Surabhi Yadav1,2, Salman Akhtar1, Surendra K Agarwal2, Gauranga Majumdar2 and Suman Vimal2
1Department of Bioengineering,Integral University, Lucknow, India.
2Department of Cardiovascular and Thoracic surgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Corresponding Author E-mail: kumaragarwalsurendra@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1202
Abstract
Atrial fibrillation (AF) is an arrhythmia which also occurs after the cardiac surgery. Apart from clinical factors some genetic factors are also involved. To know whether a genetic variant has any role or not, this study has been designed in the North Indian patients. An ion-channel gene KCNE1G38S (rs1805127) was selected to investigate its association between genetic variant and postoperative AF. The study included age and sex matched 78 postoperated-AF rhythm patients as cases and 99 postoperated-patients as controls with sinus rhythm admittedin Cardiovascular and Thoracic Surgery Department of SGPGIMS, Lucknow. The SNP detection of KCNE1G38S was geno typed by using the polymerase chain reaction based restriction fragment length polymorphism method. The geno type frequencies of the AA, AG and GG were19.20%, 56.40%, and 24.40%, respectively, in cases, whereas in controls had frequencies of 23.20%, 56.60% and 20.20% respectively. The observed frequencies were almost similar in cases and controls. The chi-square results were not statistically significant (χ2=0.668, p=0.716) and the frequency of G allele between cases and controls did not vary (52.56% vs. 54.31%). In multivariate analyses, the KCNE1G38S variant was independently associated with a significant predisposing effect on AF after adjusting for related risk factors and the odds ratio for case was 1.272 (95 % CI: 0.594–2.726, p = 0.389). The study revealed that there is no association of AF with the genetic variant ofion channel gene KCNE1G38S in the North Indian population.
Keywords
Atrial fibrillation; cardiac surgery; KCNE1G38S; RFLP-SNP; genotyping
Download this article as:| Copy the following to cite this article: Yadav S, Akhtar S, Agarwal S. K, Majumdar G, Vimal S. Genetic Association of KCNE1G38S Polymorphism in Postoperative Atrial Fibrillation of North Indian Population: A Case-Control Study. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Yadav S, Akhtar S, Agarwal S. K, Majumdar G, Vimal S. Genetic Association of KCNE1G38S Polymorphism in Postoperative Atrial Fibrillation of North Indian Population: A Case-Control Study. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16308 |
Introduction
Atrial arrhythmias and atrial fibrillation (AF), a rhythm disorder, commonly occur postoperatively.1,2 With recent developments in molecular biology techniques, exploration of the pathology of AF at the genetic level has become an emerging topic worldwide. In 2013, over 1.27 crore Indians suffered from Atrial Fibrillation, an increase by 40 lakhs from 2012 and 60 lakhs from 2011.3 AF is increasing with advancing age, diabetes, obesity, Coronary Artery Diseases and Valvular Heart diseases. AF increases the risk of heart attack and stroke. Several studies of secular trends have already documented increasing prevalence of AF over the past several decades. In case of India study is limited.4
Previous studies done in AF showed that gene exerted great influence on its pathogenesis. The study done by Lai et al.5 found that gene polymorphism in KCNE1 was one of the AF risk factors in a Taiwanese population. However, in Chinese population there was no relationship between the KCNE1G38S and AF.6 In 2006, in European populations showed that the KCNE1G38S was associated with AF. 7,8 In Uygur population also AF was associated with genetic polymorphism.9 Recently, more and more pieces of evidence indicated that AF is a multifactorial disease resulting from the interaction between environmental factors and genetics. Several studies demonstrated that the mutations in genes coding for ion-channels may be associated with parts of the familial AF.10,11 Whereas, in Off-pump Coronary artery bypass graft study revealed that gene variants has role in postoperative-AF development12
KCNE1 is a potassium ion channel coding gene for humans, it is located in chromosome 21q22.1–21q22.2 which encodes for the β-subunit of the potassium ion-channel (IKs).13,14 It is a slowly activating component of the delayed rectifier channel current (IKs), which plays an important role in atrial repolarization.15 Whereas, the IKs is important for ventricular repolarization and KCNE1 plays an important role in atrial repolarization.15 Studies have shown that when there is a gain of function, early onset of AF is seen and when there is loss of function, long QT syndrome develops.16 Several single-nucleotide polymorphisms (SNPs) have been identified in the KCNE1 gene, while the KCNE1G38S polymorphism (rs1805127 G>A; G38S) is the most widely investigated variant.17 It is well accepted that the KCNE1 polymorphism results in a glycine or serine amino acid substitution at codon 38 and is responsible for stronger IKs currents and high expression of KCNQ1.18,19 This gene variant has shown to be a risk factor for AF in several populations studies. Till, today there is no study shown regarding genetic polymorphism in the North Indian Population.
