Srinivasan V1, Sheela D2 and Mridula D1
1Department of Pharmacology, Saveetha Medical College and Hospital, Chennai-602105, India.
2M.B.B.S, Saveetha Medical College and Hospital, Chennai-602105, India.
Corresponding Author E-mail: dr.seeni23@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1251
Abstract
The Pharmacovigilance Programme of India (PvPI) aims at sensitizing the healthcare professionals towards strengthening the Spontaneous reporting system in order to protect the lives of millions of people living in a vast country like India. Currently India’s contribution to global drug safety database is about 3%, which is meagre in comparison with the huge population. In terms of number India has reported 1,81,656 ADR’s over the period from April 2011 – March 2016 to National Coordination Center (NCC) for Pharmacovigilance Programme of India (PvPI).This present study was done to identify the possible factors responsible for underreporting (UR) of adverse drug reactions (ADRs) and encourage the healthcare professionals to substantiate the Pharmacovigilance Programme of India (PvPI). The present study was a cross-sectional questionnaire-based study to assess the knowledge, attitude, and practice (KAP) of pharmacovigilance among practicing healthcare professionals working in the Saveetha Medical College & Hospital, Thandalam, Chennai. The statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 23 software. The result shows difference in explicit knowledge and tacit knowledge among healthcare professionals. Attitude questions have identified the affective behaviour of the respondents and practice questions shows evidence of a paradigm shift towards an organized pharmacovigilance constructivism. KAP of the healthcare professionals highlights the under-reporting of ADR, Multimodality interventions are needed to improve spontaneous ADR reporting.
Keywords
Attitude; Pharmacovigilance; Knowledge;Practices; Underreporting
Download this article as:| Copy the following to cite this article: Srinivasan V, Sheela D, Mridula D. Knowledge, Attitude, and Practice of Pharmacovigilance Among the Healthcare Professionals in A Tertiary Care Hospital – A Questionnaire Study. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Srinivasan V, Sheela D, Mridula D. Knowledge, Attitude, and Practice of Pharmacovigilance Among the Healthcare Professionals in A Tertiary Care Hospital – A Questionnaire Study. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16563 |
Introduction
Pharmacovigilance is the pharmacological science relating to the collection, detection, assessment, monitoring and prevention of adverse effects or any other drug-related problem, mainly long term or short term side effects of pharmaceutical products.1 Pharmacovigilance Programme of India (PvPI) was formed in July 2010. A combined initiated by Central Drugs Standard Control Organization (CDSCO), New Delhi, MoHFW, Government of India. The All India Institute of Medical Sciences (AIIMS) was established as the National Coordinating Centre (NCC) under which 22 ADR monitoring centers (AMCs) all over India were formed for monitoring Adverse Drug Reactions (ADR) in India. In order to strengthen the programme , and for better implementation , the National Coordinating Centre was relocated to Indian Pharmacopoeia Commission (IPC), Ghaziabad, (U.P.) from the All India Institute of Medical Sciences (AIIMS).2
The committees under the National Coordinating Centre (NCC-PvPI) are the Steering committee, working group, Quality review panel, Signal review panel and the Core training panel (Fig.1). At present, there are 202 ADR monitoring centers under Pharmacovigilance Programme of India (PvPI). The eventuality of ADR’s contributes significant burden to the country’s economy and also loss of quality of life. The foremost responsibility of the Health care professionals is to report the ADR’s arising out of the drugs promptly and efficiently. In our country, there is a huge divergence in the population with regard to genetic and cultural traditions. So these in formations shape the future of the government policies to protect the well-being of the people.3
The Uppsala Monitoring Centre (UMC) in Sweden has the international database of suspected adverse drug reaction reports from all over the world.4 Studies conducted among hospitalized patient populations have revealed staggering facts of incidence of serious ADR’s. These studies judged that 6.7% of hospitalized patients of the total admissions incidence of serious adverse drug reaction with a fatality rate of 0.32% 5. The present issue of concern is underreporting of ADR’s due to various confounding factors.6 The knowledge, attitude, and practice (KAP) is the best tool to assess ADR reporting among healthcare professionals and their perspective towards Pharmacovigilance and patient’s safety. 7,8,9 Hence there is a need for spontaneous reporting habit among healthcare professionals as a long term goal in order to strengthen the PvPI.8
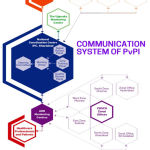 |
Figure 1: Hierarchical system of Pharmacovigilance Programme of India (PvPI)
|
Materials and Methods
Study Setting
This study was conducted at Saveetha Medical College Hospital, Thandalam, a multispecialty tertiary care hospital in Chennai. The approval for conducting this study was obtained from the Institutional Ethics Committee [012/01/2016/IEC/SU] prior to the study. The study was conducted during the period from April 2016 to September 2016.
