Rizal Maarif Rukmana1,4 , Nyoman Puniawati Soesilo1
, Nyoman Puniawati Soesilo1 , Rumiyati2
, Rumiyati2 and Rarastoeti Pratiwi3
and Rarastoeti Pratiwi3
1Faculty of Biology, Universitas Gadjah Mada, Jl.Teknika Selatan, Sekip Utara Yogyakarta 55281, Indonesia.
2Faculty of Pharmacy, Universitas Gadjah Mada, Jl.Teknika Selatan, Sekip Utara, Yogyakarta 55281, Indonesia.
3Biochemistry Laboratory, Faculty of Biology, Universitas Gadjah Mada, Jl.Teknika Selatan, Sekip Utara, Yogyakarta 55281, Indonesia.
4Departement of Medical Laboratory Technology, Faculty of Health Science, Setia Budi University, Jl. Letjend Sutoyo, Mojosongo, Surakarta.
Corresponding Author E-mail: rarastp@ugm.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1279
Abstract
Black rice bran has a number of health benefits and it contains a phytochemical that is associated with a decreased cancer risk. This research aimed to examine the ability of ethanolic extract fractions of ‘Woja Laka’ black rice bran to prevent the growth of liver carcinoma HepG2 cells. Ethanolic extracts of ‘Woja Laka’ and ‘IR 64’ (white rice) bran were fractionated by preparative thin layer chromatography. The cytotoxic activities of the fractions were conducted on HepG2 cell lines by using the MTT assay. When the IC50 value of the fraction showed lower than 500 µg/ml, it was tested for anti-proliferation using the doubling time method. Apoptotic induction and cell cycle arrest were measured by a flow cytometry method. The selectivity cells respon was performed on Vero cells. Result of this study showed that six fractions (F) of F1, F2, F3, F4, F5 and F6 from the ‘Woja Laka’ and three fractions of F1, F2 and F3 from the ‘IR 64’ were examined for their preventive activities on HepG2 cells. The selected fractions F3 and F6 from ‘Woja Laka’ showed highly cytotoxic activity (184.35 ± 16.81 and 283.64 ± 58.13 µg/ml respectively) on HepG2 cells. Furthermore, the fractions of the white rice bran extract showed IC50 > 500 µg/ml. We found that the fractions F3 and F6 from ‘Woja Laka’ were able to inhibit cell proliferation, induce apoptosis and cause G0/G1-phase arrest in the HepG2 cells, while the Vero cells were less responsive to these fractions (IC50 > 470 µg/ml). These results suggest that the compounds found in fractions F3 and F6 of the ethanolic extract of ‘Woja Laka’ black rice bran inhibit the growth of liver carcinoma HepG2 cells.
Keywords
Apoptosis; Black Rice;Cell Cycle Cytotoxicity;HepG2 Cells; Proliferation;
Download this article as:| Copy the following to cite this article: Rukmana R. M, Soesilo N. P, Rumiyati R, Pratiwi R. Chemopreventive Activities of ‘Woja Laka’ Black Rice Bran Fractions on Liver Carcinoma HepG2 Cells. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Rukmana R. M, Soesilo N. P, Rumiyati R, Pratiwi R. Chemopreventive Activities of ‘Woja Laka’ Black Rice Bran Fractions on Liver Carcinoma HepG2 Cells. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17271 |
Introduction
Cancer is a cellular disease caused by abnormal cell proliferation and it thus prohibits the normal functioning of the organ in which these cells are located. Cancer can develop in multicellular organisms due to a range of predisposing factors, including poor diet, unhealthy lifestyle, heredity and continuous exposure to various carcinogenic elements.1 The important characteristic of cancer is that it leads to abnormal tissue growth and the ability to invade the surrounding tissues and then metastasize.2 In Indonesia, cancer is the second leading cause of death after heart disease. Indeed, for every 100,000 residents in Indonesia, there are 257 new cancer patients.3
Liver cancer is the most common cancer worldwide with approximately 500,000 new cases every year, representing the second largest cause of cancer-related death in men and the sixth leading cause in women.4 The incidence of human liver cancer can be due to various risk factors, such as chronic viral hepatitis B or hepatitis C, cirrhosis, alcohol consumption, aflatoxin exposure and iron overload. This disease usually occurs in people aged 50 or above and it is more common in developing countries (85%).5,6
Numerous attempts have been made to develop safer and more affordable remedies for cancer and to increase the number of preventive measures that are available. Many methods for cancer treatments are done using radiotherapy, surgical intervention and chemotherapy. However, the success rate of these methods is still less than 30%.7 Prospective cancer treatments can be found by exploring the bioactive compounds found in natural ingredients. More than 60% of cancer drugs are obtained from natural materials such as plants, microbes, fungi, invertebrates and vertebrates. Many of bioactive compounds from natural ingredients have specific targets and few side effects.8
Black rice is one of the functional foods that has been shown to possess a number of functions that benefit health. The aleurone layer of black rice (the rice bran component) contains various compounds such as phytosterols, γ-oryzanol, tocopherol, tocotrienols, simple phenolic compounds and anthocyanins (cyanidin-3-glucoside, peonidin-3-glucoside, malvidin and pelargonidin-3,5-diglucoside). These compounds have the potential to be anti-oxidative, anti-cancer, anti-mutagenic, anti-inflammatory, anti-bacterial, anti-diabetic, anti-cholesterol, anti-allergenic and anti-carcinogenic.9 The bioactive compounds from the black rice bran can be obtained by extraction and fractination method.
