Manuscript accepted on :June 14, 2017
Published online on: --
Plagiarism Check: Yes
Esam Qnais1, Yousra Bseiso1, Mohammad Wedyan1 and Hakam Alkhateeb2
1Department of Biology and Biotechnology, Faculty of Science, Hashemite University Zarka, Jordan.
2Faculty of Medicine, Yarmouk University, Irbid, Jordan.
Corresponding Author E-mail: Esamqn@hu.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/1216
Abstract
Arum palaestinum has a wide use in traditional medicine. It used in folk medicine as a remedy for treatment of post-delivery pain, inflammation, and internal infections. The analgesic activity of the methanolic extract of Arum palaestinum was evaluated using various experimental models in mice and rats. Antinociceptive effect of methanolic extract of Arum palaestinum was carried out both peripheral and central models. The possible role of opioid receptors in analgesic activity of methanolic extract of Arum palaestinum was also examined in hot plate test using naloxone. Methanolic extract of Arum palaestinum at all three test doses (5, 10 and 20 mg/kg body weight) significantly (p < 0.05) decreased the pain response in all nociceptive models in dose-dependent fashion. Pretreatment with naloxone (2 mg/kg) caused significant (P < 0.05) change in the analgesic activity of 20 mg of methanolic extract of Arum palaestinum. In conclusion, the antinociceptive effect of methanolic extract of Arum palaestinum in volves activation of peripheral (partly related to lipoxygenases and/or cyclo-oxygenases) and central mechanism (partly, via activation of opioid receptors).
Keywords
Arum palaestinum; Analgesic activity; Analgesy-meter; Centrally mediated; Formalin test;Hot-plate test; Randall–Selitto tests; Peripherally mediated; Rats, Mice
Download this article as:| Copy the following to cite this article: Qnais E, Bseiso Y, Wedyan M, Alkhateeb H. Evaluation of Analgesic Activity of the Methanol Extract from the Leaves of Arum Palaestinum in Mice and Rats. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Qnais E, Bseiso Y, Wedyan M, Alkhateeb H. Evaluation of Analgesic Activity of the Methanol Extract from the Leaves of Arum Palaestinum in Mice and Rats. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16399 |
Introduction
Arum palaestinum Boiss (Araceae), popularly known in our region as “Al-Louf”, is a perennial flowering plant native to levant and other Mediterranean region.1 The Arum genus comprises about 26 species, Arum palaestinum is one of these species.1 Arum palaestinum has a wide use in traditional medicine. It ingested as herbal remedy to treat post-delivery pain, inflammation, and internal infections.2,3 The aerial parts of Arum palaestinum are cut up in Jordan and thoroughly cooked with olive oil and lemon after being drying because it is perceived to protect from cancer (recommended by the traditional healers).2 The plant is also used as animal feed resources. Few studies have demonstrated that Arum palaestinum has phytochemical and biological activities. Of theses, Afifi et al., (1999)4 showed that Arum palaestinum extract reduces muscle contraction of uterus in rat and guinea pig. Further, Arum palaestinum has been shown to have anti-proliferation and antioxidant effect.5,6 Phytochemical screening of the Arum palaestinum demonstrated the presence of (S)-3,4,5-trihydroxy-1 H-pyrrol-2(5H)-one alkaloid, caffeic acid, isoorientin, luteolin, vicenin 11, 3,6,8-trimethoxy, 5,7,3′,4′-tetrahydroxy flavone, vitexin (apigenin 8-C-glycoside), isoorientin (luteolin 6-C glucoside), chrysriol, and chryseriol-7-glucoside.5,4
Materials and Methods
Preparation of Plant Material and Extracts
Plant Material
In this study, the plant part utilized was the leaves. The fresh leaves of Arum palaestinum were obtained from Ajloun (Jordan) between the months of March and May. The plant material was identified and authenticated at the Herbarium section of the Department of biological sciencs, Hashemite University. A voucher specimen was deposited at the Hashemite University Herbarium for future reference.
