Ayla Bahramian1, Parisa Falsafi2, Mahdi Rahbar3, Fatemeh Dabaghi-Tabriz4, Mohammadreza Daeioughli-LeilAbadi5 and Masoumeh Mehdipour6
1,2Department of Oral Medicine, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran.
3,4Department of Operative and Esthetic Dentistry, Faculty of Dentistry, Tabriz University of Medical Science, Tabriz, Iran.
5Private Practitioner, Tabriz, Iran.
6Department of Oral Medicine, Faculty of Dentistry, Shahid Beheshty University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: Fatemeh.dabaghitabriz@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1337
Abstract
This study aimed to investigate Association of ABO blood grouping in patients with recurrent aphthous stomatitis referring to Oral Medicine department of Tabriz Dental Faculty. In this cross sectional study blood group (ABO) of 75 patients with recurrent aphthous stomatitis was determined and the data obtained from the study were analyzed by using descriptive statistics (frequency-percent). The software used for statistical analysis was SPSS 17. 31 patients (41.3%) with RAS had blood group A, 21 patients (28%) had blood group B, 3 (4%) had blood group AB and 20 patients (26.6%) had blood group O. The frequency of A blood group was highest among patients with RAS and AB blood group was lowest. The ABO blood group system is very important in organ transplantation, blood transfusion and in other cases such as determination of the immunologic characteristics of the body, in relation to various diseases, cancers etc. we can use this property for diagnosis of diseases.
Keywords
Blood Group; Recurrent Aphthous Stomatitis; Tabriz Dental Faculty
Download this article as:| Copy the following to cite this article: Bahramian A, Falsafi P, Rahbar M, Dabaghi-Tabriz F, Daeioughli-LeilAbadi M. , Mehdipour M. Association of ABO Blood Grouping with Recurrent Aphthous Stomatitis. Biomed Pharmacol J 2017;10(4). |
| Copy the following to cite this URL: Bahramian A, Falsafi P, Rahbar M, Dabaghi-Tabriz F, Daeioughli-LeilAbadi M. , Mehdipour M. Association of ABO Blood Grouping with Recurrent Aphthous Stomatitis. Biomed Pharmacol J 2017;10(4). Available from: http://biomedpharmajournal.org/?p=18261 |
Introduction
Recurrent aphthous stomatitis (RAS) is a prevalent disorder that affects the oral mucosa and approximately 20% of the population is affected by it to different degrees.1 The prevalence rate of RAS has been reported to be approximately 5‒66%.1,2 Such a condition is more common in females than in males and is manifested during the third decade of life in the majority of patients.3,4 The recurrent ulcers are painful and appear individually or in aggregates. Oral aphthous ulcers are symmetrical round or oval ulcers that are shallow and are covered with a gray membrane.4 These ulcers are classified into minor, major and herpetiform based on their clinical manifestations, with the minor being the most common form that affects almost 80% of patients with RAS. 4,5
Despite the studies carried out on RAS, the etiology of this condition is unknown6‒8 and it is hypothesized that this condition is multifactorial.9 An imbalance has been reported in the immune system of individuals affected by RAS compared to healthy individuals, in the form of changes in the ratio of TCD4+ lymphocytes to CD8+ lymphocytes.10 In addition, at least 40% of subjects with aphthous stomatitis have had a positive familial history for this condition, indicating that some patients have a genetic susceptibility to these oral ulcers.9 HLA-B12, HLA-B51, HLA-DR2 and HLA-A11 are some of the human leukocyte antigens (HLA) that are associated with RAS.2,10
ABO blood groups are one of the most stable characteristics of the population and are different in different socioeconomic, geographic and ethnic groups.11,12
Since the time when a relationship between the blood group A and gastric cancer was reported in 1953, several studies have been undertaken to evaluate the relationship between blood groups, cancers and other conditions.13,14 Studies have shown a significant relationship between some HLA antigens and blood groups on one hand and some autoimmune diseases such as rheumatoid arthritis, pemphigus and psoriasis.15‒17
Some studies have evaluated the relationship between oral diseases and blood groups Thomopoulou-Dukoudakis et al (1983) evaluated the frequencies of ABO blood groups in patients with oral lichen planus and reported that the hypertrophic form of OLP had a greater association with blood groups compared to the atrophic and erosive type.18 Jaleel and Nagarajappa (2012) reported an increased risk of oral cancers in individuals with blood group A.14 In addition, in a recent study the relationship between ABO blood typing and OLP was evaluated by Kumor et al; the results showed that individuals with blood group A exhibited a 1.28-fold higher risk for OLP, indicating that determination of blood group might be considered as an adjunct to the diagnosis of this disease .19
Considering the confirmed relationship between blood group antigens and inflammatory autoimmune diseases and the major effect of heredity in the etiology of RAS, and since only one study to date has evaluated the frequencies of ABO blood system in patients with RAS, the present study was undertaken to determine the frequencies of ABO blood groups in patients with RAS, referring to the Department of Oral Medicine, Faculty of Dentistry, Tabriz University of Medical Sciences, so that the results could help determine the etiology of this disease.
