Faculty of Pharmacy and Health sciences Universiti Kuala Lumpur Royal College of Medicine Perak, 30450 Ipoh, Perak, Malaysia.
Corresponding Author E-mail: shahnazmajeed5@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1283
Abstract
The present study focusses on the extracellular synthesis of silver nanoparticles (AgNPs) from Alternaria alternate isolated from soil at the Unikl RCMP tasek campus. The colour of the fungal filtrate has been changed into dark brown upon the addition of 1mM silver nitrate (AgNO3) suggest the nanoparticle formation. These nanoparticles were characterized by UV-vis spectrophotometric showed the absorption peak at 370nm. High resolution electron transmission electron (HRTEM) was used to determine the structure, size and shape of nanoparticles which were spherical, polydispersed and size was15 to 30 nm. These nanoparticles were evaluated for antibacterial activity against Escherichia coli and Staphylococcus aureus showed excellent antibacterial activity and also enhanced the antibacterial property of gatifloxacin and also it showed good antifungal activity against Aspergillus niger and candida albicans subsequently and enhances the antifungal activity of fluconazole.
Keywords
Alternate Alternaria; Antibacterial Activity; Antifungal Activity HRTEM;
Download this article as:| Copy the following to cite this article: Majeed S. Biosynthesis and Characterization of Nanosilver from Alternata Alternaria and its Antifungal and Antibacterial Activity in Combination with Fluconazole and Gatifloxacin. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Majeed S. Biosynthesis and Characterization of Nanosilver from Alternata Alternaria and its Antifungal and Antibacterial Activity in Combination with Fluconazole and Gatifloxacin. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=17443 |
Introduction
Nanotechnology is a multidisciplinary science of chemistry, biology, physics, engineering and technology conducted at nanoscale from 1nm until 100nm of dimensional size where practiced widely in various areas such as in food processing industry, innovative fabric, agriculture production as well as in sophisticated medicinal techniques.1 Nanoparticles have unique characteristics that vary from their bulk particle and their properties changed due to decreasing in their dimension size which resulted in high total surface area.2,3
Resistance emerged in the fungal and bacterial population to various antibiotics available in the market is a matter of concern, hence researcher shifted focus on the use of nanoparticles to counter the resistance Different types of metallic and non-metallic nanoparticles were biosynthesized like titanium, gold , copper, zinc, silver and magnesium etc. Among all these silver and silver nanoparticles were extensively studied because of their unique chemical and physical properties like antibacterial, anticancer property, burn treatment, water disinfectant, prevent colonization of bacteria on the catheters etc.4,5,6,7
Now a days different method are available for the synthesis of nanoparticles like physical, chemical and biological method, but biological approach for the synthesis of nanoparticles is superior as compared to other methods. In biological method biological organisms are used for the biosynthesis of nanoparticles like plants, bacteria, fungi and algae etc. which act as nanofactories for the nanoparticle synthesis as they contain lot of enzymes, hence reduces the metal ions.
In this study silver nanoparticles (AgNPs) were biosynthesized extracellularly from Alternate alternaria. These AgNPs were investigated and characterized by UV- Vis spectrophotometry followed by HRTEM to determine the size and shape of nanoparticles. These nanoparticles were further evaluated for anti-bacterial and antifungal activity against various pathogenic bacterial and fungal strains and then compared with gatifloxacin and fluconazole were studied.
Material and Methods
Collection of Sample
Fungal species was isolated from soil collected from the the campus of UniKL RCMP Tasek Premise. The soil was put into the sterile plastic bags and taken to the research laboratory. The soil was kept outside and dried at normal room temperature.
Isolation of Fungi
The soil sample was serially diluted from 10-3 to 10-5. The 0.1 ml of sample spreaded on the potato dextrose agar media and incubate for 2- 3 days. The fungal culture of Alternata alternaria was isolated from the petri plate and pure culture was done by using microscope and also shape, morphology of the colony. The pure culture of Alternata alternaria was confirmed by microscopically and morphological nature of culture by using various laboratory manuals to confirm the fungal culture.
