Radhakrishna Nagumantri1, Animisha Mokkapati1, Radha Tatapudi2, Madhuri Chintala2, Satyanarayana Rentala1 and Aruna Lakshmi Komarraju1
1Department of Biotechnology, Institute of Technology, GITAM University, Visakhapatnam 530 045, Andhra Pradesh, India.
2Department of Gynecology and Obstetrics, Andhra Medical College, Visakhapatnam 530 002, Andhra Pradesh, India.
Corresponding Author E-mail: mail.dr.satya@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1268
Abstract
Prenatal diagnosis uses a variety of techniques to find out the health of the fetus. Without the information acquired by prenatal diagnosis, there could be an inappropriate result for the fetus or the mother or both. Several non-invasive and invasive techniques are available for prenatal examination. Any of these techniques can be applied depending on the time periods of the gestational age. Most of the current methods of fetal diagnosis are invasive and carry accuracy, albeit small procedure-related risks are possible. Due to the risks and testing costs, only the women older than 35 years and having a high risk for fetal aneuploidy are currently screened for prenatal testing. The isolation and analysis of fetal cells isolated from maternal circulation would allow non-invasive prenatal diagnosis. Due to the scanty number of fetal cells culturing fetal cells would benefit to increase the number of fetal cells in vitro. Design and development of culture conditions to expand fetus-derived cells may lead to a new selection process for obtaining prenatal diagnosis data through fetal cells. In this paper several ex vivo expansion techniques for fetal cells isolated from maternal circulation were discussed. Cultured fetal cells could be a tool for metaphase analysis for the diagnosis of chromosomal abnormalities.
Keywords
Prenatal diagnosis; fetal cells; markers for fetal cells; isolation of fetal cells and culturing of fetal cells
Download this article as:| Copy the following to cite this article: Nagumantri R, Mokkapati A, Tatapudi R, Chintala M, Rentala S, Komarraju A. L. Ex-Vivo Expansion of Fetal Cells Isolated from Maternal Circulation. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Nagumantri R, Mokkapati A, Tatapudi R, Chintala M, Rentala S, Komarraju A. L. Ex-Vivo Expansion of Fetal Cells Isolated from Maternal Circulation. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=15967 |
Introduction
World Health Organization (WHO) recently reported more than 270000 deaths world-wide during the first 28 days of life. March of Dimes (MOD) reported that 7.9 million births (6% of total births) occur annually worldwide with serious birth defects. 94% of these births occur in the middle and low-income countries. According to WHO and MOD meeting report, birth defects account for 7% of all neonatal mortality. India stands sixth place in the world population and with ethnical, geographical and genetic diversity. In several Indian communities, birth defects are moderately high due to consanguineous marriage in families. The occurrence of birth defects in India is 6-7% which translates to around 1.7 million birth defects annually. The common birth defects include congenital heart disease (8-10 per 1000 live births), congenital deafness (5.6-10 per 1000 live births), and neural tube defects (4-11.4 per 1000 live births). These birth defects are clinically deceptive at birth; other defects may be diagnosed later. Common genetic disorders occur at birth are Down syndrome, sickle cell disease and ß-thalassemia etc. These disorders are due to gene mutations or chromosomal abnormalities.1
Prenatal Diagnosis
Control and management of the genetic disorders depend on prenatal diagnosis of the disease. Recent developments in disease genetics has proved that at least 70% of birth defects can be prevented if the evidence based chromosomal studies are made available. Prenatal genetic testing may help to detect genetic disorders. This assists the parents to choose the correct option for a probable course of medication to arrest, prevent its progress or management of its consequences. Prenatal care motivates the detection of anatomic and physiologic disorders with the zygote, embryo or fetus as early as possible, either before the initiation of gestation (pre implantation genetic analysis) or either early in gestation. Prenatal tests diagnostic techniques are available to diagnose the genetic disorders such as Down syndrome, spina bifida, cleft palate, Tay–Sachs disease, sickle cell anemia, thalassemia, cystic fibrosis, muscular dystrophy and fragile X syndrome.1
Nowadays most of the treatments are focused on adults rather at fetal stage. For example treatment for Down’s syndrome in adulthood will have less clinical impact (Table 1).
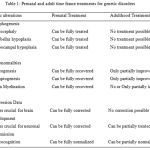 |
Table 1: Prenatal and adult time frame treatments for genetic disorders |
Types of Prenatal Diagnostic Tests
Prenatal tests are mainly categorized into two, based on the mode of the sample collection – Invasive and noninvasive prenatal diagnostic tests.