Materials and Methods
The present descriptive cross-sectional study was carried out on patients referring to the Department of Oral Medicine, Faculty of Dentistry, Tabriz University of Medical Sciences, with a clinical diagnosis of recurrent aphthous stomatitis. All the patients referring to the Department from October 2015 to March 2016, with RAS, who were eligible to be included in the present study, were evaluated. The census method was used to determine the sample size.
Inclusion criteria
Patients with a definitive diagnosis of recurrent aphthous stomatitis
Consent o be included in the study
Exclusion criteria
Affliction with systemic diseases such as cyclic neutropenia, agranulocytosis, Behçet’s disease and celiac disease that produce aphthous-like lesions
Use of medications such as streptomycin, tetracycline and gold salts, which result in lichenoid reactions that are mistaken for aphthous lesions.1,7,10,20,21
Study procedures
All the patients with RAS, who referred to the Department of Oral Medicine, Faculty of Dentistry, Tabriz University of Medical Sciences, from October 2015 to March 2016, and were diagnosed with RAS by an oral medicine specialist, were included in this study based on inclusion and exclusion criteria and evaluated in relation to the blood group in the Central Laboratory of Imam Reza Hospital in Tabriz. All the subjects signed an informed consent form to be included in the present study.
Statistical analysis
Data were analyzed with descriptive statistics (frequencies and percentages) with the use of SPSS 17.
Results
Of 75 patients evaluated in the present study, 69 (92%) were female and 6 (8%) were male. The mean age of females and males were 29.93 years (with a range of 16‒53 years) and 34.6 years (with a range of 23‒44 years), respectively (Table1).
Table 1: The frequency distributions of the subjects in terms of gender
| Number | Percentage | Valid percentage | Cumulative percentage | |||||
| Male | 6 | 8% | 8% | 8% | ||||
| Female | 69 | 92% | 92% | 100% | ||||
| Total | 75 | 100% | 100% | |||||
A total of 31 subjects (41.3%) with RAS exhibited blood group A, 21 (28%) exhibited blood group B, 3 (4%) exhibited blood group AB and 20 (26.6%) exhibited blood group O (Table2).
Table 2: Frequency distribution of subjects in terms of blood type
| Number | Percentage | Valid percentage | Cumulative percentage | |
| Blood group A | 31 | 41.3 | 41.3 | 41.3 |
| Blood group B | 21 | 28 | 28 | 69.3 |
| Blood group AB | 3 | 4 | 4 | 73.3 |
| Blood group O | 20 | 26.7 | 26.7 | 100 |
| Total | 75 | 100 | 100 |
As shown in the Table above, the frequency of blood group A was the highest in patients with RAS, with blood group AB exhibiting the lowest frequency (Graph1).
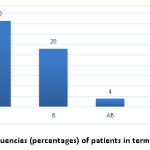 |
Figure 1: The frequencies (percentages) of patients in terms of blood groups
|
Discussion
Aphthous ulcers are the most prevalent oral ulcers that appear as painful ulcers with an erythematous margin in round or ovoid shapes on the internal buccal mucosa, lips, ventral aspect of the tongue, soft palate and pharynx. These ulcers can severely annoy the patients and interfere with the patients’ daily routines. The precise mechanism of initiation of aphthous ulcers is not known; however, some factors have been reported to be responsible for such ulcers, including trauma, emotional stress, heredity, genetic factors and nutritional deficiencies such as vitamin deficiency, especially vitamin B12 and folic acid and minerals such as iron.4,5,21
Studies estimate blood group “A” incidence more repeated in patients with gastric, laryngeal, hypopharynx, pancreatic, breast, testicular, and bone cancers.22 Literature quotes that Blood-group antigens can be existing on crucial receptors controlling cell proliferation, adhesion, and motility, such as epidermal growth factor receptor, integrins, cadherins, and CD44. Althought their role in human cancer is reported in literature, their function in normal stratified oral epithelium is unclear.23 Although prevalence of blood groups have been mentioned in literature, studies of the relative incidence of the ABO blood group in different lesions have failed to provide a unifying and testable hypothesis as to the basis for the associations observed. Therefore, we emphasize on considering blood group types together with other risk factors in various oral lesions including aphthous ulcers.
In the present study, all subjects were evaluated in relation to their blood groups. The results showed that 31 subjects (41.3%) with RAS had blood group A, 21 (28%) had blood group B, 3 (4%) had blood group B and 20 (26.6%) had blood group O. Therefore, the frequency of blood groups A and AB were the highest and lowest in patients with RAS, respectively.