Biosynthesis of AgNPs
The pure culture of Alternata alternaria was employed for the biosynthesis of AgNPs.The pure culture was incubated in 100 ml of potato dextrose broth and put on the shaker at 140 rpm for three days. After three days the fungal biomass was washed with ddH2O for two to three times so that media component and other debris should be removed. The washed fungal biomass was again put into conical flask containing 100 ml of ddH2O and put on the shaker at 140 rpm for three days again. After three days the cell filtrate extract of Alternata alternaria was challenged with 1Mm AgNO3 and keep it in dark place for overnight .The colour change confirms the formation AgNPs.
Characterization of AgNPs
The AgNPs were analyzed by UV –Vis spectrophotometry to determine the absorbance (lambda max) .1ml of AgNPs solution was taken into cuvette to observe the wave length and spectrum was taken from 300- 600 nm and absorption peak indicates the presence of nanoparticles.
TEM analysis was used to determine and investigate the shape, size and dispersity of AgNPs. For TEM analysis 2ml of AgNPs solution was diluted and sonicated for 10 minutes. After sonication one drop of AgNPs solution was put on carbon coated grid air dried it and subjected for TEM analysis.
Antibacterial and Antifungal Activity
These biologically synthesized AgNPs were evaluated for it antibacterial activity against S. aureus and E. coli on nutrient agar media by using disc diffusion method. The inhibition zone was measured in millimetre (Mm) at 37°C after overnight incubation .Antifungal activity was carried out against Candida albicans and Aspergillus niger on potatota dextrose agar media by disc diffusion method. The zone of inhibition was measured after 2 to 3 days of incubation at 25°C.
Results and Discussion
The extract of Alternata alternaria was used as a source of reducing agent for the synthesis of silver nanoparticles. The colour changes into dark brown after the addition of silver nitrate suggests the production of AgNPs. These AgNPs were characterized and investigated by UV-Vis spectrophotometry which showed the peak of absorption at 370 nm due to surface plasmon resonance and inter band transition among the nanoparticles. Fig 1 & Fig 2.8,9
 |
Figure 1: Biosynthesis of AgNPS from Fungal filtrate, Colour change
|
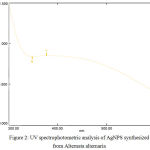 |
Figure 2: UV spectrophotometric analysis of AgNPS synthesized from Alternata alternaria
|
HRTEM analysis were used to determine the size, shape and dispersity of nanoparticles which showed that particles were well dispersed, spherical and size ranges from 15 nm to 30 nm Fig 3.
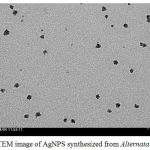 |
Figure 3: TEM image of AgNPS synthesized from Alternata alternaria
|
Theses biologically syntheiszed AgNPs were evaluated for its antibacterial activity against S. aureus and E. coli by disc Kirby method. Each disc was loaded with 30µg/ml showed the zone of inhibition 17mM against E.coli while for gatifloxacin showed 22mM zone of inhibition and for synergistic effect it showed the 25mM zone of inhibition followed by Staphylococcus aureus showed 13 mM zone, gatifloxacin 19 zone of inhibition and combined effect between AgNPs and gatifloxacin showed 22 mM zone of inhibition respectively as shown in table 1 which means these nanoparticles enhances the antibacterial property quite significantly.10,11
These biologically synthesized AgNPs were investigated for its antifungal activity against A. niger and C. albicans by disc diffusion method. Each disc was loaded with 30 µg/ml which showed the 19mM zone of inhibition for Aspergillus niger followed by candida albicans showed 16mM zone while fluconazole it showed 23 mM inhibition zone for Aspergillus niger and 20mM for candida albicanns respectively. For determining the synergistic effect each fluconazole disc was impregnated with 15 µg/ml AgNPs which showed the 28mM zone for Aspergillus niger followed by candida albicans 23mM zone as shown in table2. Fig 4 & Fig 5 showed the combined effect of AgNPs with gatifloxacin and flucanozole. This means that AgNPs showed good antibacterial and antifungal activity and also enhances the antifungal property of gatifloxacin and flucanozole.
