A. A. Kubanov1, 2, Yu. A. Gallyamova1 and O. A. Korableva2
1Russian Medical Academy of Continuous Professional Education, the Ministry of Healthcare of the Russian Federation, Barrikadnaya St., 2/1, Moscow, 123995, Russia.
2State Research Center of Dermatovenereology and Cosmetology, Ministry of Healthcare of the Russian Federation, Korolenko St., 3/1, Moscow, 107076, Russia.
Corresponding Author E-mail: yulya.gallyamova.69@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/1224
Abstract
Studying the causes of hair loss and improving the methods of alopecia therapy are among the most important trends in dermatology. The interest is determined by the fact that pathogenic mechanisms of hair loss are poorly studied, current methods of therapy are not always effective enough, and the existing theories and assumptions do not reveal fully the mechanisms of hair loss. Undoubtedly, the development of new pharmacological means and methods of alopecia therapy is possible only owing to better understanding of hair loss patterns at the pathophysiological level. The purpose of this study was to investigate the role of the VEGF, KGF, EGF, TGF-β1 growth factors in the development of androgenic alopecia in men and women. This study demonstrates gender differences in the pathogenesis of androgenic alopecia. The obtained data give reason to assume the influence of other factors on the development of androgenic alopecia in men.
Keywords
Androgenic alopecia; hair follicle; hair cycle; hair growth; VEGF; KGF; EGF; TGF-β1
Download this article as:| Copy the following to cite this article: Kubanov A. A, Gallyamova Y. A, Korableva O. A. The Study of Growth Factors in Patients with Androgenic Alopecia. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Kubanov A. A, Gallyamova Y. A, Korableva O. A. The Study of Growth Factors in Patients with Androgenic Alopecia. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16120 |
Introduction
Hair follicles develop from ectodermal and mesenchymal cells. The first morphological feature of follicles formation is the appearance of thickenings in epidermis, so-called “placodes”, located at equal distances. Placode formation begins with local bulge of epithelium and the associated condensation of mesenchymal cells. Intussusception of placodes into the dermis leads to the formation of the hair follicle, which occurs approximately on the 5th week of prenatal development.1,2 Completely formed hair follicles can be seen in a fetus already at 9-10 weeks.3-5 The morphogenesis of the hair follicle is a strictly regulated process, which is based on signaling pathways, including the Delta/Notch, Wnt/Frizzled, Hedgehog/Patched, TGFβ/BMP and FGF signaling, which provide a balance of stimulating and inhibiting effects.6-7
Owing to fundamental researches in the field of cytogenetics and biochemistry, notions of morphology and physiology of the hair follicle have increased significantly. Currently 6 periods of anagen phase are described; the so-called “bulge” zone has been revealed that is thickening under the sebaceous gland, called by some authors as the secondary hair germ; biochemical and proliferative activity has been established in “bulge” zone even during the telogen phase; it is proved that the hair growth cycle in follicles occurs not only asynchronously, but also irrespective of adjacent follicles and so on.1-2,5-9
At present several growth factors that can control the development and the cycle of the hair follicle are identified. Some participate in the anagen phase initiation (IGF-1, HGF, KGF, VEGF, FGF-2, FGF-18), others suppress growth and differentiation of a follicle in the telogen and catagen phases (TGF-b, FGF-5, EGF).10-12 Thus, growth factors can be key molecules that participate in the initiation and suppression of hair growth.
Nevertheless, many of today’s multiple studies are only experiments by nature and there are still unsolved questions concerning growth factors role in pathogenesis of alopecia and their impact on the disease severity. It is worth mentioning that most studies devoted to the influence of the growth factors on the hair follicle cycle were conducted with separate growth factors, while in the body they work in synergy with each other.13-19 Thus, it is relevant to conduct studies of various combinations of growth factors in order to establish their ability to participate in the development of hair loss. Further clinical and histochemical studies of growth factors will serve as a basis for the development of new methods for restoring the signal function between cells in various forms of alopecia.