Narang et al (2015) reported that 52 patients (26%) of patients with RAS exhibited blood group A, 53 (26.5%) had blood group B and 44 (22%) had blood group AB, with 51 (25.5%) exhibiting blood group O. Contrary to our study, in that study the highest frequency was related to blood group B; however, consistent with the results of our study, the lowest frequency was related to blood group AB. 21
Nikawa et al (1991) evaluated 442 patients wearing dentures and concluded that the patients’ blood group was an important etiologic factor for denture stomatitis; in this context, individuals with blood group O exhibited greater susceptibility to denture stomatitis. 24 Jesch et al (2007) showed that men with blood group O and women with blood group A exhibited greater risk of intestinal tumor.25 Koley et al (2008) carried out a study on 511 patient with diabetes mellitus and 475 healthy subjects and concluded that there was no relationship between the patients’ blood group and their diabetes mellitus.26
Abdollahzadeh et al (2009) carried out a study on 100 patients with denture stomatitis which had been diagnosed with the use of clinical examination and staining and concluded that the frequency of blood group O was higher in such patients.27 Yuzhalin et al (2012) carried out a study on 1163 patients with ovarian, endometrial and cervical cancers and concluded that patients with blood groups other than O had a higher risk for being affected with ovarian cancers and its spread.28
Jaleel and Nagarajappa (2012) showed a higher risk of oral cancers in subject with blood group A.14 Vinek et al (2013) evaluated 220 patients with periodontitis and reported that the majority of the patients (i.e. 65.6%) had blood group O and were Rh+, while the least among AB blood group individuals.29
Mortazavi et al carried out a case‒control study (2014) on 104 patients with oral cancers and 99 healthy subjects and concluded that the risk of oral cancers was higher in individuals with blood type B.30
Cihan et al (2014) carried out a study on 329 females and 6 males with breast cancer and showed that patients using blood groups A and O had better prognosis. 31
Valikhani et al. and Shahker et al. also did not find any relationship between ABO blood groups ad pemphigus variants in Iranian inhabitants. 32,33 Another study in Iran, didn’t show relation between esophageal cancers and blood groups. However such association has been clarified the etiology and pathophysiological aspects of some disorders, the explanation for the relationship between ABO blood groups and special disease is still unclear. 32,33
The ABO blood group system is very important in organ transplantation, blood transfusion and in other cases such as determination of the immunologic characteristics of the body, in relation to various diseases, cancers etc.30,21 In this context, even some studies have reported that the blood group antigens can bond to key receptors effective in proliferation, adhesion and cellular death including integrin, adhesion and CD44.34,23 The results of studies in this respect have sown a possible relationship between various pathologic entities and blood groups; however, these studies and the scientific evidence, especially in relation to oral ulcers such s RAS, are not sufficient to reach a definitive conclusion. Therefore, further studies with larger sample sizes are recommended.
Conclusion
The frequency of blood group A was higher in patients with RAS, with the lowest frequency belonging to blood group AB.
References
- Glick M. Burket’s Oral Medicine, 12th ed: PMPH-USA. 2015.
- Scully C. Oral and maxillofacial medicine the basis of diagnosis and treatment: Elsevier Health Sciences. 2012.
- Pongissawaranun W., Laohapand P. Epidemiologic study on recurrent aphthous stomatitis in a Thai dental patient population. Community Dent Oral Epidemiol. 1991;19(1):52-3.
CrossRef - Porter S. R., Scully C., Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 1998;9(3):306-21.
CrossRef - Field E. A., Brookes V., Tyldesley W. R. Recurrent aphthous ulceration in children–a review. Int J Paediatr Dent. 1992;2(1):1-10.
CrossRef - Eversole L. R. Immunopathology of oral mucosal ulcerative, desquamative and bullous diseases. Selective review of the literature. Oral Surg Oral Med Oral Pathol. 1994;77(6):555-71.
CrossRef - Porter S., Scully C. Aphthous stomatitis—an overview of aetiopathogenesis and management. Clin Exp Dermatol. 1991;16(4):235-43.
CrossRef - Scully C., Porter S. Recurrent aphthous stomatitis current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18(1):21-7.
CrossRef - Brocklehurst P., Tickle M., Glenny A. M., Lewis M. A., Pemberton M. N., Taylor J., et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database of Systematic Reviews. 2012(9):CD005411.
CrossRef - Neville B. W., Damm D. D., Chi A. C., Allen C. M. Oral and maxillofacial pathology Elsevier Health Sciences. 2015.
- Bhasin M. K., Chahal S. A laboratory manual for human blood analysis: Kamla-Raj Enterprises Delhi. 1996.
