil R. Estimation and comparative study of serum total sialic acid levels as tumor markers in oral cancer and precancer. J Cancer Res Ther. 2010;6:263-6.
CrossRef - Taqi S. A. Clinical evaluation of total and lipid bound sialic acid levels in oral precancer and oral cancer. Indian J Med Paediatr Oncol. 2012;33:36‑41.
CrossRef - Achalli S., Madi M., Babu S. G., Shetty S. R., Kumari S., Bhat S. Sialic acid as a biomarker of oral potentially malignant disorders and oral cancer. Indian J Dent Res. 2017;28:395-9.
CrossRef - Yadav A., Gahlaut V., Gahlaut P. S. Serum total sialic acid as a marker for prognosis of chemotherapy in patients of acute leukemia. Res J Pharm Biol Chem Sci. 2011;2:220.
- Dadhich M., Prabhu V. R., D’souza J., Harish S., Jose M. Serum and salivary sialic acid as a biomarker in oral potentially malignant disorders and oral cancer. Indian J Cancer. 2014;51:214-18.
CrossRef - Harvey H. A., Lipton A., White D., Davidson E. Glycoprotein’s and human cancer: II Correlation between circulating levels and disease status. Cancer. 1981;47:324-7.
CrossRef - Bose K. S., Gokhale P. V., Dwivedi S., Singh M. Quantitative evaluation and correlation of serum glycoconjugates Protein bound hexoses sialic acid and fucose in leukoplakia oral sub mucous fibrosis and oral cancer. J Nat Sci Biol Med. 2013;4:122–5.
CrossRef - Rajaram S., Danasekaran B. P., Venkatachalapathy R., Prashad K. V., Rajaram S. Nacetylneuraminic acid: A scrutinizing tool in oral squamous cell carcinoma diagnosis. Dent Res J. 2017;14:267-71.
CrossRef - Manjiri J., Ranjithkumar P. Estimation and comparative study of serum total sialic acid levels as tumour markers in oral cancer and precancer. Journal of Cancer Research and Therapeutics. 2010;6:263-66.
CrossRef - Ningappa S. C., Ashok L., Chandrashekhar K. V., Mysore S. K. S., Eraiah G. N and Garg R. Evaluation of serum sialic acid, fucose levels and their ratio in oral squamous cell carcinoma. J Int Soc Prev Community Dent. 2015;5(6):446–450. doi: 4103/2231-0762.169211.
- Krishnan K and Balasundaram S. Sialic Acid a Prognostic Marker in Premalignancy. Journal of Clinical and Diagnostic Research. 2017;11(3):25-27.