In this study, we investigated the role of the VEGF, KGF, EGF, TGF-β1growth factors in the development of androgenic alopecia in men and women. The research tasks were: to study the expression of the VEGF, KGF, EGF, TGF-β1 growth factors in patients with androgenic alopecia and healthy volunteers; to determine the growth factors expression in case of androgenic alopecia, taking into account the patients’ age, sex and alopecia progression; to assess the influence of individual growth factors on the development of androgenic alopecia.
Materials and Methods
The study involved 60 patients diagnosed with androgenic alopecia. As a control, samples of scalp from 16 healthy volunteers (8 men and 8 women) were used. Distribution of respondents by groups is given in table 1.
Table 1: Distribution of patients by sex and age
| Group | Diagnose | Sex | Age | Total |
| Main | Androgenic alopecia | Men | 28.5 (25.25; 34.75) | 30 |
| Women | 40.6 ± 8.98 | 30 | ||
| Control | Healthy volunteers | Men | 27 ± 5.14 | 8 |
| Women | 38.5 ± 7.4 | 8 |
Criteria for Inclusion in the Study
Informed consent of patients to participate in the study;
Age of patients from 18 to 60;
Confirmed diagnosis of androgenic alopecia.
Exclusion Criteria from the Study
Allergic reactions;
The presence of thyroid gland disease;
Existence of concurrent somatic diseases without adequate medical correction, heavy
Current or Neoplastic Character
The presence of skin diseases in the acute stage;
An active bacterial, viral or fungal infection at the place of the alleged treatment;
Infectious diseases;
Hyperandrogenism;
Other types of alopecia;
Lack of the patient’s desire to continue the study and treatment;
Clinical Examination of Patients With Hair Loss
Written informed consents were obtained from all participants with the requirements of Good Clinical Practice (GCP).
Clinical research of patients was conducted in accordance with specially developed individual card of the patient including data on age and sex of the patient, the anamnesis of a disease, the features of hair loss, heritable predisposition, postponed and associated diseases, provocative factors, early conducted treatment and its effectiveness, etc.
Assessment of the severity of the alopecia manifestations in men was performed by Norwood-Hamilton scale (1975) with determination of the stage of androgenic alopecia. To assess the severity of the clinical manifestations of alopecia in women, the scale Sinclair (2004) was applied, taking into account the 5 degrees of severity of hair thinning in the midline.
Hair measurement was performed using a special micro camera “Aramo SG” (company “Aram HUVIS Co., Ltd.”, the Republic of Korea) and two lenses (magnification x60, magnification x200) in conjunction with specialized computer diagnostic program “Trichoscience v. 1.7” (Russia) in the fixed area of scalp with the application of indelible mark (Tribal black, Startbrite Color, USA).
In the course of the study, the biopsy samples from the scalp served as the research materials. Biopsies were performed under compulsory voluntary consent of the patient with the use of 4-mm disposable biopsy punch (“Medaks, Srl.” Italy) under anesthesia with 1% lidocaine hydrochloride (Egis Pharmaceuticals PLC, Hungary) from the parietal region.
As a control, 16 samples of scalp were taken from the consenting participants, aged from 21 to 52 years, with no reports of hair loss and clinical symptoms of alopecia, during surgery or routine operations of nonmalignant lesions.
Tissue sections were dewaxed in xylene and rehydrated; then they were graded in ethanol and washed in the distilled water. The procedure of antigen retrieval was performed as follows: container with glasses was incubated in citrate buffer (PH 6.08-6.10) for 20 minutes at high pressure and temperature (95-98°C). After cooling, the glasses were washed in the Wash-buffer (Dako). Nonspecific binding ProteinBlock (abcam) was blocked. After washing in Wash-buffer (Dako) the incubation was carried out with the specific primary antibody. Glasses were transferred in a moist chamber and incubated (see Table 2).