The exact mechanism of yet to known that how these AgNPs works but some studies suggest that these nanoparticles being a small in size easily enters into the cell and interfere with DNA by the production of reactive oxygen species (ROS) which damages the cell and hence cause the cell death.12.13
Table 1: Inhibition zone in mM of AgNPs with galtifloxacin. Gat – Gatifloxacin
| S. No. | Pathogens | AgNPs 30 µg/ml | Gatifloxacin5mcg/disc | Gat + AgNPs |
| 1 | S.aureus | 13 | 19 | 22 |
| 2 | E,coli | 17 | 22 | 25 |
Table 2: Inhibition zone mM of AgNPs with flucanozole. Flu – Flucanozole
| S. No. | Pathogens | AgNPs 30 µg/ml | Flucanozole | Flu+ AgNPs |
| 1. | A.niger | 19 | 23 | 28 |
| 2. | C.albicans | 16 | 20 | 23 |
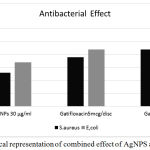 |
Figure 4: Graphical representation of combined effect of AgNPS and gatifloxacin.
|
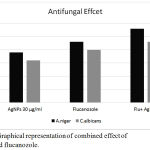 |
Figure 5: Graphical representation of combined effect of AgNPS and flucanozole.
|
Conclusion
Alternata alternata acts as a good source for the production of AgNPS. TEM analysis showed nanoparticles are well dispersed and nano in size. These nanoparticles showed excellent antibacterial and antifungal activity and also enhances the antifungal and antibacterial property of fluconazole and gatifloxacin, hence could be used as strong antibacterial and antifungal material but needs further cytotoxic study before comes into the market.
Acknowledgement
The author would like to thank Centre of Research and innovative Universiti Kuala Lumpur for providing me necessary support to carry out this study.
Conflict of Interest
There is no conflict of interest.
References
- Datar R.H & Richard J.C. Nanomedicine: Concepts, status and the future. Med Innov Business. 2010;2:6-17.
- Ahmad T, Wani I.A, Manzoor N, Ahmed J, Asiri A.M. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf.B. 2013;107:227.
CrossRef - Upadhyay L.S.B, Verma N. Synthesis and characterization of cysteine functionalized silver nanoparticles for biomolecule immobilization. Bioprocess Biosyst. Eng. 2014;37:21-39.
CrossRef - Parikh D.V, Fink T, Rajasekharan K, Sachinvala N.D, Sawhney A.P.S, Calamari T.A,Parikh A.D. Antimicrobial silver/sodium carboxymethyl cotton dressings for burn wounds. Text Res .J. 2005;75:134–138.
CrossRef - Ulkur E, Oncul O, Karagoz H, Yeniz E, Celikoz B. Comparison ofsilver-coated dressing (Acticoat TM), chlorhexidine acetate 0.5% (Bactigrass®),and fusidic acid 2% (Fucidin®) for topical antibacterial effect in methicillin-resistant Staphylococci-contaminated, full-skin thick nessrat burn wounds. Burns. 2005;31:874–877.
CrossRef - Chou W.L, Yu D.G, Yang M.C. The preparation and characterization of silver-loading cellulose acetate hollow fibre membrane for water treatment. Polym Adv Technol. 2005;16:600–607.
- Rupp M.E, Fitzgerald T, Marion N, Helget V, Puumala S, Anderson J.R, Fey P.D. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness antimicrobial resistance. Am J Infect Control. 2004;32:445–450.
CrossRef - Jagtap U.B, Bapat V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. Seed extract and its antibacterial activity. Crops Prod. 2013;46:132–137.
CrossRef - Gopinath V,MubarakAli D, Priyadarshini S, Priyadharsshini N.M, Thajuddin N, Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces. 2012;96:69–74.
CrossRef - Annamalai J, Nallamuthu T. Green synthesis of silver nanoparticles: Characterization and determination of antibacterial potency. Appl. Nanosci. 2016;6:259–265.
CrossRef - Kim S,Choi J.E, Choi J, Chung K.H, Park K, Yi J. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Vitro. 2009;23:1076–1084.
CrossRef - Morones J.R, Elechiguerra J.L, Camacho A, Holt K, Kouri J.B,Ramfrez J.T,Yacaman M.J. Thebactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353.
CrossRef - Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008;4:141–144.
CrossRef