Previous studies have reported that the ethanolic extract of the black rice bran cultivars ‘Woja Laka’, ‘Toraja’ and ‘Cempo Ireng’ contained phytochemicals, such as phenolics, flavonoids, terpenoids and steroids, while no alkaloids could be detected. The ethanolic extract of these three black rice bran cultivars has varied cytotoxic activity on HepG2 and Raji cell lines. The IC50 values of the ethanolic extract of these black rice bran cultivars in respect of HepG2 cells were 857.23±99.19; 1,896.55±83.8 and 1,494.47±87.81 µg/ml respectively, while the IC50 values for the Raji cells were 816.61±85.31; 1,079.93±28.31 and 1,627.82±119.82 µg/ml respectively.10 The ethanolic extract of the black rice bran cultivar ‘Woja Laka’ had greater cytotoxicity on HepG2 and Raji cell lines than the other extracts were tested.10 Previous studies have shown that the ethanolic fraction of the black rice bran ‘Woja Laka’ was able to inhibit cell proliferation, induce apoptosis, and caused the arrest condition of the G2/M phase in the WiDr cell lines.11 This present research performed on HepG2 cell line which is a model of hepatocellular carcinoma. The aims of present study were to discover the chemopreventive activities of ‘Woja Laka’ black rice bran fractions on HepG2 cells. Black rice bran ‘Woja Laka’ and white rice bran ‘IR 64’ were fractionated using preparative thin layer chromathography (TLC). The chemopreventive analysis in this research consists of cytotoxic activity, anti-proliferation, induce apoptosist and cell cycle arrest tests.
Materials and Methods
Rice Materials
Samples of the black rice bran cultivar ‘Woja Laka’ were collected from an organic farmer in Kepanjen, Malang, East Java Province, Indonesia. Meanwhile, the white rice bran cultivar ‘IR 64’ was obtained from an organic farmer in Minggir, Sleman, Yogyakarta, Indonesia. All the rice samples were collected in February 2016.
Cell Lines
HepG2 cells (ATCC@HB-8065) and Vero cells (ATCC@CCL-81) were obtained from the Laboratory of Parasitology, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Chemicals
DMEM medium, M199 medium, FBS (Fetal Bovine Serum) 10% v/v, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide), trypsin-EDTA and buffer kit annexin-V-FLUOS were purchased from Gibco®, Grand Island, USA. Penicillin-streptomycin 2%, RNase, PI staining and phosphate buffer saline (PBS) were obtained from Sigma/Aldrich, St. Louis, MO, USA. Dimethyl sulfoxide (DMSO), stopper reagent SDS 10% in 0.01N HCl, ethanol, HCl, water, glacial acetic acid and n-butanol were acquired from Merck Darmstadt, Germany. Preparative thin layer chromatography plates were purchased from the Biology Department, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Methods
Fractionation of Ethanolic Extract of Rice Bran
The ethanolic extracts of the black rice bran ‘Woja Laka’ and the white rice bran ‘IR 64’ were fractionated using preparative thin layer chromatography with mobile phase n-butanol: glacial acetic acid: water (4:1:5) and stationary phase plates silica gel G60F254. The plates were activated for use in an oven for 2 h at 80°C. A streak of ethanolic extract was smeared manually onto a preparative TLC glass plate. Then, the plate was developed in a saturated glass chamber. With regard to the separated spot zones, the marked separated zones were scrapped off the plates with a spatula onto a conical tube. The spot zones were dissolved in ethanol and centrifuged at 3000 rpm for 10 minutes in order to remove the silica. The supernatant was collected and evaporated in micro tubes.
