Sara Siadat1, Soosan Sadeghian1, Majid Heidarpour2, Sayed Mohammad Razavi3 and Seyed Sadra Izadi4
1Department of Orthodontics, School of Dentistry, Islamic Azad University, Isfahan (Khorasgan) Branch, Isfahan, Iran.
2Department of Orthodontics, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3Department of Oral and Maxillofacial Pathology, Dental Implants Research Center, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran.
4Depatment of Surgery and Radiology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
Corresponding Author E-mail: drsadeghian@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1263
Abstract
Amitriptyline is widely used in the treatment of depression and different types of pain. Since it has anti-inflammatory and antagonist effects on prostaglandin, it can affect the orthodontic treatment. The current inquiry examined the effects of amitriptyline on tooth movement. A case-control experimental study design was conducted. Six male dogs were randomly divided into two experimental and control groups. Amitriptyline(2 mg/kg/day, orally) was prescribed to the experimental group. A nickel titanium spring (200 gr) was used between the second premolar and canine after the 1st premolar extraction. After 2 months, the reduction of distance between the 2nd premolar and canine was measured. The percentages of root resorption and bone formation were determined. The data were analyzed using repeated measures analysis at significance level of 0.05. The average tooth movements were 3.17±0.56 mm in the experimental group and 3.68±0.92 mm in the control group. On the other hand, the average percentages of external root resorption and bone formation in experimental group were 7.97±1.75 and 9.54 ±1.84, respectively. Moreover, the average percentages of external root resorption and bone formation in control group were 8.33±2.41 and 9.79 ±1.99,respectively. All the differences between two groups were insignificant at the significant level of 0.05. The rate of tooth movement and the percentages of bone formation and root resorption in dogs decreased with systemic administration of amitriptyline although this reduction was not statistically significant in comparison with the control group. This decrease could be related to anti-inflammatory and anti-prostaglandin effects of amitriptyline.
Keywords
Amitriptyline;Bone remodeling; Tooth movement; Root resorption
Download this article as:| Copy the following to cite this article: Siadat S, Sadeghian S, Heidarpour M, Razavi S. M, Izadi S. S. The Effects of Systemic Prescription of Amitriptyline on Orthodontic Tooth Movement Rate, Root Resorption and Alveolar Bone Remodeling in Dog. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Siadat S, Sadeghian S, Heidarpour M, Razavi S. M, Izadi S. S. The Effects of Systemic Prescription of Amitriptyline on Orthodontic Tooth Movement Rate, Root Resorption and Alveolar Bone Remodeling in Dog. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16196 |
Introduction
The orthodontic tooth movement is an outcome of organized periodontal remodeling due to exertion of mechanical forces. This process at the cellular level involves resorption of alveolar bone next to the periodontal fibers in the compression zone, deposition of bone in the tensile zone, and reorganization of periodontal fibers. It has been found that many factors are involved in this complex process.1
During orthodontic tooth movement, PDL and its related structures are subjected to tensile and compressive forces. Following this change, certain types of neuropeptides,2 cytokines3,4 prostaglandins, or leukotrienes5,6 have been found at high frequency in the PDL. The presence of these factors formed by cells originating from immune and neural system in the periodontium indicates that such materials may be involved in the process of bone tissue remodeling after exertion of orthodontic forces.6
It is also possible to influence the biological activity through the application of pharmacologic agents, thereby to affect the tooth movement both in term of decreasing tooth movement (when it is desirable to augment anchorage) and increasing it. 7
Prostaglandin E plays an important role in the initiation of signaling cascades associated with tooth movement. It seems that the Prostaglandin E inhibitors affect tooth movement. 7
Amitriptyline is a serotonergic and noradrenergic reuptake inhibitor (SNRI) widely used in the treatment of major depression and different types of pain, including neuropathic pain and migraine. Since it has anti-inflammatory and antagonist effects on prostaglandin, it can affect the orthodontic treatment. 8-12 Some researchers including Rafie et al.13 indicated the decreased amount of tooth movement by other antidepressant drugs such as fluoxetine.
The inflammation during tooth movement can activate odontoclasts, which can cause external root resorption due to release of tissue degenerative enzymes. Since the degree of inflammation is subject to alteration after amitriptyline prescription, the change in the degree of root resorption is also probable.
Given the increasing tendency of adults toward orthodontic treatment in recent years and also due to the increasing rate of depression and subsequent use of antidepressants in the society, a significant percentage of people nominated for orthodontic treatment may take these drugs for their depression.