Table 2: The antibodies used in immunofluorescence method
| Antibodies | Company | Dilution | Incubation conditions |
| Anti-VEGF antibody (mouse) | Abcam (UK) | 1:50 | 30 min at room temperature |
| Anti-TGF beta 1 antibody (rabbit) | Novus Biologicals (UK) | 1:100 | 1 hour at room temperature |
| Anti-KGF antibody (mouse) | Abcam (UK) | 1:200 | 1 hour at room temperature |
| Anti-EGF antibody (mouse) | Abcam (UK) | 1:100 | 30 min at room temperature |
After washing in the Wash-buffer (Dako) glasses were held with the secondary antibodies conjugated with fluorochrome Alexa 488 (1:1000, Abcam) and Alexa 647 (1:1000, Abcam) for 30 minutes at room temperature in the dark. After washing in the Wash-buffer (Dako) nucleuses were counterstained Hoechst 33258 (Sigma, USA). The ready medication was placed under cover glasses in matting area Dako Fluorescent Mounting Medium (Dako, USA).
For the assessment of the results of immunofluorescent staining the morphometric research was conducted using the system of the computer analysis of microscopic images which comprised the confocal microscope Olympus FlueView1000 and the personal computer on the basis of IntelPentium 4 and the software “Videotest Morphology 5.0” (Russia). In each case, 5 visual fields were analyzed at 200x magnification of.
A statistical analysis was performed using R version v3.2.0. The distribution of patients according to the values of indicators was determined using the Shapiro-Wilk test (W). Descriptive statistics were calculated according to generally accepted methods. Data are present as mean ± standart deviation (if the distribution is recognized as normal) or median and range (if the distribution was different from the normal). Analysis of the effect of growth factors on the development of alopecia was performed using unifactorial and multifactorial linear regression. The power of growth factors influence on the alopecia development was analyzed using univariate and multivariate dispersion analysis of the previously obtained linear models. The strength of the effect of growth factors on the development of alopecia was determined by the value of the partial ETA-squared (η2). Statistical significance of results was established at P ≤ 0.05.
Results of the Research
During the study 60 patients aged from 20 to 56 with the diagnosis of androgenic alopecia (30 women and 30 men) were under our supervision. Table 3 demonstrates the distribution of patients in accordance with the onset and duration of the disease.
Table 3: Distribution of patients in accordance with the diagnosis, age and the duration of the disease.
| Diagnosis | Sex | Average age | Average age of the disease onset | Average duration of the disease | Total |
| Androgenic alopecia | women | 40.6 ± 8.98 | 31.8 ± 9.53 | 8.83 ± 4.47 | 30 |
| men | 32.3 ± 7.36 | 25.67 ± 6.38 | 5.57 ± 2.83 | 30 | |
| Total | 36 ± 10.4 | 28.69 ± 9.83 | 7.2 ± 4.89 | 90 | |
In women with androgenetic alopecia, the 2nd degree of hair loss was diagnosed in 12 (40%) patients, and the 3rd degree in 18 (60%) patients. In men with androgenic alopecia, the II stage of hair thinning was diagnosed in 6 (20%) patients, the IIa stage – in 3 (10%), the III stage – in 7 (23.3%), and the III vertex stage in 14 (46.67%) patients. Based on the anamnesis data, it was established that women with androgenic alopecia correlated the first manifestations of the disease with stress (n=14; 46.66%), with childbearing (n=5; 16.67%), with perming (n=3; 10%) and with hair extension (n=1; 3.33%).
Men with androgenic alopecia mainly indicated psychological stress (n=4; 3.33%).
At the same time 7 women (23.33%) and 26 men (86.67%) with androgenic alopecia could not associate the onset of the disease with a specific precipitating factor.
The anamnesis enabled to establish that among patients with androgenic alopecia 19 (63.33%) women were hereditary tainted, namely: 16 patients (53.33%) in paternal line of descent and 5 patients (16.67%) in maternal line of descent; including 2 patients in paternal and maternal line of descent and 18 patients (60%) in the first line of parentage. Among men 24 patients (80%) were hereditary tainted, namely: 21 patients (70%) in paternal line of descent and 6 patients (20%) in maternal line of descent, including 3 patients in paternal and maternal line of descent and 23 patients (76.67%) in the first line of parentage.