Cytotoxic Assay of ‘Woja Laka’ and ‘IR 64’ Fractions (MTT Assay Method)
Each HepG2 and Vero cell culture was plated at a density of 104 cells/100µL media and distributed into 96-well plates and allowed to incubate overnight in a 5% CO2 incubator at 37°C. Then, 100 μL of ‘Woja Laka’ and ‘IR 64’ fractions in varied series of concentration (62.50; 125; 250; 500; 1000 μg/mL) was added into the wells and incubated for 48 hours. As a positive control, 100 μL of doxorubicin (a comercial anti-cancer drug) in different concentrations (10; 5; 2.5; 1.25; 0.625 μg/mL) was treated into the wells, which contained 100 μL of HepG2 cells suspension. The treatment medium was discarded after 48 h and substituted with an MTT solution (sterile stock solution of 5 mg/ml) which was added to the cell media at a final concentration of 100 μg/ml. This solution was incubated for 4–6 h at 37°C in a 5% CO2 incubator. The MTT reaction was terminated with the stopper reagent (SDS 10% in 0.01N HCl) and then incubated overnight at room temperature. The test result was measured at 595 nm (microplate ELISA reader). The percentage of cell viability was calculated using the formula:
![]()
in which X= cell viability, A = absorbance of cell control, B = absorbance of media control and C = absorbance of fractions.12
Cell Proliferation Test
The method used for the proliferation test was the cytotoxic test. The dosages used included 1/4 IC50, 1/2 IC50, IC50 and 2 IC50 cytotoxicity (Table 4), and incubation was conducted at time intervals of 0, 24, 48 and 72 h.
Apoptosis Test (Flow Cytometry Method)
HepG2 cell cultures were plated at 5 x 105 cells/ml medium and distributed into 6-well plates and allowed to incubate overnight in a 5% CO2 incubator at 37°C. After incubation, the HepG2 cells were treated using active fractions (1/2 IC50 dosage and IC50 dosage) and incubated for 48 hours. The cells were harvested using trypsin and washed using PBS. The cells were stained using 100 μL of Annexin-VFLUOUS staining kit and incubated in a dark room for 30 minutes at a temperature of 25°C–27°C. Apoptotic and necrotic cells were analyzed using the flow cytometer FACSCalibur.
Cell Cycle Test (Flow Cytometry Method)
In order to study the stages of the cell cycle influenced by the active fractions, a flow cytometry analysis of the cell cycle was carried out. HepG2 cells were seeded in a 6-well plate at an initial density of 5 x 105 cells per well and allowed to incubate overnight in a 5% CO2 incubator at 37°C. The cells were treated with a different concentration of active fractions (1/2 IC50 dosage and IC50 dosage) and incubated for 48 hours. Then, the attached cells were collected after trypsinization and centrifugation at 2000 rpm for 5 minutes. The cells were then fixed with 70% ethanol at 4°C for 30 minutes. Finally, the cell suspension was placed into a flow cytometry tube and analysed by flow cytometer.
Data Analysis
The higher activity of the fractions was evaluated by their lower IC50 value. Probit analysis was used to calculate the IC50 values. The proliferation test was analyzed using a correlation test of observation time versus absorbance. The apoptosis data were analyzed by comparing treatment and control. The cell cycle data were analyzed by using Modfit LT 3.0 to detect the cell distribution in each phase of G0/G1, S and G2/M.