Invasive Prenatal Diagnostic test
Invasive procedures involve collection of cells of fetal origin by obstetricians with expertise. These are considerably safe and accurate, but may have mild discomfort to the mother and risk of miscarriage. Chorionic villus sampling, amniocentesis and percutaneous umbilical cord blood sampling are major invasive procedures of prenatal diagnosis. Chorionic villus sampling may be used to determine chromosomal disorders in the fetus. It can be carried out at 10 to 12 weeks of gestation. The procedure involves aspiration of trophoblastic tissue under continuous ultrasound monitoring. taking a tissue sample from the placenta through the cervix or abdomen.2 Amniocentesis can be performed at 15 weeks of gestational age and involves obtaining of amniotic fluid sample by passing a needle through the mother’s abdomen into the uterus.3 Percutaneous umbilical cord blood sampling (PUBS), is used to determine the fetal abnormalities by investigating the blood from the umbilical cord.4 PUBS provide a process of rapid chromosomal analysis. PUBS is useful when information cannot be obtained through amniocentesis, chorionic villus sampling, or ultrasound. In the above procedures, prenatal diagnosis involves aspiration of tissue / cells of fetal origin under continuous ultrasound monitoring. The rate of miscarriage associated with PUBS, CVS and amniocentesis is 1 to 2 %. Despite the risks, on average 5 – 10% of pregnant women chose to undergo these tests, averaging approximately. PUBS has been predominantly replaced by non-invasive prenatal diagnostic techniques to investigate the chromosomal abnormalities.5 The development of fluorescence in situ hybridization (FISH) and highly sensitive polymerase chain reaction (PCR) have made pre-natal diagnosis safe, simple and rapid. Trisomy 21, 13, and 18 and sex chromosome aneuploidy may be accurately measured using the above procedures.
Non-Invasive Prenatal Diagnostic Techniques
Noninvasive prenatal diagnostic techniques involve risk-free procedure and it is mainly based on the isolation of cells or DNA of fetal origin from maternal circulation. The damage or degeneration of feto-maternal tissues can be minimized by using safe and simple non-invasive prenatal diagnostic techniques. These techniques may be classified into three types – analysis of preimplantation genetics, cell-free DNA and fetal cells in maternal blood.
Preimplantation Genetic Diagnosis (PGD)
In the course of in vitro fertilization (IVF) procedure, human embryos are isolated prior to implantation. These cells are analyzed using FISH and PCR techniques for chromosomal abnormalities. Though PGD is a non-invasive technique, but IVF generally implicate invasive procedures like transvaginal oocyte retrieval.6
Cell-Free Fetal DNA in Maternal Blood
This technique involves the analysis of circulating fetal DNA in maternal blood. Testing of fetal DNA can potentially identify fetal aneuploidy7 even before six weeks of pregnancy. Fetal DNA occupies approximately 2-10% of total in maternal circulation. Whole genome sequencing of the fetus is also possible with cell-free DNA in maternal circulation.8
Fetal Cells in Maternal Blood (FCMB)
All the genetic information of the fetus is present in fetal cells and so fetal cells can be employed to perform prenatal diagnosis.9 The presence of fetal cells in maternal circulation is yet to be studied. Recent evidences show that fetal cells in maternal circulation protect feto-maternal tissues by the mechanism of Microchimerism. Microchimerism is common in placental mammals. The conflict between mother and offspring over transport of the cells can be studied using Microchimerism. The analysis of fetal cells in maternal circulation play crucial role in placental diversity of evolutionary consequence.10
Types of Fetal Cells
Fetal trophoblasts, leukocytes and erythroblasts or nucleated red blood cells (NRBC), haematopoietic progenitors and stem cells11 are the variety of fetal cell types in maternal circulation. These cells were identified as possible candidates for improvement for further analysis. Trophoblasts are large in size, placental mosaicism and frequently multinucleated or enucleated.12 These cells show the pregnancy specificity; these cells are challenging targets due to difficulty in isolation from maternal blood. Enrichment of Trophoblasts is restrained due to having the inadequate availability of antibodies for placental antigen specificity and its multinucleated morphology.13 Leukocytes are prolonged cells, which leads to exists from previous pregnancies.13 Leukocytes may persevere from previous pregnancies. The isolation of fetal cells in these leucocytes is limited due to the scanty data available on fetal cell markers to differentiate maternal from fetal leukocytes.14
At an in-vitro concentration of erythropoietin (EPO) < 2 U/ml, fetal erythroblasts and fetal erythroid progenitors have shown maximal growth when compared to adult erythroid progenitors.14 Nucleated red blood cells (NRBC) have short half-life of 25 to 35 days; consequently, persistence of NRBCs of previous pregnancy is limited in maternal blood15 and present in maternal circulation even after 27 years of pregnancy. Fetal NRBC with exclusive cell morphology and complete chromosomal complement are available in the first trimester of maternal circulation. Hence, isolation of fetal NRBCs are more suitable for non-invasive prenatal diagnosis.16
Fetal Cell Markers
Colony forming definitive erythroid progenitors (BFU-E) can be isolated from early developmental stages using CD34 marker.17 Potential markers such as CD71, glycophorin A (CD235A), CD36 and epsilon globins have been used for enrichment and identification of fetus derived erythroid cells.17
CD 71
The transferrin receptor, which is known as CD71 is an integral membrane protein, which helps the uptake of transferrin-iron complexes. TfR1 is required for iron import from transferrin into cells by endocytosis. CD71 antibodies are used for isolation of fetal erythroid cells from maternal circulation.18 PCR technique may be used to confirm the fetal origin of the isolated cells.
