Zainab A. Hamid
Department of Microbiology, Baghdad Medical College, UOB, Baghdad, Iraq.
Corresponding Author E-mail: zainabhamid96@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1258
Abstract
Hepatitis B virus (HBV) which affects the liver and the Epstein–Barr virus (EBV) which consider as one of the most common viruses in humans that cause of infectious mononucleosis (glandular fever) which also associated with particular forms of cancer, those two viruses are possible etiology for rheumatoid arthritis (RA). The study extended from July 2016- February 2017 in Baghdad Teaching Hospital, Baghdad/Iraq, patients with rheumatoid arthritis (n=100) and a control group (healthy persons, n=50), and osteoarthritis patients (n=50) were tested by Enzyme-Linked Immunosorbent Assay (ELISA) for HBs Ag, IgG anti-HBs antibodies, total anti-HBc antibodies, and IgG antibodies specific to EBV. RA patients were also tested for rheumatoid factor and C-reactive protein by latex agglutination test, total White Blood Cells (WBCs), WBCs count, and Erythrocyte Sedimentation Rate (ESR or Sed. Rate). The results revealed that differences in antibody titers of HBV markers (HBs Ag, IgG anti-HBs antibodies, total anti-HBc antibodies) in serum of RA group and control group were statistically not significant while the mean antibody titers of IgG antibodies for EBV in RA patients was higher than that in control group and the difference was statistically significant (P < 0.05) which concluded that the EBV infection might be a possible factor in pathogenesis of RA, while the role of HBV infection is not evident.
Keywords
HBV; EBV; RA; Pathogenesis; Serum
Download this article as:| Copy the following to cite this article: Hamid Z. A. The Impact of Hepatitis B Virus and Epstein-Barr Virus in Pathogenesis of Rheumatoid Arthritis. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Hamid Z. A. The Impact of Hepatitis B Virus and Epstein-Barr Virus in Pathogenesis of Rheumatoid Arthritis. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16381 |
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease, which could lead to can cause bone damage, cartilage and eventually could cause disability. Early diagnosis is the key to best therapeutic accomplishment, mainly in patients with risk factors such as early joint damage, presence of autoantibodies and high disease activity. RA also considered a disease that includes extra articular appearances, such as rheumatoid nodules, pulmonary involvement or vasculitis. Despite intensive research that was taken place over many decades, the cause of RA remains unknown, but researchers belives in three sectors of interconnected are most encouraging: genetic elements, autoimmunity and immunoregulatory abnormalities, and possible microbial infection.1,2 which considereds as the major environmental factors that may cause inflammatory arthritides in genetically susceptible persons, and may related with the pathogenesis of RA through their previous or their chronic infection.3,4
Many clinical and laboratory methods used to find the role of microorganisms in the pathogenesis of RA. However, direct isolation of the microorganisms from the synovial tissue or synovial membrane almost always was negative or lacks the reproducibility.5 Many investigators demonstrated the presence of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) of some microorganisms in the synovial tissues including the synovial membrane or synovial fluid using Polymerase Chain Reaction (PCR), southern blot hybridization technique, and some other advanced nucleic acid detection methods.6,7 Studies on the synovial fluid clarified that antibodies against some microorganisms could be detected in RA patients and their titers were higher than their corresponding in the control group.8 Some studies showed that some microorganisms could be detected in the blood of patients with RA, while some investigators showed that the antibody titers for some microorganisms in the serum of patients with RA are higher than those in the control group.9
Many viruses are implicated in the etiology of RA; the most proposed to play a role in RA, include: Hepatitis B virus, Hepatitis C virus, Epstein -Barr virus, Retroviruses, Cytomegalovirus, and Human parvovirus B19.10
Method
This study was conducted to estimate the Hepatitis B virus markers (HBs antigen, IgG anti-HBs antibodies, and IgG anti-HBc antibodies) ,This test was performed using commercially available kit (DRG.ELISA). and for IgG antibodies for Viral capsid antigen (VCA) of EBV using commercially available kit (DRG.ELISA). in the patients with rheumatoid arthritis and control groups in a period between July 2016 and January 2017, using ELISA technique .One hundred Iraqi patients with rheumatoid arthritis who were attending the Rheumatology Consultation Clinic or admitted to Baghdad Teaching Hospital. All of whom met the revised criteria for RA as defined by the American College of Rheumatology 1987. Control group was age and sex matched with the patients group (RA group). The control group was sub grouped into:
Osteoarthritis (O.A.) control subgroup included (50) patients with osteoarthritis of the knee and (50) healthy individuals chosen from blood bank donors, who have no clinical evidence of any obvious disease.