Materials and Methods
The study population consisted of 99 haemodynamically stable patients as control and 78 AF patients as case evidenced by ECG undergoing cardiac surgery (coronary artery bypass graft and valvular heart disease surgery) from Cardiovascular and Thoracic surgery Department in SGPGIMS, Lucknow. The diagnosis of AF was based on the medical history and the diagnostic criteria of ECG for AF were: (1) absence of P-waves, (2) irregular atrial activity at a rate of 350–600/min, and (3) irregular ventricular rhythm. The exclusion criteria for AF patients included one of the following: symptomatic heart failure, cardiomyopathy, chronic obstructive pulmonary disease, acute medical illness and severe infections. Adopting a one-by-one matched case-control study design, the 99 control subjects matched with age (above 18yrs) and sex, enrolled in the study during the same period admitted to these hospitals undergoing either valvular heart surgery or coronary bypass graft surgery. All samples in our study were the residents of North India. The presence of smoking, diabetes mellitus, hypertension, type of surgery (CABG and VHD) were assessed on the basis of subjects’ questionnaires, blood detecting indexes and hospital records. Written informed consent was obtained from all the individuals.
Molecular Analysis
Genomic DNA extraction was performed from peripheral blood leucocytesusing the phenol–chloroform method. The genotyping of KCNE1G38S was done through PCR– RFLP analysis. The PCR reaction was conducted in a final volume of 20 μl using primers 5’-GTG ACG CCC TTT CTG ACC AA-3’ (primer sense) and 5’-CCA GAT GGT TTT CAA CGA CA-3’ (primer antisense) at an annealing temperature of 54.1°C. The 12 μl of the reaction GreentaqLucigenmix, 0.8 µl of each primers, 5.4 μl nuclease free water and 1 μl genomic DNA were used for amplification. Cycling conditions included an initial denaturation at 94°C for 3 min followed by 35 cycles with a fast denaturation at 94°C for 40 s, an annealing step at 54.1°C for 30 s and an extension step at 72°C for 30 s, with a final incubation at 72°C of 5 min. The amplification reaction was followed by a digestion with the reaction enzyme, MspA1I (NEB) at 37°C for overnight and electrophoresis on 2.0% agarose gel. The polymerase reaction product was 318 base pairs in size. Amplification product was cut with MspA1I to produce 232–base pair and 86– base pair fragments.
Statistical Analysis
Statistical analysis was performed using the SPSS (Statistical Package forSocial Sciences, Chicago, USA) software for Windows (Version 15.0). The χ2 -test was used to test the deviation of genotype distribution from Hardy–Weinberg equilibrium and the differences of the frequency of KCNE1. The association between the risk factors and AF was assessed using logistic regression analysis. Odds ratio (OR) with 95% confidence interval (CI) was determined. Threshold for statistical significance was a p-value of 0.05.
Results
Clinical Characteristics of Patients
In total, 177 patients were recruited in the study, with a mean age of 45.78±15.605 vs. 47.08±16.037 in cases and controls respectively.The ratio of female was higher in cases than in controls, 40% female developed postoperative AF. Out of 177 patients, 78 (44.00%) presented at least 1 qualifying episode of AF postcardiac surgery. Clinical and demographic characteristics of the study population are summarized in Table 1. Results of the odds ratios are shownin Table3.
Table 1: Demographic and clinical characteristic:
| Cases | Controls | p-value | |
| Age(yrs) | 45.78±15.605 | 47.08±16.037 | Matched |
| Sex(male/female) | 47/31 | 71/28 | Matched |
| male | 60% | 71.71% | |
| female | 40% | 28.29% | |
| Smoking | 5(6.4%) | 9(9.1%) | 0.512 |
| Hypertension | 27(34.6% ) | 41(41.4%) | 0.355 |
| Diabetes | 8(10.3%) | 25(25.3%) | 0.011 |
| Valvular heart surgery | 77.5% | 52.52% | 0.001 |
| Coronary artery bypass graft | 22.5% | 47.48% | 0.001 |
Table 2: Genotype & Allele frequencies of cases and controls
| AA | AG | GG | χ2 | p | A | G | p | |
| Case | 15 | 44 | 19 | 0.668 | 0.716 | 74 | 82 | 0.247 |
| 19.2% | 56.4% | 24.4% | 47.44% | 52.56% | ||||
| Control | 23 | 56 | 20 | 102 | 96 | |||
| 23.2% | 56.6% | 20.2% | 45.69% | 54.31% |
Table 3: Multivariable analysis for KCNE1 polymorphism according to conditional logistic regression model in North Indian Population
| Variable | β | SE | Wald | OR (95 % CI) | p-value |
| Smoking | 0.072 | 0.624 | 0.013 | 1.075(0.317-3.647) | 0.908 |
| Hypertension | 0.158 | 0.332 | 0.227 | 1.172(0.611-2.248) | 0.634 |
| Diabetes | 1.035 | 0.464 | 4.971 | 2.814(1.133-6.987) | 0.026 |
| KCNE1 | 0.408 | 0.474 | 0.384 | 1.272(0.594-2.726) | 0.389 |
Results of Allele, Genotype Frequencies, and Hardy-Weinberg Equilibrium
A 318-bp fragment of the coding sequence of KCNE1 gene was observed by PCR-RFLP genotyping. Genotype and allele frequencies in the study’s population are summarized in Table 2. Observed allele and genotype frequencies were in accordance with expected frequencies by the Hardy-Weinberg equilibrium in the total cohort study.