The disease had out-of-seasonal clinical course in 19 (63.33%) women with androgenic alopecia and in 29 (96.67%) men.
Chronic-persistent nature of hair loss prevailed in women and men with androgenic alopecia (n=25; 83.3% and n=29; 96.67%, respectively).
Most patients carried out self-treatment using external means for hair care and drugs for oral administration: 16 (53.33%) and 17 (56.67%) women with androgenic alopecia, respectively and 18 (60%) and 1 (3.33%) men, respectively. Various cosmetic lotions, shampoos and folk remedies were used externally as well as complex vitamins and mineral agents were ingested. Among them 15 (50%) women and 2 (6.67%) men were under mesotherapy treatment.
Despite the conducted therapy a temporary effect was noted in 10 (37%) women and 2 (10.5%) men. The positive dynamics from the conducted therapy was absent in 9 (33.33%) women and 13 (68.4%) men.
Among study population, 3 (10%) women with androgenic alopecia and 11 (36.67%) men have not received any treatment previously.
All patients had not undergone any medical treatment for hair loss for the past 6 months.
Trichoscopy and Phototrichography Data
According to trichoscopy and phototrichography data, it was found that women with androgenic alopecia had the reduction in the total amount of hairs per square centimeter in the parietal region (242.74 ± 68.63), change of terminal and vellus hair percentage ratio in the parietal and occipital regions towards the increase of vellus hairs (33 (32; 38.5) in parietal region, 25 (21; 27.75) in occipital region), violation of the anagen and telogen hair percentage ratio towards the increase of hair that are in telogen (44.28 ± 15.56 in the parietal region, 24.87 ± 15.07 in the occipital region).
According to trichoscopy and phototrichography data, it was found that men with androgenic alopecia had the reduction in the total amount of hairs per square centimeter in the parietal region (233.06 ± 57.59), change of terminal and vellus hair percentage ratio in the parietal region towards the increase of vellus hair (33.53 ± 9.42), violation of normal ratio of anagen and telogen hairs towards the increase of hairs that are in telogen (55.51 ± 13.59). Percentage ratio of terminal and vellus hairs, as well as anagen and telogen hairs in the occipital region did not have any considerable deviations from norm and conformed to the norm in majority of patients.
Growth Factor Expression in Patients With Androgenic Alopecia
It was reported that women with androgenic alopecia had reduction of the baseline of expression levels for VEGF (32.24 (30.59; 33.83), KGF (22.32 (21.93; 23.81)) and the increase of expression for TGF-β1 (56.11 ± 1.77) in comparison with indicators of healthy individuals (p<0.05). The level of EGF expression in women with androgenic alopecia (20.96 (20.12; 21.7) did not differ from the indicator of healthy women (P =0.204) (Table 4).
Table 4: Relative area indicators of the growth factor expression in the scalp skin in women with androgenic alopecia and healthy individuals
| Growth factor
Trial groups |
VEGF
(relative area of expression, %) |
KGF
(relative area of expression, %) |
EGF (relative area of expression, %) | TGF-β1 (relative area of expression, %) |
| Women with androgenic alopecia (n=15) | 32.24 (30.59; 33.83)* | 22.32 (21.93; 23.81)* | 20.96 (20.12; 21.7) | 56.11 ± 1.77* |
| Healthy women (n=8) | 68.53 ± 1.08 | 47.68 ± 0.93 | 19.96 ± 3 | 51.72 ± 2.21 |
Values are mean (SD) or median and range. *- statistically significant differences: P < 0.05
When comparing indicators of growth factor expression in men having androgenic alopecia with these indicators in healthy men, the statistically significant differences were also established.
It was reported that men with androgenic alopecia had reduction of the expression level baseline for VEGF (56.27 ± 1.36). At the same time the initial levels of expression for EGF (65.05 ± 2.49), KGF (61.33 ± 1.01) and TGF-β1 (82.38 ± 1.05) in men with androgenic alopecia exceeded the indicators of healthy men (P <0.05) (Table 5).