Results
Fractionation of the Ethanolic Extract of the Black Rice Bran ‘Woja Laka’ and the White Rice Bran ‘IR 64’
The ethanolic extract of the black rice bran ‘Woja Laka’ and the white rice bran ‘IR 64’ was separated by preparative thin layer chromatography with mobile phase n-butanol: glacial acetic acid: water (4:1:5) and stationary phase plates silica gel G60F254. The black rice bran ‘Woja Laka’ extract resulted in separation of a total of six fractions, as shown in Figure 1A. Meanwhile, the ‘IR 64’ extract resulted in separation of a total of three fractions, as shown in Figure 1B. The characteristics of the fractions including their yield and Rf values are presented in Table 1
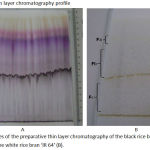 |
Figure 1: Profiles of the preparative thin layer chromatography of the black rice bran ‘Woja Laka’ (A) and the white rice bran ‘IR 64’ (B).
|
1: fraction 1, 2: fraction 2, 3: fraction 3, 4: fraction 4, 5: fraction 5, 6: fraction 6 from ‘Woja Laka’. Fa: fraction 1, Fb: fraction 2 and Fc: fraction 3 of ‘IR 64’.
Table 1: Characteristics of the fractions of ‘Woja Laka’ and ‘IR 64’
| Fractions of ‘Woja Laka’ | Quantity (mg) | Rf values | PTLC Coloration |
| F1 | 80 | 0.971 | Yellow |
| F2 | 90 | 0.853 | Pink |
| F3 | 100 | 0.758 | Purple |
| F4 | 105 | 0.679 | Violet |
| F5 | 270 | 0.601 | Reddish brown |
| F6 | 280 | 0.404 | Dark brown |
| Fractions of ‘IR 64’ | Quantity (mg) | Rf values | PTLC Coloration |
| F1 | 220 | 0.781 | Yellow |
| F2 | 280 | 0.718 | Violet |
| F3 | 380 | 0.437 | Purple brown |
Cytotoxic Activity of the ‘Woja Laka’ and ‘IR 64’ Fractions
Six fractions of ‘Woja Laka’ and three fractions of ‘IR 64’ were obtained and tested for cytotoxic activity on HepG2 cell. Fraction 3 (F3) of ‘Woja Laka’ showed the most cytotoxic effect on the HepG2 cell lines, followed by fraction 6 (F6) of ‘Woja Laka’, with IC50 values of 184.35±16.81 µg/ml and 283.64±58.13 µg/ml respectively. The IC50 values of the fractions against the HepG2 cells are shown in Table 2. Then, F3 and F6 of ‘Woja Laka’, which had demonstrated a cytotoxic effect, were selected to test for cytotoxic activity on the Vero cell line. The IC50 values of the fractions against the Vero cells are shown in Table 3.
Table 2: The IC50 values of the fractions against the HepG2 cells
| Fractions of ‘Woja Laka’ | IC50 Values (µg/ml) |
| F1 | 3146.32 ± 124.11 |
| F2 | 3112.35 ± 260.7 |
| F3 | 184.35 ± 16.81 |
| F4 | 1698.14 ± 220.77 |
| F5 | 846.41 ± 99.59 |
| F6 | 283.64 ± 58.13 |
| Fractions of ‘IR 64’ | IC50 Values (µg/ml) |
| F1 | 822.95 ± 112.97 |
| F2 | 1307.08 ± 125.85 |
| F3 | 1239.6 ± 37.26 |
| Doxorubicin | 6.43 ± 0.12 |
Table 3: The IC50 values of fraction 3 and fraction 6 of ‘Woja Laka’ against the Vero cells
| Fractions of ‘Woja Laka’ | IC50 Values (µg/ml) |
| F3 | 473.15 ± 6.20 |
| F6 | 571.04 ± 2.53 |
Cell Proliferation
Proliferative kinetic assay of fractions F3 and F6 of ‘Woja Laka’ at dosages of ¼.IC50, ½.IC50, IC50 and 2.IC50 (Table 4) with incubation periods of 0, 24, 48 and 72 hours showed a significant influence on the proliferation of the HepG2 cells (Figure 2).
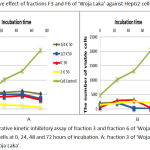 |
Figure 2: Proliferative kinetic inhibitory assay of fraction 3 and fraction 6 of ‘Woja Laka’ against HepG2 cells at 0, 24, 48 and 72 hours of incubation. A: fraction 3 of ‘Woja Laka’. B: fraction 6 of ‘Woja Laka’.
|
Apoptosis Induction
Table 5 shows the percentage of apoptotic, necrotic and viable cells in a population of HepG2 cells treated with different concentrations (1/2 IC50, IC50) from F3 and F6 of ‘Woja Laka’. The IC50 concentrations of F3 had the highest apoptotic induction activity compared to the other concentrations and fractions.