- Dilek İ., Demir C., Bay A., Akdeniz H., Oner A. ABO and Rh blood groups frequency in men and women living in eastern Turkey.Int J Hematol Oncol. 2006;16(1):23.
- Aird I., Bentall H. H., Roberts J. F. Relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1(4814):799.
CrossRef - Jaleel B. F., Nagarajappa R. Relationship between ABO blood groups and oral cancer. Indian J Dental Res. 2012;23(1):7.
CrossRef - Eiermann T. H., Vejbaesya S., Prestel H., Roepke A., Muller-Myhsok B., Schmitt-Egenolf M. Association and linkage of human leukocyte antigens with psoriasis – Revisited. Transfus Med Hemother. 2002;29(6):326-30.
CrossRef - Tirado-Sanchez A., Ponce-Olivera R. ABO and Rhesus blood groups and their non-existent relationship with pemphigus vulgaris. Acta Dermatovenerol APA. 2010;19(3):47-8.
- Valikhani M., Kavand S., Toosi S., Kavand G., Ghiasi M. ABO blood groups, Rhesus factor and pemphigus. Indian J Dermatol. 2007;52(4):176.
CrossRef - Thomopoulou‐Doukoudakis A., Squier C., Hill M. Distribution of ABO blood group substances in various types of oral lichen planus. J Oral Pathol Med. 1983;12(1):47-56.
CrossRef - Kumar T., Puri G., Laller S., Bansal T., Malik M. Association of ABO blood grouping with oral lichen planus. Univers Res J Dent. 2014;4(2):93.
CrossRef - Hudson J. Recurrent aphthous stomatitis: diagnosis and management in primary care. J Patient Cent Res Rev. 2014;1(4):197-200.
CrossRef - Narang D., Rathod V., Shishodiya S., Sur J., Khan F., Jain R., et al. correlation of ABO blood group and apthous ulcers and epidemiological study. J Adv Med Dent Sci Res. 2015;3(4):9.
- Tursen U., Tifik E. N., Unal S., Gunduz O., Kaya T. I., Camdeviren H., et al. Relationship between ABO blood groups and skin cancers. Dermatol Online J. 2005;11:44‑9.
- Gao S., Bennett E. P., Reibel J., Chen X., Christensen M. E., Krogdahl A., et al. Histo‐blood group ABO antigen in oral potentially malignant lesions and squamous cell carcinoma–genotypic and phenotypic characterization. APMIS. 2004;112(1):11-20.
CrossRef - Nikawa H., Kotani H., Sadamori S., Hamada T. Denture stomatitis and ABO blood types. J Prosthet Dent. 1991;66(3):391-4.
CrossRef - Jesch U., Endler P. C., Wulkersdorfer B., Spranger H. ABO blood group. Related investigations and their association with defined pathologies. Sci. World J. 2007;7:1151-4.
CrossRef - Koley S. The distribution of the ABO blood types in patients with diabetes mellitus. Anthropol. 2008;10(2):129-32.
CrossRef - Abdollahzadeh S., Abdolsamadi H., Vahedi M. M. The frequency of ABO blood groups among patients with denture stomatitis. Avicenna J Dent Res. 2009;1(1):21-3.
- Yuzhalin A. E., Kutikhin A. G. ABO and Rh blood groups in relation to ovarian, endometrial and cervical cancer risk among the population of South-East Siberia. Asian Pac J Cancer Prev. 2012;13(10):5091-6.
CrossRef - Vivek S., Jain J., Simon S. P., Battur H., Supreetha S., Haridas R. Association of ABO Blood Group and Rh factor with Periodontal Disease in a Population of Virajpet, Karnataka: A Cross-Sectional Study. J Int Oral Health. 2013;5(4):30.
- Mortazavi H., Hajian S., Fadavi E., Sabour S., Baharvand M., Bakhtiari S. ABO blood groups in oral cancer: A first case-control study in a defined group of Iranian patients. Asian Pac J Cancer Prev. 2014;15(3):1415-18.
CrossRef - Cihan Y. B. Significance of ABO-Rh blood groups in response and prognosis in breast cancer patients treated with radiotherapy and chemotherapy. Asian Pac J Cancer Prev. 2013;15(9):4055-60.
CrossRef - Cerović R., Juretić M., Balen S., Belušić M., Caser L., Rogić M. Examining the presence of ABO (H) antigens of blood types in the saliva of patients with oral cancer. Collegium antropologicum. 2008;32(2):509-12.
- Moshaverinia M., Rezazadeh F., Dalvand F., Moshaverinia S., Samani S. The relationship between oral lichen planus and blood group antigens. World J Med Sci. 2014;10:103-5.
- Demir T., Tezel A., Orbak R., Eltas A., Kara C., Kavrut F. The effect of ABO blood types on periodontal status. Eur J Dent. 2007;1(3):139.