Since the effects of antidepressants on bone have been proven, and the effects of these drugs on the alveolar bone and tooth movement have not been examined and considering the high prevalence of antidepressants such as amitriptyline, to the current study examined the effect of antidepressants on orthodontic tooth movement and alveolar bone in dogs.
Materials and Methods
A case-control experimental study was conducted on dog modal. A total of sixmale dogs from mixed breeds with an age range of 10-12 months, weighing approximately 25±2 kg, with full health and full dentition and without taking any medication were selected.
The samples were randomly divided into two groups: The experimental group received amitriptyline while the control group did not receive any drugs.
Because of the thin and short roots of first premolars in these dogs, the second premolars were selected as the unit of movement, and the canine teeth (due to their long roots) were selected as the unit of anchorage. Then, the first premolars were extracted due to proximity to the second premolars and possibility of interfering with the movement.
The designed appliance consisted of two loops made from 0.014 inches ligatures wires(Ligature Ties, 0.14 Orthotechnology, USA) placed around the canine teeth and the second premolars and bonded on the tooth surface by dental composite( Fig. 1).
Then, a nickel titanium spring (Close Coil Spring with Eyelets, size 9F, G&H Wire Co.) with a spring force of 200 g was stretched between two loops. Finally, the distance between two teeth on the two designated points on the buccal surface of the canine teeth and the second premolars ‘marked by a pointed bur’ was measured by a digital caliper.
Medication in the experimental group was prescribed orally on a daily basis at 2 mg/kg from the first day of study. In each dog, the upper right second premolar (UR), the lower right second premolar (LR), the upper left second premolar (UL), and the lower left second premolar (LL) were moved during two months. At the end of the second month, the distances between the canine teeth and premolars and the reduction in these distances were measured. Then, the second premolars were removed along with the surrounding bone for histological studies using Hematoxyline and Eosin (H&E) technique after decalcification in 10 percent formic acid. The percentages of root resorption and bone formation rates were determined by a pathologist who repeated the histological examination after 4 weeks (Figs. 2-4).
The data were statistically analyzed using SPSS 22. For this purpose, the rate of tooth movement, the percentage of bone formation, and the percentage of external root resorption in the animals of the two groups were reported. Given that four measurements were made on each dog, the data were analyzed via repeated measures analysis at a significance level of 0.05.
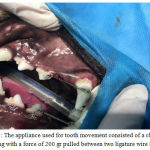 |
Figure 1: The appliance used for tooth movement consisted of a close coil spring with a force of 200 gr pulled between two ligature wire loops
|
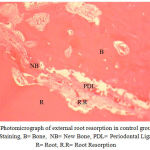 |
Figure 2: Photomicrograph of external root resorption in control group (X100) H&E Staining, B= Bone, NB= New Bone, PDL= Periodontal Ligament, R= Root, R.R= Root Resorption
|
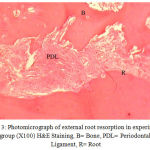 |
Figure 3: Photomicrograph of external root resorption in experimental group (X100) H&E Staining, B= Bone, PDL= Periodontal Ligament, R= Root
|
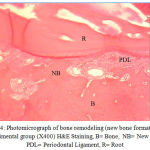 |
Figure 4: Photomicrograph of bone remodeling (new bone formation) in experimental group (X400) H&E Staining, B= Bone, NB= New Bone, PDL= Periodontal Ligament, R= Root
|
Results
According to the results, the average tooth movement was 3.17±0.56 mm in the experimental group and 3.68±0.92 mm in the control group. According to the repeated measures analysis at a significance level of 0.05, the difference between the two groups was not significant (P= 0.193).
On the other hand, the average percentage of external root resorption was 7.97±1.75 in the experimental group and 8.33±2.41 in the control group. According to the analysis of repeated measures, the effect of group on external root resorption was insignificant (P= 0.642).
Moreover, the average percentage of bone formation was 9.54 ±1.84 in the experimental group and 9.79 ±1.99 in the control group. According to the results of repeated measures analysis, the percentage of bone formation in two groups was not statistically different (P= 0.642) (Table 1).
Table 1: Comparing the average rate of tooth movement, root resorption, and bone formation in the experimental and control groups
| Variable | group | Mean±SD | P |
| Amount of Tooth movement(mm) | Control
Experimental |
3.68±0.92
3.17±0.56 |
0.193 |
| Percent of Root Resorption(%) | Control
Experimental |
8.33±2.41
7.96±1.75 |
0.642 |
| Percent of bone formation (%) | Control
Experimental |
9.79±1.99
9.54±1.84 |
0.642 |
Discussion
This study investigated the effect of amitriptyline as a classic tricyclic antidepressant drug (TCA) on the rate of tooth movement, root resorption, and bone formation during the orthodontic tooth movement in animal models.