The study protocol also included: serological tests for detection of rheumatoid factor (RF) and C- Reactive Protein (CRP) by latex test, hematological tests to enumerate the total WBCs count (cell count/ mm3 ) using hemocytometer manual cell counting, and measurement the erythrocyte sedimentation rate (ESR mm/hr) by westergren method.
Data have been analyzed statistically using SPSS program ver.10. Results were expressed using simple statistical parameters such as mean ±SD. Analysis of qualitative data was assessed using Χ² Chi square test. Analysis of quantitative data was estimated using t-test and ANOVA (analysis of variance). The strength of linear relationship between X and Y variables was determined by sample correlation coefficient (r).
Results
The RA group involves 100 patients with rheumatoid arthritis; their diagnosis based on the American Rheumatism Association 1987 revised criteria for the classification of RA. The frequency distribution of these criteria in the RA group is demonstrated in table (1).
Table 1: Frequency distribution for the criteria of classification rheumatoid arthritis in patients study group.
| Criterion | Frequency (%) |
| Morning stiffness | 79 (79%) |
| Arthritis of three or more joints | 71 (71%) |
| Arthritis of hand joints | 80 (80%) |
| Symmetrical arthritis | 63 (63%) |
| Rheumatoid nodules | 15 (15%) |
| Serum rheumatoid factor | 88 (88%) |
| Radiographic changes | 69 (69%) |
Other clinical features revealed that only 31 RA patients (31%) have extra-articular manifestations, only 3 RA patients (3%) have other autoimmune diseases (one patient with systemic lupus erythematosis, while the other two with insulin dependent diabetes and alopecia areata; depending on history taking), while of the O.A. control subgroup, only one patient (2%) have autoimmune disease (vitiligo). Of the RA group, 15 patients have joint effusion (15%), while the O.A. control subgroup only 2 patients (4%) have joint effusion. The RA group involved 100 patients, their age ranged between (16-76 years); with mean age (44.8 ±10.2 years). The age of highest incidence is between 35-40 years. There were 82 females and 18 males with female to male ratio was (4.5:1.0) as shown in figure (1).
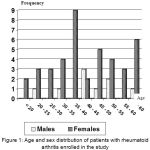 |
Figure 1: Age and sex distribution of patients with rheumatoid arthritis enrolled in the study
|
In the RA group the rheumatoid factor was positive in 88 patients (88%) as mentioned in table (1), while it was positive in only one O. A. patient subgroup and none in the healthy control subgroup (P<0.0001).
The results revealed that CRP was positive in 89 RA patients (89%), and negative in 11 RA patients (11%). The CRP was positive in 2 patients with osteoarthritis (4%) and negative in 48 (96%) patients with osteoarthritis, while all the healthy control subgroup was negative. There was statistically significant differences between the RA group and the control group (P<0.0001), table (2).
Table 2: Results of C-reactive protein measured by latex agglutination in study groups.
| Study groups | Positive No. (%) | Negative No. (%) | Total |
| RA patients | 89
(89%) |
11
(11%) |
100
(100%) |
| O.A. patients | 2
(4%) |
48
(96%) |
50
(100%) |
| Healthy control | 0
(0%) |
50
(100%) |
50
(100%) |
|
Total
|
91
|
109
|
200
|
The mean of total white blood cells count in the patients group (RA) was 6.548× 10/L (±2.207), while those for the healthy control subgroup and O. A. patient’s subgroup were 7.354× 10/L (±1.877) and 7.204×10/L (±3.361) respectively. The results showed no significant differences (P=0.408).
Erythrocyte sedimentation rate (ESR) was found to be elevated in RA group in comparison to the O.A. control subgroup and healthy control subgroup. There was statistical significant differences between the RA group and control group (P=0.042), table (3).
Table 3: The difference in mean and range of ESR count between RA group and control group.
| ESR (Mm/hr) | RA patients group (n=100) | O. A. patients Subgroup (n=50) | Healthy control Subgroup (n=50) |
|
Range
|
4-105
|
4-45
|
2-28
|
|
Mean
|
45
|
17
|
12
|
The seroprevalence of HBs Ag in RA group with mean Optical Density (O.D.) measured at 450 nm was 0.5173(±0.2009); while those for the healthy control subgroup and osteoarthritis (OA) control subgroup was 0.4892 (±0.1808) and 0.5426 (±0.2143) respectively. The results were statistically not significant (p=0.410); figure (2). The seroprevalence of anti-HBs Abs in patients group showed that the mean O.D. measured at 450 nm was 1.0900 (±0.3822); while those for the healthy control subgroup and OA control subgroup were 1.0512 (±0.3357) and 0.9612 (±0.3359) respectively. The difference between RA and control groups was statistically not significant (p=0.121); figure (3).