Glycophorin A
Glycophorin A (GPA) also known as CD235A, is widely available glycoprotein on the surface of erythrocytes. It is a definitive marker for all types of erythroid cells. Its expression is highly limited to lineage-specific manner that is from late in terminal erythrocyte differentiation to mature erythrocytes, reticulocytes, and bone marrow and circulating erythroblasts. The genetics of Glycophorin A have not been studied in fetal cells.19
CD36
CD36 is known as platelet glycoprotein 4 or fatty acid translocase (FAT) or scavenger receptor class B member 3 (SCARB3) or glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV). CD36 is expressed on several cell types including epithelial, endothelial cells, platelets, monocytes erythrocytes and macrophages and some macrophage-derived dendritic cells. CD36 is an early marker of erythroid differentiation.20
HBF
Fetal hemoglobin (Hb F) also known as α2γ2 is one of the most studied oxygen transport protein in the fetus. It helps the fetus for oxygen transport during the last seven months of the delivery in the uterus and also until 6 months old of the post delivery. Fetal hemoglobin has greater affinity for oxygen when compared to adult hemoglobin. This results in supplying enough oxygen to the developing fetus. Recent studies have shown the application of intracellular HbF to isolate fetal nucleated RBCs.21 Fluorescence in situ hybridization (FISH) or other molecular techniques may be employed to study fetal nucleated RBCs to identify chromosomal abnormalities in the fetus. This diagnostic approach provides a safer alternative to amniocentesis or chorionic villus sampling procedures.
Epsilon Globin
Epsilon globin gene (HBE) is normally expressed in the embryonic yolk sac. PCR amplification and fluorescence in situ hybridization (FISH) of Y-chromosome specific sequences for ε-and γ-globin may confirm the fetal cells in maternal circulation. This analysis provides the information about DNA polymorphisms with STR (short tandem repeats) of mother and child genotype.22
CD34
CD34 antigen is a protein that in humans is encoded by the CD34 gene.CD34 is one of the fetal cell surface marker present in the maternal blood.30 It was observed that CD34 positive fetal cells may contribute from past pregnancies.30 This tempered interest for CD34 positive cells as potential prenatal diagnostic tools.
Current Methods for Isolation of Fetal Cells from Maternal Blood
Fetal cells from maternal circulation may be isolated by density gradient centrifugation followed by fluorescent associated cell sorting (FACS) or magnetic associated cell sorting (MACS) (Table 2). Fluorescence-activated cell sorting (FACS) is a specialization of flow cytometry. FACS is useful to sort two or more cell types in heterogeneous mixture of cells. FACS works on the principle of one cell at a time at specific light scattering and fluorescence. Presently Magnetic cell sorter (MACS) is being used as it is more advanced, simple to operate and reliable method.23 This method is performed by labeling the cell surface markers with magnetic beads. Using this method, one can separate positive and negative fractions of the cells. The separated cells may be subjected to labeling of other surface markers using magnetic beads as shown in Figure 1.