Antidepressant drugs, especially tricyclic antidepressants such as amitriptyline, nortriptyline, and doxepin are commonly used to relieve pain, including pain of inflammatory origin (such as rheumatoid arthritis and fibromyalgia) and neuropathic pain (e.g. postherpetic neuralgia). This class of drugs is prescribed in addition to treating mood disorders.14-16 Amitriptyline is a common antidepressant with dual effects of serotonergic and noradrenergic reuptake inhibition (SNRI) widely prescribed to treat major depression. 8-12
Abdul Salam et al. found that amitriptyline had an anti-inflammatory effect on artificially induced paw inflammation in rats.17 Similarly, Hajhashemi et al. 18 found that amitriptyline hadsignificant anti-inflammatory effects on paw edema induced by external object injection in rats. They suggested that this anti-inflammatory effect is comparable to the effects of indomethacin,a drug with proven anti-inflammatory effects. It is noteworthy that indomethacin is one of the drugs which can reduce the rate of tooth movement.18 Considering the anti-inflammatory effect of amitriptyline which is comparable to indomethacin, the interference of amitriptyline with orthodontic tooth movement is probable.
Amitriptyline was examined in this study because of its anti-inflammatory effects reported in the above studies and the prevalence of its use in the treatment of depression, neuropathic pain, and migraine. Regarding the anti-inflammatory mechanism of amitriptyline, few studies have been conducted. However, the predominant theory states that the antidepressant effect of amitriptyline can alter the concentrations of monoamine neurotransmitters such as norepinephrine and serotonin in synapses in the CNS. The same mechanism may also change the level of peripheral inflammation. In several studies, including Hajhashemi et al.18 and Saynak1 et al.,20 the anti-inflammatory effects of amitriptyline were found associated with sites above the spinal cord, i.e. CNS. The sedative effects of amitriptyline may also lead to anti-inflammatory effects of these drugs in systemic administration.
During the exertion of orthodontic force in the compression zone, cytokines such as prostaglandin E2 and TNF-α and IL 1,6 are released from PDL cells. This leads to inflammation and increased RANKL level, subsequently activating osteoclasts, bone resorption, and tooth movement in the compression zone.21 If the inflammatory process on the compression side is reduced, the rate of tooth movement can be curtailed. Several studies have explored the effects of antidepressants on prostaglandin, including Lee22 and Metabaji.23 Lee et al. demonstrated that antidepressants prevent the biosynthesis of prostaglandins. Metabaji et al. reported that tricyclic antidepressants act as antagonists against prostaglandin E2.
Several studies have shown that NSAIDs are effective in reducing the pain caused by orthodontic tooth movement, while affecting the sequence of tooth movement by inhibiting or at least reducing inflammatory reactions and bone formation associated with tooth movement.24-29 This effect of NSAIDs is similar to the inhibitory effect of amitriptyline on the production of inflammatory mediators.
As noted earlier, amitriptyline is a serotonergic drug increasing the levels of serotonin in the brain.8-12 In addition to functioning as a neurotransmitter, serotonin has several other physiological functions in the peripheral organs.30 In the human body, there are more than 17 serotonin receptors identified. Examples of serotonin receptor cells are osteoblasts and osteoclasts.31 The effects of amitriptyline on the bone formation and resorption originate from indirect effect on osteoblasts and osteoclasts.
Hence, there is a possible interference between anti-inflammatory effects and the inflammation initiating orthodontic tooth movement, the theoretical significance of which has been discussed in this study. The results revealed that the average rate of tooth movement was reduced only slightly in the experimental group prescribed with amitriptyline even though this reduction was not statistically significant.
In other words, assuming the fact that the inflammation caused by the compression and tension in the PDL leads to orthodontic tooth movement, it can be argued that the anti-inflammatory effects of amitriptyline somewhat reduced the inflammation in the PDL. This led to an insignificant difference between the experimental and control groups.
The effects of other anti-depressant drugs on orthodontic tooth movement have been explored in similar studies. Rafiei et al.13 reported an insignificant reduction of the amount of tooth movement because of fluoxetine prescription in rats. This effect can be justified based on similar anti-inflammatory effects of anti-depressants like fluoxetine demonstrated in several animal models.32-33
The classic histologic studies by Rytan 34 on orthodontic tooth movement in animals revealed that root resorption basic and advanced lesions are similar to trabecular bone remodeling during the deposition phase. These areas are subsequently repaired by cellular cementum. The inflammation during tooth movement can activate odontoclasts, which can cause external root resorption due to release of tissue degenerative enzymes.