Table 4: The percentage of apoptotic and necrotic HepG2 cells after treatment with fractions F3 and F6 of ‘Woja Laka’.
| Cell Control | 1/2 IC50 F3 | IC50 F3 | 1/2 IC50 F6 | IC50 F6 | |
| Viable cells | 86.84±0.064 | 34.525±0.29 | 15.4±0.089 | 30.57±0.3 | 39.875±0.36 |
| Apoptosist | 6.27±1.22 | 51.76±0.012 | 73.16±0.05 | 61.32±0.011 | 46.235±0.10 |
| Necrosist | 6.93±0.073 | 13.945±0.14 | 11.51±0.14 | 8.125±0.021 | 14.175±0.03 |
Cell Cycle Arrest
To detect the effect of fractions F3 and F6 of ‘Woja Laka’ on cell cycle, the cells were treated with different concentrations of these fractions for 48 hours. Cell cycle analysis was performed by flow cytometry. The results show that factions F3 and F6 of ‘Woja Laka’ induced G0-G1 phase arrest (Figure 3 and Table 6). After 48 hours of treatment with various concentrations of F3 of ‘Woja Laka’, there was an increase in the number of cells arrested at the G0-G1 growth phase compared with the other treatment.
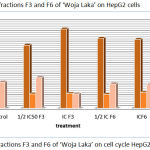 |
Figure 3: Effect of fractions F3 and F6 of ‘Woja Laka’ on cell cycle HepG2 cells.
|
Table 5: Cell cycle assay for fractions F3 and F6 of ‘Woja Laka’ against HepG2 cells (%)
| Cell control | ½IC50 F3 | IC50 F3 | ½IC50 F6 | IC50 F6 | |
| GO/G1 | 61.35 | 58.72 | 73.8 | 65.22 | 64.06 |
| S | 14.83 | 13.26 | 13.66 | 12.91 | 14.61 |
| G2/M | 24.28 | 28.53 | 13.35 | 22.6 | 22.03 |
Discussion
Silica gel G60F254 was used as a stationary phase because it has a greater polarity than the compounds in the sample. Accordingly, the desired active compounds could be separated from the other compounds.12 In the present study (Table 1), the ethanolic extract of ‘Woja Laka’ and ‘IR 64’ was fractionated using mobile phase n-butanol: glacial acetic acid: water (4:1:5). The best mobile phase for fractionation of the ethanolic extract of the black rice bran ‘Woja Laka’ is n-butanol: glacial acetic acid: water (4:1:5).11
The present study demonstrated that fractions F3 and F6 of the black rice bran ‘Woja Laka’ have high cytotoxic activity on HepG2 cells (Figure 4). However, the fractions of the white rice bran ‘IR 64’ appear to have IC50 values > 500 µg/ml. Doxorubicin, a cancer therapeutic drug standard, was used as a positive control in this experiment and showed great toxicity on the HepG2 cells (Table 2). Fractions F3 and F6 of ‘Woja Laka’ were evaluated for their cytotoxicity activities against Vero cell lines, as a model of non-human carcinoma cells. The IC50 values of fractions F3 and F6 on the Vero cells were 473.15 ± 6.20 and 473.15 ± 6.20 µg/ml respectively (Table 3). These fractions did not show any cytotoxicity on the Vero cells. This result is consistent with the previous study which suggested that the ethanolic extract of the black rice bran ‘Woja Laka’ is more cytotoxic on the HepG2 and Raji cells but less cytotoxic on the Vero cells. This signifies that the black rice bran extract has better selectivity on both cells compared to the white rice bran extract.10
Previous studies suggested that the methanolic extract of the black rice bran cultivars ‘Nan’, ‘Doi Saket’ and ‘Payao’, and the white rice bran cultivar ‘KK6’, have been evaluated for their cytotoxic effect on HepG2 cells. The results of this study indicate that the methanolic extract of the black rice ‘Payao’ was the most cytotoxic in respect of the HepG2 cells compared to the other extracts. The IC50 value of the ‘Payao’ extract against the HepG2 cells was 175.95±8.02 µg/ml. From HPLC analysis, the active components of this rice are the anthocyanins: cyanidin 3-glucoside and peonidin 3-glucoside.13 Two bioactive compounds, peonidin 3-glucoside and cyanidin 3-glucoside from Oryza sativa L. indica, showed growth inhibition activity on the HS578T cells14 and HeLa cells15. Cyanidin and malvidin from Oryza sativa cv. Heugjinjubyeo (Gramineae) mediate cytotoxicity on U937, human monocytic leukemia cells had IC50 values of 60 and 40 µg/mL respectively.16