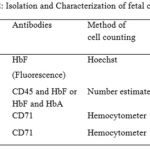 |
Table 2: lsolation and Characterization of fetal cells |
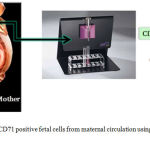 |
Figure 1: Isolation CD71 positive fetal cells from maternal circulation using magnetic cell sorter (MACS)
|
Cell Culture Systems
Culture systems are mainly applied to scale-up the number of cells. This will improve self-renewal /proliferation capacity of the cells. Culture systems are generally characterized based on the matters related to expansion of cells – two dimensional (2D) and three dimensional (3D) culture system. These culture systems provide an artificial microenvironment and mimic the in vivo conditions for better expansion of the cells.24
Two Dimensional Culture Systems
The advancements in polymer technology opened the doors for the invention of Petri dish. As shown in Figure 2 several plastic vessels and flasks are used for cell culture with and without coatings.
 |
Figure 2: Two dimensional culture system for the expansion of isolated fetal cells
|
Surface Coatings
For some cell types, to increase the ability of cell binding to the surface, coatings are generally applied (for example, gelatin). Specific ECM proteins such as fibronectin, collagen or laminin are used for the above purpose. It was observed that cells proliferate faster on ECM coated plates compared to uncoated surfaces.25 This type culture system is termed as 2.5 dimensional culture systems.
Three Dimensional Culture Systems
To mimic the in vivo conditions cells may be cultured in 3 dimensional (3D) culture systems. 3D culture system is an artificial micro-environment for cells to grow in all three dimensions. Unlike 2D culture system, a 3D cell culture system is very close to in vivo situation.26 At present two types of 3D culture systems are available. They are scaffold based and non-scaffold culture systems.
Scaffold Based Culture Systems
Scaffolds are the materials which are engineered for required cellular interactions for tissue regeneration. Cells are ‘seeded’ to produce three-dimensional tissue. The main purpose of the scaffold is to mimic the extracellular matrix of the native tissue and allow the cells to influence their own microenvironments).27
Scaffold-Free Techniques
Micropatterned surfaces, rotating bioreactors, and magnetic 3D bioprinting are some of the scaffold-free methods. 28
Bioreactors
The bioreactors are a type of 3D cell culture systems with specifically engineered plastic cylindrical chambers to grow the cells in three dimensions. Polyethylene terephthalate membranes are used in bioreactors to provide an environment that maintains high levels of nutrients. The rotating bioreactors ensure equal cell growth in all three dimensions.29
Conclusions
In this paper several prenatal diagnostic methods were discussed. The cell surface makers for fetal cells isolated from maternal circulation were known to express CD71, CD36, HbF, epsilon globin and Glycophorin A. The fetal cells may be isolated using FACS and MACS methods. These methods are robust and sensitive. The isolated cells can be expanded in several culture systems parse two and three dimensional culture systems. Expansion systems for fetal cells will surely help the researchers and physicians for better understanding of the origin and function of fetal cells in maternal circulation.
Acknowledgements
We are thankful to SERB, DST, Govt of India for the financial support (F.No.SR/SO/HS – 225/2012, dated: 11.09.2013.) under the extramural grant to Department of Biotechnology, Institute of Technology, GITAM University, Visakhapatnam.
References
- Seeds J. W. Diagnostic mid trimester amniocentesis: how safe? American Journal of Obstetrics and Gynecology. 2004;191(2):607–15.
CrossRef - Gardner R. J. M., Grant S., Lisa S., eds. Chromosome Abnormalities and Genetic Counseling (4 ed.). Oxford, New York: Oxford University Press. 2012:423.
- Fan H. C., Blumenfeld Y. J., Chitkara U., Hudgins L., Quake S. R., Quake B. C. H. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc. Natl. Acad. Sci. U.S.A. 2008;105(42):16266–71.
CrossRef - Yurkiewicz I. R., Korf B. R., Lehmann L. S. Prenatal whole-genome sequencing is the quest to know a fetus’s future ethical? New England Journal of Medicine. 2014;370(3):195–7.
CrossRef - Herzenberg L. A., Bianchi D. W., Schröder J., Cann H. M., Iverson G. M. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(3):1453–5.
CrossRef - Khosrotehrani K., Johnson K. L., Cha D. H., Salomon R. N., Bianchi D. W. Transfer of fetal cells with mul- tilineage potential to maternal tissue. JAMA. 2004;292:75-80.
CrossRef - Mueller U. W., Hawes C. S., Wright A. E., Petropoulos A., DeBoni E., Firgaira F. A., Morley A. A., Turner D. R and Jones W. R. Isolation of fetal trophoblast cells from peripheral blood of pregnant women.Lancet. 1990;336:197-200.
CrossRef - Sargent I. L., Choo Y. S and Redman C. W. G. Isolating and analyzing fetal leukocytes in maternal blood. Ann. N. Y. Acad. Sci. 1994a;731:147-153.
CrossRef - Bianchi D. W., Flint A. F., Pizzimenti M. F., Knoll J. H. M and Latt S. A. Isolation of fetal DNA from nucleated erythrocytes in maternal blood. Proc. Natl Acad. Sci. USA. 1990;87:3279-3283.