Table 5: Relative area indicators of the growth factor expression in the scalp skin in men with androgenic alopecia and healthy individuals
| Growth factor
Trial groups |
VEGF
(relative area of expression, %) |
KGF
(relative area of expression, %) |
EGF
(relative area of expression, %) |
TGF-β1
(relative area of expression, %) |
| Men with androgenic alopecia (n=15) | 56.27 ± 1.36* | 61.33 ± 1.01* | 65.05 ± 2.49* | 82.38 ± 1.05* |
| Healthy men (n=8) | 66.53 ± 1.42 | 49.76 ± 1.31 | 22.56 ± 0.5 | 54.45 ± 0.59 |
Values are mean (SD) or median and range. *- statistically significant differences: P < 0.05
Most clearly, the difference in the level of growth factors expression in different clinical forms of alopecia is shown in Fig. 1.
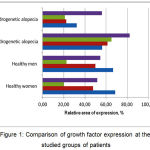 |
Figure 1: Comparison of the growth factors expression at the studied groups of patients |
The Level of the Growth Factors Expression (VEGF, KGF, EGF, TGF-Β1) Depending on the Progression and Clinical Picture of Alopecias
For a detailed study of the influence of the growth factors expression on the progression of alopecia, a correlation analysis was performed and the power of influence of each factor was calculated.
When comparing the indicators of the growth factors expression level in the scalp skin of patients with androgenic alopecia with the indicators of the disease onset age, the disease duration and the patients’ age, negative correlation dependencies were detected between the levels of expression for VEGF (r = -0.22, P=0,039 ), KGF (r = -0.33, P<0,001), EGF (r = -0.28, P=0,007) and TGF-β1 (r = -0.35, P<0,001) in the scalp skin and the patients’ age.
Thus, in young patients, the level of expression of these factors was higher than in patients of the senior age group. Perhaps, this testifies to higher activity of biosynthetic processes in patients of younger age.
A negative correlation dependence was found between KGF expression levels (r = -0.24, P=0,022), TGF-β1 (r = -0.23, P=0,031) and disease duration. It was established that the less the disease duration, the higher the expression level of KGF and TGF-β1. This correlation may indicate the role of these factors in the initiation of the pathological process.
A positive correlation dependence was found between the severity of hair thinning on the median line in women (Sinclair scale (2004)) and the levels of the growth factors expression for EGF (r = 0.36, P=0,004), VEGF (r = 0.75, P<0,001), as well as a negative correlation dependence between the levels of the growth factors expression KGF (r = -0.85, P<0,001), TGF-β1 (r = -0.75, P<0,001). Thus, it may be concluded that in female patients with the severe course of alopecia, the levels of the EGF and VEGF growth factors expression are higher and levels of the KGF and TGF-β1 expression are lower.
The Power of Influence of Growth Factors (VEGF, KGF, EGF, TGF-Β1) in the Scalp Skin on the Development of Alopecias
The analysis of the influence of the VEGF, KGF, EGF, TGF-β1 growth factors in the scalp skin on the development of androgenic alopecia in women displayed statistically important influence of the VEGF, KGF, TGF-β1 growth factors on the development of the androgenic alopecia in women. Thereby, the possibility of the development of androgenic alopecia in women increases with the reduction of indicators for VEGF (η2 = 99%), KGF (η2 = 97.7%) and their rise for TGF-β1 (η2 = 37.7%) (P <0.05).
The multifactorial model analyses showed, that η2 indicators are statistically significant (P <0.05) for the VEGF and KGF predictors. At the same time the VEGF growth factor (η2 = 0.595864, P<0.05) influences the development of androgenic alopecia in women to a greater degree, and KGF exerts influence to a lesser degree (η2 = 0.127408, P <0.05), whereas TGF-β1 (η2 = 0.016131, P = 0.417) and EGF (η2 = 0.039376, P = 0.202) are not statistically significant in this model as the predictors of androgenic alopecia development in women (Table 6).