The KCNE1G38S Polymorphism and Incidence of Postoperative AF
The frequency of G allele was observed same in the postoperative AF group compared with the group without postoperative AF (52.56% vs. 54.31%, respectively, p = 0.247). The genotype frequencies did not deviated from Hardy-Weinberg law of equilibrium. The results of the multivariate regression analysis revealed that only diabetic patients had relation with AF while smoking, hypertension and type of surgery (CABG and VHD surgery) were not significant as shown in Table 3.
Discussion
AF shows differential incidence rates among different ethnic groups. The first study by Lai et.al. emphasized on polymorphism study in KCNE1G38S gene variant in AF subjects. In 2002, for the first time, he reported that the KCNE1G38S is related to AF in a Taiwanese population. Therefore,KCNE1G38Spolymorphism has been considered as one of the risk factors for AF.5 A similar study of Zhiyu et al. in a Chinese population revealed that there was no relationship between the KCNE1G38S and AF, which totally differed from the study published by former. He stated that the difference possibly resulted from the different subjectsi. eethinicity.6 However, two studies in European populations demonstrated that the KCNE1G38S was a higher risk of AF.20 Due to the relationship between the KCNE1G38S and AF is different among different ethnicities. The KCNE1G38S variant was associated with increased risk of AF among Uygur people. Yao et al. in 2011 showed that the KCNE1 gene (rs1805127) polymorphism increases the AF risk in Xinjiang Uygur individuals, which still remained significant after adjustment for related risk factors.21 The results manifested that the Uygur exhibited more European features in heredity than the Asian population. Hence, they inferred that this is one of the possible reasons why results showed that the distribution of KCNE1G38S genotype and allele frequency among Uygur AF individuals was similar to that in European AF subjects instead of Chinese people.
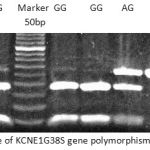 |
Figure 1: Gel picture of KCNE1G38S gene polymorphism |
As far our findings suggests that the KCNE1G38S gene polymorphism confers no susceptibility to AF in the North Indian Population. The allele and genotype frequencies did not deviated from Hardy-weingberg law of equilibrium. The G allele in cases came 52.56% and in controls 54.31% which is almost similar. The genotype frequency in cases of AG+GG came 80.80% and in controls it is 76.80%. Here, also difference is less i.e. 4%. The results suggests that KCNE1 gene variant has no association with postoperative AF in north Indian population.
The study has some limitations. Firstly, few risk factors of AF were selected. Secondly, we cannot completely exclude the presence of asymptomatic AF in the control group though the standard interview were done. Thirdly, we relied on a clinical history, ECG and obtained evidence in the hospitals to assess coronary artery disease and valvular heart diesease. However, the symptoms and ECG are sufficient to diagnose the most of the patients in clinics. The experiment is not a large-scale study. Therefore, what the results mean cannot support general consideration on the genetic background of the whole population it may vary. Lastly, AF episodes that occurred after hospital discharge were missed. In conclusion, we found that the KCNE1G38S was not a risk factor for post-AF in an north Indian population. The KCNE1G38S might have different impact on AF in different ethnicities. Further researches among different parts of India may potentially reveal new avenues for explaining the pathogenesis of the important disease.Understanding the risk factors for atrial fibrillation would promote the development of improved therapies and preventive measures to lessen this public-health burden.
Conflict of Interest
The authors declared that there is no conflict of interest.
Financial Support
There was no financial support from any agencies.
References
- Maesen B., Nijs J., Maessen J., et al. Post-operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14:159-74.
CrossRef - Crystal E., Garfinkle M. S., Connolly S. S., et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2004;4. bCD003611.