CrossRef - Tilesi F., Coata G., Pennachi L., Lauro V., Tabilio A and Di Renzo G. C. A new methodology of fetal stem cell isolation, purification, and expansion: preliminary results for noninvasive prenatal diagnosis. J. Hematother. Stem Cell Res. 2000;9:583-590.
CrossRef - Goldberg J. D and Wohlferd M. M. Incidence and outcome of chromosomal mosaicism found at the time of chorionic villus sampling. Am. J. Obstet. Gynecol. 1997;176:1349-1353.
CrossRef - Covone A. E., Johnson P. M., Mutton D and Adinolfi M. Trophoblast cells in peripheral blood of pregnant women. Lancet ii. 1984;841–843.
CrossRef - Bianchi D. W., Zickwolf G. K., Weil G. J., Sylvester S and DeMaria M. A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum.Proc. Natl Acad. Sci. USA. 1996;93: 705-708.
CrossRef - Shroder J and de la Chapelle A. Fetal lymphocytes in maternal blood. Blood. 1972;39:153-162.
- Weinberg R. S., He L. Y and Alter B. P. Erythropoiesis is distinct at each stage of ontogeny. Pediatr. Res. 1992;31:170-175.
CrossRef - Zheng Y. L., Carter N. P., Price C. M., Colman S. M., Milton P. J., Hackett G. A., Greaves M. F and Ferguson-Smith M. A. Prenatal diagnosis from maternal blood: simultaneous immunophenotyping and FISH of fetal nucleated erythrocytes isolated by negative magnetic cell sorting. J. Med. Genet. 1993;30:1051-1056.
CrossRef - Zimmermann S., Hollmann C., Stachelhaus S. A. Unique monoclonal antibodies specifically bind surface structures on human fetal erythroid blood cells. Exp Cell Res. 2013;319(17):2700-2707.
CrossRef - Kavanagh D. M., Kerhoas M. K., Dhariwal R. S., Desmulliez Y. M .P. Current and emerging techniques of fetal cell separation from maternal blood. J. Chromatogr. B. 2010;878:1905–1911.
CrossRef - Ashoor G., Syngelaki A., Wagner M., Birdir C., Nicolaides K. H. Chromosome-selective sequencing of maternal plasma cell-free DNA for first trimester detection of trisomy 21 and trisomy 18. Am. J. Obstet. Gynecol. 2012;31:237–243
CrossRef - Kitagawa M., Sugiura K., Omi H., Akiyama Y., Kanayama K., Shinya M. New technique using galactose-specific lectin for isolation of fetal cells from maternal blood. Prenat. Diagn. 2002;22: 17–21.
- CrossRef
- Bigbee L. W and grant G. S. Use of Allele-Specific Glycophorin A Antibodies to Enumerate Fetal Erythroid Cells in Maternal Circulation, Annals New York Academy Of Sciences. 1994;731:128-32.
CrossRef - Edelman P., Vinci G., Villeval J. L.,Vainchenker W., Henri A., Miglierina R., Rouger P.,Reviron J., Breton-Gorius J., Sureau C. A monoclonal antibody against an erythrocyteontogenic antigen identifies fetal and adult erythroidprogenitors. Blood. 1986;67(1):56-63.
- Bromilow I. M and Duguid J. K. Measurement of feto-maternal haemorrhage a comparative study of three Kleihauer techniques and two flow cytometry methods. Clinical & Laboratory Haematology. 1997;19(2):137-42.
CrossRef - Cheung M., Goldberg J and Kan Y. Prenatal diagnosis of sickle cell anaemia and thalassaemia by analysisof fetal cells in maternal blood. Nature Genetics. 1996;14:264–68.
CrossRef - Lewis D. E., Schober W., Murrell S., Nguyen D., Scott J., Boinoff J. Rare event selection of fetal nucleated erythrocytes in mater- nal blood by flow cytometry. Cytometry. 1996;23:218–227.
CrossRef - Hall J. M., Williams S. J., Layton T. J., Adams S., Molesh D. Puri- fication of fetal cells from maternal blood using an avidin-biotinimmunoaffinity column. Am J Hum Genet. 1993;53:1416.
- Thomson R. C., Yaszemski M. J., Powers J. M., Mikos A. G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci Polym Ed. 1995;7(1):23–38.
- CrossRef
- Mooney D. J., Baldwin D. F., Suh N. P., Vacanti J. P., Langer R. Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17(14):1417–1422
CrossRef - Agrawal C. M., Ray R. B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–150.
CrossRef