Table 6: The power of influence of free variables (growth factors) on the probable development of androgenic alopecia in women
| Indicator | η2 | Sum of squares (SS) | Fisher test (F) | P |
| VEGF | 0.595864 | 65.63259 | 60.45109 | 0.000 |
| KGF | 0.127408 | 6.499599 | 5.986474 | 0.019 |
| EGF | 0.039376 | 1.824644 | 1.680594 | 0.202 |
| TGF- β1 | 0.016131 | 0.729848 | 0.672228 | 0.417 |
The analysis of the influence of the VEGF, KGF, EGF, TGF-β1 growth factors in the skin of the scalp on the development of androgenic alopecia in men with the help of unifactor linear regression showed that each of the growth factors (VEGF, KGF, EGF TGF-β1) has the statistically important influence on the development of the androgenic alopecia in men. Thus, the reduction of indicators VEGF (η2 = 93.5%), and the rise of KGF (η2 = 96.2%), EGF (η2 = 98.7%) and TGF-β1 (η2 = 98.9%) increase the possibility of the androgenic alopecia development in men (P > 0.05).
When analyzing the multifactorial model, it was established that none of the studied factors has dominating influence on the development of this type of alopecia. Moreover, the TGF-β1 (η2 = 0.128601), EGF (η2 = 0.108276) and VEGF (η2 = 0.087867) growth factors influence the development of androgenic alopecia in men almost equally. The KGF (η2 = 0.008052, P = 0.567) growth factor is not statistically significant for this model as a predictor of androgenic alopecia development in men (Table 7).
Table 7: The power of influence of free variables (growth factors) on the probable development of androgenic alopecia in men
| Indicator | η2 | Sum of squares (SS) | Fisher test (F) | P |
| VEGF | 0.087867 | 4.737589 | 3.949606 | 0.054 |
| KGF | 0.008052 | 0.399201 | 0.332803 | 0.567 |
| EGF | 0.108276 | 5.971608 | 4.978375 | 0.031 |
| TGF- β1 | 0.128601 | 7.257967 | 6.050779 | 0.018 |
Discussion
Changes in the hair cycle are one of the key elements of the pathophysiological process of androgenic alopecia.20,21 Hair thinning occurs as a result of shortened duration of anagen and prolonged stay of follicles in the latent phase – kenogen.2,22 The research has established the fact of changing the expression of the VEGF, KGF, EGF, and TGF-β1 growth factors in patients with androgenic alopecia as compared to healthy persons that has not been previously described in the scholarly publications. In this study we established that the expression of growth factors of VEGF, KGF, EGF, TGF-β1 in the hair follicle is disturbed in patients with alopecia.
The study findings show that in patients with alopecia the expression of the VEGF, KGF, EGF, TGF-β1growth factors is disturbed in the hair follicle.
It was discovered that women with androgenic alopecia had statistically significant reduction for the VEGF, KGF growth factors and the increase for TGF-β1. The difference in the indicators of the EGF expression level is not statistically reliable. Abnormalities in the expression of growth factors are combined with the reduction in the total amount of hairs in the parietal region, change of terminal and vellus hair percentage ratio in the parietal and occipital regions towards the increase of vellus hairs, violation of the anagen and telogen hair percentage ratio towards the increase of hair that are in telogen.
It was found that men with androgenic alopecia had statistically significant reduction of VEGF and the increase of KGF, EGF and TGF-β1 growth factors. This abnormality in the expression of growth factors is combined with the reduction in the total amount of hairs in the parietal region, change of terminal and vellus hair percentage ratio in the parietal region towards the increase of vellus hair, violation of normal ratio of anagen and telogen hairs towards the increase of hairs that are in telogen.
The growth factors expression has different levels depending on the patients’ age and the onset of the disease. The VEGF, KGF, EGF, TGF-β1 growth factors are reliably higher in the younger age, at the same time KGF and TGF-β1 are reliably higher at the beginning of the disease.