The adverse effects of osteoclasts and odontoclasts on root resorption have been found in studies regarding the effect of stress on root resorption during orthodontic tooth movement.
Stress can affect the axis of hypothalamic-pituitary-adrenal, thereby altering the immune system. Since the osteoclasts and osteoblasts are derived from the immune system, any alteration in their activity through stress may affect the root resorption. 35 For example, a recent study showed that patients with high stress levels were highly susceptibleto root resorption.36
Hence, reduced inflammation by taking amitriptyline can curtail resorption of roots by reducing the activity of osteoclasts. In the current study, reducing the extent of root resorption was observed following the use of amitriptyline. A slight decrease in root resorption was observed in the experimental group, which was consistent with the slight decrease in tooth movement in this group even though this decrease, similar to the amount of tooth movement, was not enough to be statistically significant.
In this study, bone formation rate was only marginally reduced due to prescription of amitriptyline. This may be due to a slight decrease in PDL inflammation consistent with the slight decrease in the external root resorption. Moreover, as the bone formation curtailed, there was lower external root resorption. There is a relationship between bone density and root resorption; the teeth moved next to dense cortical bone tolerate great amount of root resorption in comparison with the teeth moved in trabecular bone.37
The reduction in bone formation in the experimental group compared to the control group as well as reduction in the external root resorption in this group was consistent with the inflammation reduction in PDL during tooth movement in dogs. Such reduction in bone formation and root resorption paralleled a decrease in the amount of tooth movement in the experimental group.
Conclusion
The results of the present study showed that the rate of tooth movement and the percentage of bone formation and root resorption in dogs decreased with systemic administration of amitriptyline although this reduction was not statistically significant in comparison with the control group. This decrease could be related to anti-inflammatory and anti-prostaglandin effects of amitriptyline reported in pharmaceutical studies.
Acknowledgement
Hereby, the authors appreciate the invaluable efforts of Torabinejad Research Center Animal Lab members; specially Dr Manshai, for their warm support in conducting this research.
Conflict of interest
There is no conflict of interest.
References
- Blesta A.,Berggreen E., Brudvik P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur J Oral Sci. 2006;114(5):423-429.
CrossRef - Vandevska-Radunovic V., Kvinsland S., Kvinsland I. H. Effect of experimental tooth movement on nerve fibers immunoreactive to calcitonin gene related peptide,protein gene product 9.5 and blood vessel density and distribution in rats. Eur J Orthod. 1997;19(5):517-529.
CrossRef - Saito M., Saito S., Ngan P. W., Shanfeld J., Davidonitch Z. Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod. Dentofac. Orthop. 1991;99:226-240.
CrossRef - Greive W. G., Johnson G. K., Moore R. N., Rinhardt R. A., Dubois L. M. Prostaglandin E (PGE) and interleukin-1β (IL-1β) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod. Dentofac. Orthop. 1994;105:369-374.
CrossRef - Mohammad A. H., Tatakis D. N., Dziak R. Leukotriens in orthodontic tooth movement. Am J Orthod. Dentofac. Orthop. 1989;95:231-237.
CrossRef - Davidovitch Z.., Nicolay O., Ngan P. W., Shanfeld J. L. Neurotransmitters, cytokines and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988;32:411-434.
- Proffit W. R., Henry W. F., Sarver D. M., Ackerman J. L. Contemporary Orthodontics. 5th St. Louis: Elsevier. 2013;278-312.
- Bansal D., Bhansali A., Hota D., Chakrabarti A., Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med. 2009;26:1019-26.
CrossRef - Briley M., Moret C. Treatment of comorbid pain with serotonin norepinephrine reuptake inhibitors. CNS Spectr. 2008;13:22-6.
CrossRef - Galletti F., Cupini L. M., Corbelli I., Calabresi P., Sarchielli P. Pathophysiological basis of migraine prophylaxis. Prog Neurobiol. 2009;89:176-92.
CrossRef - Nishishinya B., Urrutia G., Walitt B., Rodriguez A., Bonfill X., Alegre C., et al. Amitriptyline in the treatment of fibromyalgia a systemic review of its efficacy. Rheumatology (Oxford). 2008;47:1741-6.
CrossRef - Young L. Post-herpetic neuralgia: a review of advances in treatment and prevention. J Drugs Dermatol. 2006;5:938-41.
- Rafiei M., Sadeghian S., Torabinia N., Hajhashemi V. Systemic effects of fluoxetine on the amount of tooth movement, root resorption, and alveolar bone remodeling during orthodontic force application in rat. Dental Research Journal. 2015;12(5):482-7.
CrossRef - Goldenberg D., Mayskit M., Mossey C., Ruthazer R., Schmid C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996;39:1852-9.