Study Design
This was a cross-sectional questionnaire-based study. The study participants consisted of all the practicing healthcare professionals (doctors, nurses and pharmacists) who gave their informed consent and who were working at the hospital during the study period.7,8,9 KAP questionnaire was designed to assess the demographic details of the healthcare professionals, their knowledge of pharmacovigilance, attitudes towards pharmacovigilance, and their practice on ADR reporting.7,8,9 There were 20 questions in the questionnaire to assess the knowledge on ADR, attitude towards pharmacovigilance and their practice on reporting ADR. The study instrument was a self-administered KAP questionnaire designed by the faculty of the Department of Pharmacology based on previous studies.
Study Data Collection
A total of 230 healthcare professionals participated in this cross sectional questionnaire based study. A time period of one day was given for the participants to read, understand and answer the questions.
Statistical Analysis
Information from the returned questionnaire were entered and analyzed by Statistical Package for Social Sciences (SPSS) version 23 software.
Results and Discussion
Of the 300 KAP questionnaires circulated, a total of 230 healthcare professionals (Doctors 147, Nurses 83) gave consent to participate in this study and responded to the questionnaire. The demographic details of the healthcare professionals with baseline characteristics are summarized in (Table 1)
Table 1: Baseline characteristics of the study
| Characteristics | Frequency
(number) |
| Gender | |
| Male | 128 |
| Female | 102 |
| Age wise distribution(in years) | |
| 20-30 | 56 |
| 30-40 | 133 |
| >40 | 41 |
| Health care professionals | |
| Doctors | 147 |
| Nurses | 83 |
Healthcare Professionals Knowledge Regarding ADR Reporting
Healthcare professionals knowledge was gauged based on important parameters, 53.4 % of the respondents gave correct response regarding the definition of Pharmacovigilance. 51.7 % of the respondents were aware of the importance of pharmacovigilance. 61.7 % of the healthcare professionals had knowledge about Post Marketing Surveillance (PMS). 59.5 % of the respondents were aware of the regulatory body responsible for monitoring ADR’s in India (Table 2). The results of the present study are slightly lower with regard to knowledge when compared to a similar study done by Gupta et al9 and Dharmadhikari PP et al.10 Response rate was around 72.1 % regarding banned drugs due to ADR. The awareness about Pharmacovigilance Programme of India (PvPI) was around 83.1 % among healthcare professionals which leads to constructivism towards pharmacovigilance (Table 3), this value is slightly higher than the similar study done by Gupta et al.9
Table 2: Knowledge among healthcare professionals regarding ADR reporting
| Knowledge Related Questions | Correct response
n=230, % |
Incorrect response
n=230, % |
| Define Pharmacovigilance?
a) The science of monitoring ADR’s happening in a Hospital b) The process of improving the safety of Drugs c) The detection, assessment, understanding & prevention of adverse effects* d) The science detecting the type & incidence of ADR after drug is marketed. |
53.4 % | 46.6 % |
| The important purpose of Pharmacovigilance is?
a) To identify safety of drugs* b) To calculate incidence of ADR’s c) To identify predisposing factors to ADR’s d) To identify unrecognized ADR’s |
51.7 % | 48.3 % |
| Which of the following methods is commonly employed by the pharmaceutical companies to monitor adverse drug reactions of new drugs once they are launched in the market?
a)Meta analysis b) Post Marketing Surveillance (PMS) studies* c) Population studies d) Regression analysis |
61.7 % | 38.3 % |
| In India which Regulatory body is responsible for monitoring of ADR’s?