CrossRef - Bohra V., Sharma G., Juneja R. Burden of atrial fibrillation in India. J Pract. Cardiovasc Sci. 2015;1:230-2.
CrossRef - Deore R., Vora A. Epidemiology and risk factor for atrial fibrillation in India. J of Preventive Cardiology. 2014;3:505-7.
- Lai L. P., Su M. J., Yeh H. M., Lin J. L., Chiang F. T., Hwang J. J., et al. Association of the human minK gene 38G allele with atrial fibrillation evidence of possible genetic control on the pathogenesis of atrial fibrillation. Am Heart J. 2002;144(3):485–90.
CrossRef - Zeng Z., Tan C., Teng S., Chen J., Su S., Zhou., et al. The single nucleotide polymorphisms of IKs potassium channel genes and their association with atrial fibrillation in a Chinese population. Cardiology. 2007;108(2):97–103.
CrossRef - Fatini C., Sticchi E., Genuardi M.,Sofi F., Gensini F., Gori A. M., et al. Analysis of minK and eNOS genes as candidate loci for predisposition to non-val- vular atrial fibrillation. Eur Heart J. 2006;27(14):1712–8.
CrossRef - Prystupa A., Dzida G., Myslinski W., Malaj G., Lorenc T. MinK gene polymorphism in the pathogenesis of lone atrial fibrillation. Kardiol Pol. 2006;64(11):1205–11.
- Haijun M., Xiaohui Z. Ting Association between KCNE1 non-valvular atrial fibrillation in an Uygur population. 2012;124(21-22):737-41.
- Otway R., Vandenberg J. I., Guo G., Varghese A., Castro M. L., Liu J., et al. Stretch-sensitive KCNQ1 mutation A link between genetic and environmental factors in the pathogenesis of atrial fibrillation? J Am CollCardiol. 2007;49:578–86.
CrossRef - Ellinor P. T., Nam E. G., Shea M. A., Milan D. J., Ruskin J. N., MacRae C. A.Cardiac sodium channel mutation in atrial fibrillation. Heart Rhythm. 2008;5:99–105.
CrossRef - Voudris V. K.,Apostolakis S.,Karyofillis P., Doukas K.,Zaravinos A. et al. Genetic diversity of the KCNE1 gene and susceptibility to postoperative atrial fibrillation.American Heart Journal. 2014;164:274-280.
CrossRef - Chevillard C., Attali B., Lesage F., Fontes M., Barhanin J., Lazdunski M., et al. Localization of a potassium channel gene (KCNE1) to 21q22.1-q22.2 by in situ hybridization and somatic cell hybridization. Genomics. 1993;15:243-45.
CrossRef - Splawski I., Shen J., Timothy K. W., Vincent G. M., Lehmann M. H., Keating M. T. Genomic structure of three long QT syndrome genes: KVLQT1, HERG and KCNE 1. Genomics. 1998;51:86-97.
CrossRef - Chen Y. H., Xu S. J., Bendahhou S., Wang X. L., Wang Y., Xu W. Y et al. KCNQ 1 gain-of-function mutation in familial atrial fibrillation. Sci J. 2003;29:251–4.
CrossRef - Olesen M. S., Bentzen B. H., Nielsen J. B., Steffensen A. B., David J. P., Jabbari J et al. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC Medical Genetics. 2012;13:24.
CrossRef - Aydin A., Bahring S., Dahm S., Guenther U. P., Uhlmann R., Busjahn A., et.al. Single nucleotide polymorphism map of five long-QT genes. J. Mol. Med. (Berl). 2005;83:159-165.
CrossRef - Lai L. P., Deng C. L., Moss A. J., Kass R. S., Liang C.S. Polymorphism of the gene encoding a human minimal potassium ion channel (minK). Gene. 1994;151:339-340.
CrossRef - Ehrlich J. R., Zicha S., Coutu P., Hebert T. E., Nattel S. Atrial fibrillation-associated minK 38G/S polymorphism modu- lates delayed rectifier current and membrane localization. Cardiovasc Res. 2005;67(3):520–8.
CrossRef - Gregory M. M., Alvaro A., Carmen A. P., Lettre G., Vittinghoff E., Lubitz S. A. European ances-try as a risk factor for atrial fibrillation in African Ameri- cans. Circulation. 2010;122(20):2009– 15.
CrossRef - Yao J., Ma Y. T., Xie X., Liu F., Chen B. D. Association of rs1 805127 polymorphism of KCNE 1 gene with atrial fibrillation in Uigur population of Xinjiang. Zhonghua Yi Xue Yi ChuanXueZaZhi. 2011;28:436-40.