CrossRef - McQuay H. J., Tramer M., Nye B. A., Carroll D., Wiffen P. J., Moore R. A. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217-27.
CrossRef - Wong M. C., Chung J. W., Wong T. K. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87.
CrossRef - Abdel-Salam O. M., Nofal S. M., El Shenawy S. M. Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacol Res. 2003;48:157-65.
CrossRef - Hajhashemi V., Sadeghi H., Minaiyan M., Movahedian A., Talebi A. Central and peripheral anti-inflammatory effects of maprotiline on carrageenan-induced paw edema in rats. Inflamm Res in press. 2010.
CrossRef - Giunta D., Keller J., Nielsen F. F., Melsen B. Influence of indometacin on bone turnover related to orthodontic tooth movement in miniature pigs. Am J Orthod. Dentofacial. Orthop. 1995;108:361-6.
CrossRef - Sawynok J., Reid A. Antinociception by tricyclic antidepressant in the rat formalin test differential effects on different behaviours following systemic and spinal administration. Pain. 2001;93:51-9.
CrossRef - Richard J. B., Papaioannou A.., Adachi J. D., Joseph L., Whitson H. E., Prior J. C., et al. Effects of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188-94.
CrossRef - Lee R. E. The influence of psychotropic drugs on prostaglandin biosynthesis. Prostaglandins. 1974; 5(1):63-68.
CrossRef - Mtabaji J. P., Manku M. S., Horrobin D. F. Actions of the tricyclic anti-depressant clomipramine on response to pressure agents. Interactions with prostaglandin E2. Prostaglandins. 1977; 14(1):125-132.
CrossRef - Roche J. J., Cisneros G. J., Aivs G. The effect of acetaminophen on tooth movement in rabbits. Angle Orthod. 1997;67:231-6.
- Chumbley A. B., Tuncay O. C. The effect of indomethacin (an aspirin like drug) on the rate of orthodontic tooth movement. Am J Orhtod. Dentofacial. Orthop. 1986;89:312-4.
- Polat O.,Karaman A. L. Pain control during fixed appliance theraphy. Angle Orthod. 2005;75:210-5.
- Bernhardt M. K., Southard K. A., Batterson K. D., Logan H. L., Baker K. A., Jakobsen J. R. The effect of preemptive and/or postoperative ibuprofen therapy for orthodontic pain. Am J Orthod. Dentofacial. Orthop. 2001;120:20-7.
CrossRef - Law S. L. S., Southard K. A., Law A. S., Logan H. L., Jakobsen J. R. An evaluation of preoperative ibuprofen for treatment of pain associated with orthodontic separator placement. Am J Orthod. Dentofacial. Orhtop. 2000;118:629-33.
- Sari E., Olmez H., Gurton A. V. Comparison of som effect of acetylsalicylic acid and rofecoxinb during orthodontic tooth movement. Am J Orthod. Dentofacial. Orhtop. 2004;25:310-5.
- De Vernejoul M. C., Collet C., Chabbi-Achengli Y. Serotonin: good or bad for bone. Bonekey Rep. 2012;1:120.
CrossRef - Gustafsson B. I., Thommesen L., Stune A. K., Tommeras K., Westbroek I., Waldum H. L., et al. Serotonin and fluxetine modulate bone cell function in vitro. J Cell Biochem. 2006;98:139-151.
CrossRef - Bianchi M., Rossoni G., Sacerdote P., Panerai A. E., Berti F. Effects of chlomipramine and fluoxetine on subcutaneous carrageenin-induced inflammation in the rat. Inflamm Res. 1995;44:466-9.
CrossRef - Roumestan C., Michel A., Bichon F., Portet K., Detoc M., Henriquet C., et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35.
CrossRef - Reitan K. Tissue behavior during orthodontic tooth movement. Am J Orthod 1960;46:881.
CrossRef - Goldie R. S., King G. I. Root resorption and tooth movement in orthodontically treated, calcium deficient and lactating rats. Am J Orthod. 1984;85:424-430.
CrossRef - Davidivitch Z., Lee Y. J., counts A. L.., Park Y. G., Bursac Z. The immune system possibly modulates orthodontic root resorption. In: Davidovitch Z., Mah J., editors. Biological Mechanisms of tooth movement and craniofacial adaptation. Boston, MA: Harvard society for the advancement of orthodontics. 2000;207-17.
- Horiuchi A., Hotokezaka H., Kobayashi K. Correlation between cortical plate proximity and apical root resorption. Am J Orthod. Dentofacial. Orthop. 1998;114:311-318.
CrossRef