The anti-proliferative activity of fractions F3 and F6 was confirmed by monitoring the proliferation kinetics of the exponentially growing HepG2 cell lines. Fractions F3 and F6 inhibited the proliferation of HepG2 cells in a dose and time-dependent manner. Proliferative inhibition began at the 24-hour incubation period. Previous studies have explored the potential of fraction F6 of ‘Woja Laka’ to inhibit the proliferation of WiDr cell lines.11 Cell growth and proliferation are mediated through cell cycle progression, and defects in the cell cycle are one of the most common characteristics of cancer cells; cancer cells divide under conditions in which their normal counterparts do not. Therefore, anti-proliferation can prevent the growth and development of cancer cells.14 Previous studies also reported the potential of brewers’ rice extract to inhibit the proliferation of HT-29, Caov-3 and HepG2 cells.17
In this study, apoptosis analysis using flow cytometry showed that fractions F3 and F6 of ‘Woja Laka’ induced apoptosis of HepG2 cells. Fraction 3 with a dosage of its IC50 can induce the highest apoptosis compared to the other fractions and doses (Table 5). Previous studies on the anti-cancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo have shown that the anthocyanin of black rice induced apoptosis of MDA-MB-453 cells in a dose-dependent manner. Anthocyanin-rich extract can induce apoptosis via intrinsic pathways. Caspase-3 and caspase-9 in MDA-MB-453 cells have been activated by the anthocyanin-rich extract. In contrast, no significant cleavage of caspase-8 was monitored. The intrinsic pathway of apoptosis implicates permeabilization of the mitochondrial membrane to release apoptogenic mitochondrial proteins such as cytochrome C. Once in the cytoplasm, cytochrome C mediates the binding of Apaf-1 to pro-caspase-9, producing activation of caspase-9 and initiation of the caspase cascade.18
In the present study, the flow cytometry data indicates that fractions F3 and F6 were able to induce the G0-G1 arrest phase of the cell cycle (Figure 3). Cell cycle and apoptosis have important roles in the regulatory mechanisms of cell growth. Cell cycle checkpoints ensure the maintenance of genomic integrity by protecting dividing cells from the potentially fatal consequences of DNA damage. The presence of unrepaired DNA damage may force the cells to arrest from the cell cycle or die by apoptosis; thus, cells do not replicate chromosomes with irreparable damage.19 The evidence of cell cycle disruption in this study could be due to the anti-proliferative action of the ‘Woja Laka’ black rice bran compounds in these fractions. The inhibitory concentration of fractions F3 and F6 of ‘Woja Laka’ may act by specifically blocking the cell cycle at the G0-G1-phase and preventing the cell from entering the next phase.
Conclusion
Evidence from this study suggests that the compounds contained in fraction 3 and fraction 6 of the ethanolic extract of the ‘Woja Laka’ black rice bran inhibit the growth of liver carcinoma HepG2 cells, induce apoptosis and cause cell cycle arrest in the G0-G1 phase.
Acknowledgement
We would like to thank Ms. Dwimei Ayudewandari Pranatami, Ms. Mega Octaviana and Mrs. Ifandari for their supports in this research, also Prof. John Thomas from the University of Queensland, Australia for the valuable comments on the manuscript. We would like to thank the Ministry of Research, Technology and Higher Education for The Doctoral Research Grant with contract number: 001/LPPM-USB/PDD/IV/2017.
Conflict of Interest
The authors have no conflicts of interest to disclose
Fundiing Source
This research is funded by Doctoral Research Grants from Ministry of Research, Technology and Higher Education, Indonesia with contract number: 001/LPPM-USB/PDD/IV/2017.
References
- Lim F.P.K, Bongosia L.F.G, Yao N.B.N, Santiago L.A. Cytotoxic activity of the phenolic extract of virgin coconut oil on human hepatocarcinoma cells (HepG2). Int Food Res J. 2014;21(2):729-733.
- Chen P.N, Kuo W.H, Chiang C.L, Chiou H.L, Hsieh Y.S, Chu S.C. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem Biol Interact. 2006;163(3):218-229. doi:10.1016/j.cbi.2006.08.003.