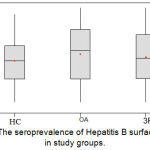 |
Figure 2: The seroprevalence of Hepatitis B surface antigen in study groups.
|
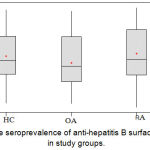 |
Figure 3: The seroprevalence of anti-hepatitis B surface antibodies in study groups.
|
The seroprevalence of anti-HBc Abs in RA group and control group showed that the mean O.D. for RA patients’ sera measured at 450 nm was 0.6244 (±0.1990); while those for the healthy control subgroup and OA control subgroup were 0.6448 (±0.1882) and 0.6752 (±0.2052) respectively. The results were statistically not significant (p=0.334); figure (4).
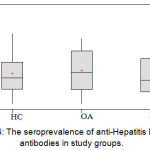 |
Figure 4: The seroprevalence of anti-Hepatitis B core antibodies in study groups.
|
The Seroprevalence of IgG Antibodies specific to EBV in RA group and control group clarify that the mean absorbance value for the RA group sera measured at 450 nm was 0.7569 (±0.1892); while those for the healthy control subgroup and O.A. control subgroup were 0.6723 (±0.1329) and 0.6962 (±0.1059). This difference between the RA and control groups was statistically significant (p=0.005), figure (5).
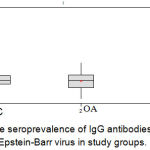 |
Figure 5: The seroprevalence of IgG antibodies specific to Epstein-Barr virus in study groups.
|
The results showed non-significant positive correlation between EBV antibodies and other parameters as shown in table (4).
Table 4: Correlation between different parameters and antibody titers against some microorganisms
| Parameters | Correlation coefficient (r factor) |
| Age | 0.197 |
| Smoking | 0.108 |
| Allergy | 0.241 |
| No. of inflamed joints | 0.171 |
| Extra-articular manifestations | 0.185 |
| Joint effusion | 0.188 |
| CRP | 0.231 |
| ESR | 0.198 |
| WBCs count in blood | 0.229 |
The numbers represent the correlation coefficient (r factor), r = +1 Perfect linear correlation, r = -1 Perfect inverse linear correlation, r = 0 No linear correlation
Discussion
In this study the age of highest incidence for RA patients is at the fourth decade (35-40years), while abroad studies had diverse data for the predominant age of disease. Female to male ratio in this study was 4.5:1 which indicates female predominance. Many other studies revealed almost similar results.11 Over presentation of women of RA is mostly linked to sex and hormonal predisposing factors.19 Past hepatitis B infection and vaccination against it have been implicated in the potential triggering or flare of some autoimmune diseases, including rheumatoid arthritis (RA).12
The results demonstrated that seroprevalence of HBs Ag, anti-HBs antibodies, and anti-HBc antibodies were of low antibody titers in RA and control groups. There were no significant differences between RA and control groups, Nevertheless, vaccination against HBV may be associated with the development of RA, which might represent the activation of RA in genetically susceptible individuals.13,14
Studies were detected the development of RA in six patients, 2 months following hepatitis B vaccination.22 On the contrary to these result, Elkayam et al. did not notice the development of RA in 44 subjects receiving hepatitis B vaccine.23 There could be no relationship between HBV (past infection or vaccination) with the development of RA. An alternative hypothesis supposed the presence of a link, thus a single or more peptides of HBV might bind to MHC class II molecules in a genetically susceptible patients leading to triggering RA.1,5,12
This study shown that the mean of IgG antibody titers specific to VCA of EBV in RA group was higher than in control group, the results were statistically significant (p=0.005). It was the first study in Iraq that looks for the role of EBV in RA., the seroprevalence of EBV is generally high among healthy adult population which indicates a previous infection with EBV may occur in early childhood.15
A similar ELISA technique was used to detect the same antibodies (anti-VCA antibodies) in serum of RA patients. They detected a twofold increase in mean of values for IgG antibodies compared with the control group, the rise in antibodies specific to EBV antigens in existing RA more than in healthy control has been reported in other studies.16,17
Possible mechanisms were suggested for the contribution of EBV in pathogenesis of RA. The sequence similarity of an EBV capsid antigen to HLA – DR1 RA susceptibility sequence QKRAA, could lead to antibody cross reaction and the production of autoimmune response.15
T cells proliferating against gp110 might represent a chronic exposure to EBV antigens, and thus leading to a chronic inflammatory response in patients with RA.18
Longitudinal data on RA patients demonstrated a non-significant positive correlation between CRP and EBV antibody titers (r = 0.231). All other inflammatory markers done in the study showed almost similar results; like ESR, and WBCs count in blood.