The level of the VEGF, KGF, EGF, TGF-β1 growth factors expression in the scalp skin depends on the clinical form of alopecia and the progression of the disease. The data obtained are comparable with the opinion of a number of authors that although the pathogenesis of androgenic alopecia is based on the paradoxical effect of circulating and local androgens on the follicles of the scalp and body, there are gender differences.20-22
Thus, the VEGF, KGF, TGF-β1 growth factors have statistically significant influence on the development of androgenic alopecia in women. The reduction of indicators of VEGF, KGF and the rise of TGF-β1 increase the possible development of androgenic alopecia in women. At the same time, VEGF has the greatest influence on the development of androgenic alopecia in women, while KGF affects to a lesser degree. The VEGF, KGF, EGF, TGF-β1 growth factors have influence on the development of androgenic alopecia in men. Thus, the reduction of the VEGF indicator and the rise of KGF, EGF and TGF-β1 increase the possible development of androgenic alopecia in men. However, when analyzing the multifactorial model, it was established that none of the studied factors has the dominating influence on the development of androgenic alopecia in men. The change in the indicator values for TGF-β1 , EGF and VEGF almost equally influences the development of androgenic alopecia in men. The KGF growth factor is not statistically significant in this model as a predictor of androgenic alopecia development in men.
According to the anamnesis data, patients with androgenic alopecia have a chronic course of the disease. Despite the earlier comprehensive treatment of these patients, a pronounced therapeutic effect was not noted, which once again confirms the urgency of the search for new therapeutic agents. The provisions formulated in the study make it possible to improve the therapy of patients with androgenic alopecia.
To obtain reliable results, not only patients with androgenic alopecia (30 women and 30 men), but also healthy individuals (8 women and 8 men) were included in the study. Modern laboratory and instrumental methods were applied; expression of the VEGF, KGF, EGF, TGF-β1 growth factors in the scalp skin was studied by an immunofluorescent method.
This study has some limitations. Firstly, the expression of only 4 growth factors was studied. Secondly, to study the expression of growth factors, we were limited to one technique – immunofluorescence.
Conclusion
In conclusion, the present study highlights the important role of the VEGF, KGF, EGF, TGF-β1 growth factors in the androgenic alopecia development through the established changes in the expression of these growth factors in patients with androgenic alopecia compared to controls. Expression of the VEGF, KGF, EGF, and TGF-β1 growth factors is impaired in patients with androgenic alopecia in comparison with healthy persons. The level of growth factors expression depends on the patient’s sex, age, duration and nature of the disease progression.
It has been found that, reduction in the VEGF expression has a greater effect on the development of androgenic alopecia in women and decrease in the KGF expression affects it to a lesser degree. None of the VEGF, KGF, EGF, TGF-β1 growth factors has a prevailing influence on the development of androgenetic alopecia in men. The level of the VEGF, KGF, EGF, TGF-β1 growth factors in the hair follicle of patients with androgenic alopecia is higher at a younger age, and that of the KGF and TGF-β1 – at the onset of the disease. In men with androgenic alopecia, the VEGF, KGF, EGF, TGF-β1 expression level is higher than in women with androgenic alopecia.
Thus, we may conclude that the VEGF, KGF, TGF-β1 growth factors influence the development of androgenic alopecia in women. Men obviously have other factors prevailing in the pathogenesis of alopecia development.
Conflict of Interest
The authors declare no conflict of interest.
The study was approved by the ethical committee of Russian Medical Academy of Postgraduate Education (Protocol No12, 8.12.2015).
Acknowledgement
The authors would like to thank Dr Anna A. Kubanova from Russian Academy of Sciences for her contribution in conducting some of the experiments and support in implementing the project.
References
- Messenger A. G., de Berker D. A. R., Sinclair R. D. Chapter Disorders of Hair. In: Burns T., Breathnach S., Cox N., Griffiths C. (Eds.) Rook’s Textbook of Dermatology. 8th ed. Oxford, UK: Blackwell Science Publications. 2010;66(16)1–66. DOI: 10.1002/9781444317633. ch66
CrossRef - Goldsmith L. A, Katz S. I., Gilchrest B. A., Paller A. S., Leffell D. J., Wolff Fitzpatrick’s Dermatology in General Medicine, 8th Edition. New York: McGraw-Hill. 2011.