CrossRef - Kimman M, Norman R, Jan S, Kingston D, Woodward M. The burden of cancer in member countries of the association of southeast asian nations (ASEAN). Asian Pacific J Cancer Prev. 2012;13(2):411-420. doi:10.7314/APJCP.2012.13.2.411.
CrossRef - Banerjee A, Sengupta A, Maji B, Nandi A, Pal S, Mukherjee S. Hepatology and Pancreatic Science Possible Cytotoxic Activity of Annona muricata Leaves in Huh-7. Human Liver Cancer Cells. 2017;1(1):1-6.
- Praveena A and Suriyavathana M. in Vitro Cytotoxicity of the Crude Alkaloids of Toddalia Asiatica . L . Against the Human Liver Cancer Cell Lines ( Hep G2 ) and Normal Liver Cell Lines ( Lo2 ). World J Pharm Pharm Sci. 2014;3(3):1781-1788.
- Begum S.S, Aruna A, Sivakumar T, Premanand C. Invitro Cytotoxic Activity On Ethanolic Extracts Of Leaves Of Ipomoea Pes-Tigridis ( Convolulaceae ) Against Liver Hepg2 Cellline. 2015;3:1778-1784.
- DeVita V.T, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643-8653. doi:10.1158/0008-5472.CAN-07-6611.
CrossRef - Iwamaru A, Iwado E, Kondo S, et al. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol Cancer Ther. 2007;6(1):184-192. doi:10.1158/1535-7163.MCT-06-0422.
CrossRef - Choi S.P, Kim S.P, Kang M.Y, Nam S.H, Friedman M. Protective effects of black rice bran against chemically-induced inflammation of mouse skin. J Agric Food Chem. 2010;58(18):10007-10015. doi:10.1021/jf102224b.
CrossRef - Rukmana R.M, Soesilo N.P, Pratiwi R. The Effect of Ethanolic Extract of Black and White Rice Bran ( Oryza sativa L .) on Cancer Cells. 2016;21(1):63-69.
- Dad P. Analisis Antosianin Dan Kandungan Nutrien Pada Bekatul Beras Hitam ( Oryza sativa L . cv cempo ireng Sayegan , Yogyakarta ). Tesis. UGM; Yogyakarta. 2016.
- Puji A, Nurhayati D, Pratiwi R, Wahyuono S. Original Research Article Cellular mechanism of anti-cancerous activity in active marine sponge Cinachyrella anomala against T47D cell. 2015;4(3):785-791.
- Banjerdpongchai R, Wudtiwai B, Sringarm K. Cytotoxic and apoptotic-inducing effects of purple rice extracts and chemotherapeutic drugs on human cancer cell lines. Asian Pacific J Cancer Prev. 2013;14(11):6541-6548. doi:10.7314/APJCP.2013.14.11.6541.
CrossRef - Chen P-N, Chu S-C, Chiou H-L, Chiang C-L, Yang S-F, Hsieh Y-S. Cyanidin 3-Glucoside and Peonidin 3-Glucoside Inhibit Tumor Cell Growth and Induce Apoptosis In Vitro and Suppress Tumor Growth In Vivo. Nutr Cancer. 2005;53(2):232-243. doi:10.1207/s15327914nc5302.
- Pratiwi R, Anindito W, Tunjung S, Amalia R. Black Rice Bran Extracts and Fractions Containing Cyanidin 3-glucoside and Peonidin 3-glucoside Induce Apoptosis in Human Cervical Cancer Cells. 2015;20(I):69-76.
- Hyun J.W, Chung H.S. Cyanidin and Malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of G(2)/M phase and induction of apoptosis. J Agric Food Chem. 2004;52(8):2213-2217. doi:10.1021/jf030370h.
CrossRef - Tan B.L, Norhaizan M.E, Suhaniza H.J, Lai C.C, Norazalina S, Roselina K. Antioxidant properties and antiproliferative effect of brewers’ rice extract (temukut) on selected cancer cell lines. Int Food Res J. 2013;20(5):2117-2124.
- Hui C, Bin Y, Xiaoping Y, et al. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr Cancer. 2010;62(8):1128-1136. doi:10.1080/01635581.2010.494821.
CrossRef - Kwan Y.P, Saito T, Ibrahim D, et al. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm Biol. 2015;209(May 2017):1-14. doi:10.3109/13880209.2015.1064451.
CrossRef