Preparation of Methanol Extract
The fine powder leaves was weighed (1000g) and soacked in 2500 ml of methanol for 36 h with constant shaking. The mixture was clarified by filtration using Whatman’s filter paper and the resultant filtrate was concentrated under low tempreture (40°C) and reduced pressure to gummy materials in rotary evaporator. The final yield was 5.92% (w/w) of the extract.
Phytochemical Analysis
Phytochemical analysis of the methanol extract of the plant was based on a standard procedure.7,8 The methanol extract was tested for alkaloids, tannins, saponins, glycocides and saponin glycosides. Thin layer chromatography was used to confirm the presence of these constituents.
Animals
Male and female Wistar rats weighing 180–250 g and Swiss albino mice weighing 18–25 g were used in this study. The experimental animals were provided the Animal House of Biology Department, Hashrmite University. Animals were maintained under ambient conditions. Rooms temperature was maintained at 22±2 ◦C Alternate 12 hr light/dark cycle was also maintained. Food and water were provided ad libitum to all animals. Prior to commencement of the experiments, the animals were fasted for Twelve hours but still had access to water. Animal care and experimental protocols were performed in accordance with NIH publication No: 85-23, revised in and approved by the Animal Care Committee at the Hashemite University.
Acute Toxicity Studies: LD50
The determination of the median (LD50) of the plant extract was perform according to the methods described by Lorke (1983) with some modification.9 Sixty mice were fasted overnight and then were divided into two sets containing 6 male and female mice in each set. Methanol plant extract was administrated to set I orally at the doses of 10,100 and 1000 mg/kg in the first stage of determination while set II was treated intraperitoneally (i.p.) at same doses. The mice were allowed food and water ad libitum and observed for 24 h after treatment for signs of acute toxicity or death. The results of this stage help us to select the doses for the next stage. 25, 50 and 100 mg/kg (p.o.) and 200, 400 and 600 mg/kg (i.p.) were the selected doses for the next stage. The final median lethal dose (LD50) value was estimated as the geometric mean of the highest non-lethal dose and the lowest lethal dose in the second stage.
Acetic-Acid Induced Abdominal Writhing Test
The writhing test was based on the method described by Koster et al. (1959) [10] with modifications in timing of observations. Animals injected with acetic acid (6%, 10 mL/kg) intraperitoneally to induce abdominal writhing. A total of 30 mice were divided into 5 groups (n = 6 per group) and were pretreated 30 min prior to acetic acid injection as follows: groups I, II and III: injected intraperitoneally with 5, 10 and 20 mg/kg of the extract, respectively; group IV: injected intraperitoneally with acetylsalicylic acid (ASA) 150 mg/kg (as positive control) and group V: injected intraperitoneally with distilled water (vehicle control). The number of writhings was counted twice (at 15 min intervals)over a period of 5 min after a 5-min lag period after the injection of acetic acid and expressed as % of constriction inhibition. The assessment was performed in a blind fashion.
Formalin Test in Rats
The test was conducted according the method of Dubuisson and Dennis (1977) [11] with slight modification. Briefly, rats were divided into six groups (six rats each) and were trated intraperitoneally with either distilled water (control), 5, 10 and 20 mg/kg extract, ASA (150 mg/kg) and morphine (4 mg/kg). 30 min later, animals were given an intraplanter injection of 50 μL of formalin (2.5%) into the right paw using 27-gauge needle. Immediately post formalin administration animals were placed individually in acrylic observation chambers (320 cm2 × 40 cm2) and monitored for 1 h. The nociceptive responses was recorded over a period of 40 min with 10min lag period (early phase, 0-5 and late phase 15-40min) and assessed using the following 4 points scoring system: (0) rats and stand or walked firmly on injected paw; (1) the injected paw was not fully lifted; (2) the injected paw was completely lifted off the floor; (3) the rat licked or chewed the injected paw.