Other clinical features and parameters used in the study like number of inflamed joints, presence of extra-articular manifestations and joint effusion, age, sex, smoking, and allergy; did not show any significance in relation to anti- EBV antibody titers. So EBV might trigger the disease but has no effect on the next steps in pathogenesis of RA, or on the activity of the disease.
In conclusion this study gave rise to that the rheumatoid factor were higher in RA patients than in control group. There was a significant difference of IgG anti-EBV (anti-VCA) in RA patients compared to control group which indicated that previous infection with EBV may trigger RA. The antibodies to Hepatitis B virus, were very low in RA patients as well as in control group, thus this virus might have no role in RA.There was no correlation between IgG antibody titers against EBV in serum with some parameters like RF, CRP,ESR, WBCs count in blood or synovial fluid , which indicated that this virus if play a role in RA, have no effect on the next steps in pathogenesis of RA.
Conclusion
Further studies including large sample size of cases are needed to reveal specific link between various infectious agents and RA
Molecular techniques including PCR and DNA hybridization test should be introduce for detection of viral infections.
Searching for other autoantibodies rather than RF, like antinuclear antibodies and anti-keratin antibodies and study their association with features of infection in RA patients.
References
- Johanna A.H, Johanna V. et al., (2017). Cytokine data obtained from synovial stromal cells of patients with rheumatoid arthritis or osteoarthritis. Data in Brief 12: 593–602.
CrossRef - Joanna K., Violaine F. et al., (2017). Lewis–Sumner syndrome in a patient with rheumatoid arthritis: Link between rheumatoid arthritis and demyelinating polyradiculoneuropathies. Joint Bone Spine 84: 485–487.
CrossRef - Kamel BS, Mongi T. and Naceur B. (2006). Epstein–Barr virus infection associated hepatic lymphoma in a patient treated with methotrexate for rheumatoid arthritis. Letters to the editor / Joint Bone Spine 73: 215–220.
- Kobayashi S, Momohara S, Kamatani N and Okamoto H. (2008). Molecular aspects of rheumatoid arthritis: role of environmental factors.FEBS J 275(18):4456–62.10.1111.
- Song Li, Yangsheng Yu, Yinshi Yue, Zhixin Zhang, and Kaihong Su. (2013). Microbial Infection and Rheumatoid Arthritis. J Clin Cell Immunol. 4(6): 174.
- Qunying H., Cuiling Y. et al., (2017). Association of genetic variation in B-cell activating factor with chronic hepatitis B virus infection. Immunology Letters 188: 53–58.
CrossRef - Yu Z, Jian-ning C. (2017). Clinical significance of spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia in Epstein-Barr virus–associated and Epstein-Barr virus–negative gastric cancer. Human Pathology, 63: 128–138.
CrossRef - Tao X., Zeli H., et al., (2015). Clinical implications of hepatitis B viral infection in Epstein–Barr virus-associated nasopharyngeal carcinoma. Journal of Clinical Virology 64: 64–71.
CrossRef - Kuipers JG, Sibilia J, Bas S, et al. (2009). Reactive and undifferentiated arthritis in North Africa: use of PCR for detection of Chlamydia trachomatis. Clin Rheumatol .28(1):11–6.
CrossRef - Témoin S,Chakaki A,Askari A et al., (2012). Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol.18(3):117-21.
CrossRef - Mahabadi M, Faghihiloo E, et al., (2016).Detection of Epstein-Barr virus in synovial fluid of rheumatoid arthritis patients. Electron Physician 8: 2181-2186.
CrossRef - Westergaard MW, Draborg AH et al., (2015).Isotypes of Epstein-Barr virus antibodies in rheumatoid arthritis: association with rheumatoid factors and citrulline-dependent antibodies. Biomed Res Int 472174.
CrossRef - Jacques P Brown and Jean G. (2016). Does Epstein-Barr Virus Infection Contribute to Disease Flares in Rheumatoid Arthritis? 5:208. doi:10.4172/2167-7921.1000208.
CrossRef - Bajraktari IH, Teuta B-Ç, Vjollca S-M et al., (2014). Demographic Features of Patients with Rheumatoid Arthritis in Kosovo. Medical Archives. 68(6):407-410.
CrossRef - Kvien TK, Uhlig T, Ødegård S and Heiberg MS. (2006). Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 1069: 212-22.
CrossRef - Ronald F van. (2009). Vollenhoven. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Medicine, 7: 12. org/10.1186/1741-7015-7-12
- Andrew A and Henry A. (2008). Foster Gbagbo. Seroprevalence of HHV-8, CMV, and EBV among the general population in Ghana, West Africa. BMC Infectious Diseases, 8 (1): 111-116.
CrossRef - Kurbanov SA and Mamedov MK. (2009). Serological markers of viral infections in patients with rheumatoid arthritis. Georgian Med News. 166: 65-7.