a) Central Drugs Standard Control Organization* b) Indian Institute of sciences c)) Pharmacy Council of India d) Medical Council of India |
59.5 % | 40.5 % |
| The international centre for adverse drug reaction monitoring is located in
a) Unites States of America b) Australia c) France d) Sweden* |
32.1 % | 67.9 % |
| Pharmacovigilance includes
a) Drug related problems b) Blood related products c) Herbal products d) All of the above* |
29.1 % | 70.9% |
ADR=Adverse drug reaction, * Correct response
Table 3: Knowledge among healthcare professionals regarding ADR reporting
| Knowledge Related Questions | Yes (%) | No (%) |
| Are you aware of any drug that has been banned recently due to ADR? | 72.1 % | 27.9% |
| Are you aware of suspected ADR reporting system in India? | 83.1% | 16.9 % |
Healthcare Professionals Attitude Towards ADR Reporting
About 33.9 % of the healthcare professionals believe that reporting of ADR is a collective responsibility of the Doctor, pharmacist, and the nurse too; this response is comparable to a similar study done by Chandrakapure AR et al.12 A total of 69.1 % of the participants were of the view that pharmacovigilance should be integrated with the undergraduate curriculum itself because they are the future prescribers. About 72.1 % healthcare professionals agreed that establishing ADR monitoring centers in every hospital is the need of the hour; it’s similar to study done by Gupta et al.9 Nearly 83.9% of the respondents feel that reporting of adverse drug reaction is necessary. 91.3% of the participants uniformly concurred with the actuality that pharmacovigilance should be taught in detail to the healthcare professionals which coincides with the study done by Gupta et al9 and Chandrakapure AR et al12 (Table 4).
Table 4: Health care professionals response towards Attitude-related questions
| Attitude‑related questions | Correct response
n=230 (%) |
Incorrect response
n=230(%) |
| The healthcare professionals responsible for reporting ADR in a hospital is/are?
a) Doctor b) Pharmacist c) Nurses d) All of the above* |
33.9 % | 66 .1% |
| Is there a need to include pharmacovigilance in undergraduate curriculum to create Awareness among the budding Doctors? | 69.1 % (Yes) | 30.9 % (No) |
| What is your opinion about establishing ADR monitoring centre in every hospital? | 72.1 % (Yes) | 27.9% (No) |
| Do you think reporting of adverse drug reaction is necessary? | 83.9% (Yes) | 16.1% (No) |
| Do you think Pharmacovigilance should be taught in detail to healthcare professionals? | 91.3% (Yes) | 8.7% (No) |
ADR=Adverse drug reaction, * Correct response
Factors Deterring ADR Reporting
The factors discouraging health care professionals from ADR were reporting non remuneration for reporting (13.9%), lack of time to report ADR (33.4%), a single unreported case may not affect ADR database (17.3%), difficult to decide whether ADR has occurred or not (35.2%)(Table 5).
Table 5: Factors discouraging ADR reporting
| Response | ||||
| Which among the following factors discourage you from reporting ADR | Non remuneration for reporting
13.9% |
Lack of time to report ADR
33.4% |
A single unreported case may not affect ADR database
17.3% |
Difficult to decide whether ADR has occurred or not
35.2% |
Health Care Professionals Practice Towards ADR Reporting
Among the entrants, 59.5% have experienced ADR in their day to day clinical practice, 64.3% have been trained how to report a ADR to CDSCO, but only 36.5% had reported a ADR, fortunately 75.2% of the health care professionals have seen a ADR reporting form, only 17.8% keep record of the ADR’s and 89.5% of the participants expressed their willingness for ADR reporting which shows signs of logical positivism (Table 6).Multi modality approach model is the best intervention to prevent under-reporting, namely reassurance among doctors that reporting has no legal implications, making ADR reporting mandatory in Medical college hospitals.14
Table 6: Health care professionals response towards practice-related questions
| Practice‑related questions | Yes (%) | No (%) |
| Have you ever experienced adverse drug reactions in your patient during Your professional practice? | 59.5% | 40.5% |
| Have you ever been trained on how to report Adverse Drug Reaction (ADR)? | 64.3% | 35.7% |
| Have you ever seen the ADR reporting form? | 75.2% | 24.8% |
| Have you ever reported adverse drug reaction (ADR) to the pharmacovigilance center? | 36.5% | 66.5% |
| Do you keep records of ADR? | 17.8% | 82.2% |
| Are you willing for ADR reporting? | 89.5% | 10.5% |
Conclusion
This study concludes that the health care professionals knowledge towards ADR reporting is better and their commendable attitude towards a uniform structured system of ADR reporting. It identified the factors discouraging ADR reporting and emphasized on spontaneous ADR reporting. The under-reporting issues can be corrected by conducting periodic educational interventional15 programs and sensitizing programs for the health care professionals working in a tertiary care hospital.13,14 The health care professionals practice towards ADR reporting showed positive trend towards improving ADR reporting and safeguarding the safety of the patients.16
Acknowledgement
We would like to express our gratitude to all the health care professionals who participated in this study.