Hot-Plate Test in Mice
This test was carried out according the method of Turner (1965).12
The mice were divided into 5 groups (n=6 per group) and treated as following: groups I, II and III: injected intraperitoneally with 5, 10 and 20 mg/kg of the extract, respectively; group IV: injected intraperitoneally with morphine (4mg/kg, as positive control) and group V: injected intraperitoneally with distilled water (vehicle control). Animals were then placed on hotplate (UGO Basile, Italy) kept at a temperature of 53 ± 0.5°C. The reaction time was then observed over the period of 90min (0, 15, 30, 60 and 90 min) after extract or drug administration. The reaction time was considered as the time elapsed between placing of the mouse on the hotplate and licking of the hind paw or jumping. The increase in reaction time was determined for each extract- and drug-treated group and is expressed as percentage.
Analgesy-Meter Induced Pain
Analgesy-meter test was carried out in rats using an Ugo Basile analgesy-meter (model 7200, Italy). A constantly increasing force was applied to the rat paw by the plunger. Pain was determined by the physical struggles of the animal to free its paw. Rats were used in groups of six per dose of plant extract (groups I, II and III received 5, 10 and 20 mg/kg, i.p.), distilled water (group IV i.p.), or morphine (4 mg/kg, group V (s.c.)). Readings were taken at intervals of 15 minutes for 30 minutes (0, 15 and 30 min) after drug and extract administration. The assessment was performed in a blind fashion. The increase in pain threshold for each extract- or drug-treated group was compared with that of contrl group and was expressed as percentage.
Randall–Selitto Test
The method of Randall and Selitto (1957)13 was used in this test. Rats were divided into 6 groups. Rats in group 1,2,3 were injected with 5,10,20mg/kg methanol extract, respectively. Rats in group 4, 5, 6 were received distilled water morphine (4 mg/kg) and ASA (150 mg/kg). 30 min later rats were received 50 µl of raw egg albumin into the subplanter surface of rat left hind paw to induce edema. The threshold pain (the force (g) applied to the dorsal surface that caused animal to withdraw the paw) to mechanical stimulus was determined for both inflamed and intact paws of each group by means of analgesiometer (model 7200). The increases in the nociceptive response for both inflamed and intact paws in comparison to control measurements were calculated and was expressed as percentage.
Evaluation of the Mechanism of Antinociceptive Action
To evaluate the mechanism of action of the methanolic extract of Arum palaestinum on pain. Animals were pre-treated with the opioidergic receptor antagonist naloxone (2 mg/kg). Naloxone was administrated intraperitoneally 10 min before administration of distilled water, plant extract (20 mg/kg) or morphine (4 mg/kg). Hot-plate test was performed as mentioned above. The reaction time was recorded over the period of 90 min after extract and drug administration.
Statistical Analysis
The data obtained were presented as Mean ± Standard Error Mean (SEM) and analysed using two-way ANOVA followed by Student’s t-test. Microsoft Excel®and STATISTICA® computer software were also used for data analysis. P < 0.05 indicates statistical significance
Results
Phytochemical Screening
Data obtained from the phytochemical analysis of Arum palaestinum indicated the presence of the all secondary metabolites tested.
Acute Toxicity Studies
The acute toxicity study shows a decreased mobility, respiratory distress (gasping) with eventual immobility but no convulsions or loss of righting reflex prior to death in the animals. The calculated LD50 of the methanolic extract of Arum palaestinum was 94.3±5.5 mg/kg i.p. and 735.1±23.5 mg/kg p.o. in mice.
Antinociceptive Studies
In this study, i.p. injection of methanolic extract of Arum palaestinum showed a dose-dependent antinociceptive activity in all nociception models used (Figs. 1–4 and Table 1).