- Millar S. E. Molecular mechanisms regulating hair follicle development. Invest. Dermatol. 2002;118:216-225.
CrossRef - Botchkarev V. A., Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55.
CrossRef - Schmidt-Ullrich R and Paus R. Molecular principles of hair follicle induction and morphogenesis. Bio. Essays. 2005;27:247-261.
CrossRef - Katsuoka K., Schell H., Wessel B., Hornstein O. P. Effects of epidermal growth factor, fibroblast growth factor, minoxidil and hydrocortisone on growth kinetics in human hair bulb papilla cells and root sheath fibroblasts cultured in vitro. Arch Dermatol Res. 1987;279:247–50.
CrossRef - Hu M. C., Qiu W. R., Wang Y. et al. FGF-18, a novel member of the fibroblast growth factor family stimulates hepatic and intestinal proliferation. Molecular and Cellular Biology. 1998;18:6063–74.
CrossRef - Jindo T., Tsuboi R., Imai R., Takamori K., Rubin J. S., Ogawa H. Hepatocyte growth factor scatter factor stimulates hair growth of mouse vibrissae in organ culture. J Invest Dermatol. 1994;103: 306–309.
CrossRef - Jindo T., Tsuboi R., Takamori K., Ogawa H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998;110:338–42.
CrossRef - Takakura N., Yoshida H., Kunisada T., Nishikawa S., Nishikawa S. I. Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J Invest Dermatol. 1996;107(5):770-777.
CrossRef - Yano K and Oura H. Angiogenesis by VEGF controls hair growth and follicle size. Cell Technol. 2001;20:852–3.
CrossRef - Woo W. M and Oro A. E. SnapShot: hair follicle stem cells. 2011;146:334-334.
- Zhang H., Nan W., Wang S. et al. Epidermal growth factor promotes proliferation and migration of follicular outer root sheath cells via Wnt/β-catenin signaling. Cell Physiol Biochem. 2016;39(1):360-370.
CrossRef - Zhang H., Nan W., Wang S. et al. Epidermal growth factor promotes proliferation of dermal papilla cells via Notch signaling pathway. Biochimie. 2016;127:10-8.
CrossRef - Alexandrescu D. T., Kauffman C. L., Dasanu C. A. The cutaneous epidermal growth factor network: Can it be translated clinically to stimulate hair growth? Dermatol Online J. 2009;15(3):1.
- Wu X. J., Zhu J. W., Jing J. et al. VEGF165 modulates proliferation adhesion migration and differentiation of cultured human outer root sheath cells from central hair follicle epithelium through VEGFR-2 activation in vitro. J Dermatol Sci. 2014;73(2):152-60.
CrossRef - Seo H. S., Lee D. J., Chung J. H. et al. Hominis Placenta facilitates hair regrowth by upregulating cellular proliferation and expression of fibroblast growth factor-7. BMC Complement Altern Med. 2016;16:187.
CrossRef - Lu G. Q., Wu Z. B., Chu X. Y. et al. An investigation of crosstalk between Wnt/β-catenin and transforming growth factor-β signaling in and rogenetic alopecia. Medicine (Baltimore). 2016;95(30):e4297.
CrossRef - Li J., Yang Z., Li Z., et.al. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Hormone & IGF Research 2014;24: 89–94.
CrossRef - Sawaya M and Price V. Different levels of 5alfa-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with and rogenetic alopecia. J Invest Dermatol. 1997;109:296-300.
CrossRef - Werner B and Mulinari-Brenner F. Clinical and histological challenge in the differential diagnosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia areata – Part I. An Bras Dermatol. 2012;87(5):742-7.
CrossRef - Werner B and Mulinari-Brenner F. Clinical and histological challenge in the diagnosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia areata – Part II. An Bras Dermatol. 2012;87(6):884-890.
CrossRef