Reference
- World Health Organization. The Importance of Pharmacovigilance–Safety Monitoring of Medicinal Products. Geneva: World Health Organization. 2002.
- Pharmacovigilance Program of India (PvPI) [homepage on the internet] .The start of the programme. Updated on Jan 30,Available from: http://www.ipc.gov.in/PvPI/pv_home. html.
- Pharmacovigilance Program of India Annual Performance Report 2015-2016.Available from: http://www.ipc.gov.in/PvPI/pub.html. 2017.
- Jayanthi C. R., Renuka M., Panchaksharimath P. An observational Study to analyze the Adverse drug Reactions among the Elderly at A Tertiary Care Hospital. Biomedical and Pharmacology Journal. 2017;10(1):345-52.
CrossRef - Lazarou J., Pomeranz B., Corey P. N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–1205.
CrossRef - Hazell L., Shakir S. A. Under-reporting of adverse drug reactions. Drug Safety. 2006;29(5):385-96.
CrossRef - Ganesan S., Vikneswaran G., Reddy K. C., Subrahmanyam D. K., Adithan C. A Survey on Knowledge, Attitude and Practice of Pharmacovigilance towards Adverse drug reactions reporting among Doctors and Nurses in a Tertiary Care Hospital in South India. J Young Pharm. 2016;8(4):471-6.
CrossRef - Desai C. K., Iyer G., Panchal J., Shah S., Dikshit R. K. An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital. Perspectives in Clinical research. 2011;2(4):129.
CrossRef - Gupta S. K., Nayak R. P., Shivaranjani R., Vidyarthi S. K. A questionnaire study on the knowledge, attitude, and the practice of pharmacovigilance among the healthcare professionals in a teaching hospital in South India. Perspectives in clinical research. 2015;6(1):45.
CrossRef - Dharmadhikari P. P., Patil K. S. Knowledge, attitude, and practice among healthcare professionals of adverse drug reactions reporting in a tertiary care center.
- Kalaiselvan V., Thota P., Singh G. N. Pharmacovigilance Programme of India: Recent developments and future perspectives. Indian Journal of Pharmacology. 201648(6):624.
CrossRef - Ajay R. C., Imran N., Giri S. P., Khan I. N., Mateenuddin M., Faheem M. Pharmacovigilance a study to evaluate knowledge, attitude, and practices of and impact of educational intervention among doctors in teaching hospital, in rural area of Jalna, India.
- Kalaiselvan V., Prasad T., Bisht A., Singh S., Singh G. N. Adverse drug reactions reporting culture in Pharmacovigilance Programme of India. Indian J Med Res. 2014;140:563–4.
- Tandon V. R., Mahajan V., Khajuria V., Gillani Z. Under-reporting of adverse drug reactions: A challenge for pharmacovigilance in India. Indian journal of pharmacology. 2015;47(1):65.
CrossRef - Ganesan S., Sandhiya S., Reddy K. C., Adithan C. The impact of the educational intervention on knowledge, attitude, and practice of pharmacovigilance toward adverse drug reactions reporting among health-care professionals in a Tertiary Care Hospital in South India. Journal of Natural Science, Biology and Medicine. 2017;8(2):203.
CrossRef - Alsbou M., Abdeen G., Batarseh A., Bawaresh N., Jaber J., Qawasmi G. Analysis of the National Pharmacovigilance Database in Jordan (2010-2014). Biomedical and Pharmacology Journal. 2017;10(1):319-28.
CrossRef