Table 1: Effects of the methanolic extract of the leaves of Arum palaestinum, ASA (150 mg/kg) and morphine (4 mg/kg) on albumin-induced edema in rats (Randall–Selitto test).
| Treatment group (dose mg/kg) | Pain threshold (g)/time (min) | |||||
| 0 | 30 | 60 | ||||
| Intact | Inflammed | Intact | Inflammed | Intact | Inflammed | |
| Control | 176.1 ± 23.3 | 162.1 ± 31.1 | 161.5 ± 32.3 | 150.5 ± 22.1 | 168.0 ± 22.4 | 152.2 ± 19.7 |
| Extract (5) | 154.2 ± 34.3 | 147.2 ± 30.4 | 241.4 ± 37.1* | 219.4 ± 20.2* | 248.1 ± 15.3* | 244.3 ± 17.9* |
| Extract (10) | 155.6 ± 25.1 | 146.6 ± 27.7 | 278.2 ± 35.9* | 225.2 ± 15.4* | 278.8 ± 17.1* | 282.3 ± 34.1* |
| Extract (20) | 156.1 ± 21.8 | 138.8 ± 33.9 | 290.9 ± 44.7* | 238.3 ± 27.9* | 282.4 ± 37.6* | 238.5 ± 39.3* |
| Morphine (4) | 149.7 ± 19.5 | 136.3 ± 22.5 | 250.6 ± 23.5* | 378.8 ± 37.6* | 292.2 ± 39.9* | 364.7 ± 57.28 |
| ASA (150) | 151.3 ± 19.9 | 144.2 ± 26.8 | 210.4 ± 21.6* | 383.7 ± 57.5* | 231.3 ± 20.8* | 362.1 ± 33.6* |
Control values indicate the animals injected with distilled water.* P < 0.05 compared with control group. Values are expressed as mean ± S.E.M. (n = 6)
Acetic-Acid Writhing Test
The administration of methanolic extract of Arum palaestinum at 5,10, and 20mg/kg had significant (P < 0.05) inhibitory effect on the number of acetic acid-induced abdominal constrictions in mice as compared with control. The effect was dose-dependent and increase with time. The inhibitory effect was (93.3%) at 20mg/kg over the first 5–10 min of monitoring (Fig. 1). The reference drug ASA also significantly (P < 0.05) inhibited the abdominal constrictions compared to control.
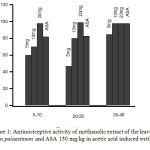 |
Figure 1: Antinociceptive activity of methanolic extract of the leaves of Arum palaestinum and ASA 150 mg/kg in acetic acid induced writhing test.
|
Control values indicate the animals injected with distilled water. (P < 0.05, n = 6).
Formalin Test in Rats
As shown in Fig. 2 the methanolic extract of Arum palaestinum had significant (P < 0.05) antinociceptive activity in the formalin test. The extract reduced significantly (P < 0.05) both the early (neurogenic, 0– 5 min) and late (inflammatory, 15–30 min) phases of the formalin-induced nociception method at 5, 10, and 20 mg/kg. In the early phase, the maximal percentage of inhibition of nociceptive response was 53.3% at a dose of 20 mg/kg. Morphine (4 mg/kg), used as reference drug, produced significant (P < 0.05) and equal reduction in the paw licking of both phases compared to control. ASA (150 mg/kg) significantly (P < 0.05) produced more inhibition of licking in the late phase than early phase.
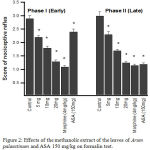 |
Figure 2: Effects of the methanolic extract of the leaves of Arum palaestinum and ASA 150 mg/kg on formalin test.
|
Control values indicate the rats injected with distilled water . * P < 0.05 compared with control group (n = 6)
Hot-Plate Test in Mice
The pretreatment of animals with the methanolic extract of Arum palaestinum (5–20 mg/kg) resulted in a significant dose-dependent increase in the reaction time to thermal stimulation compared with control group (Fig. 3). The highest increase in reaction time was observed with the dose of 20 at 60-min post-treatment (69.8%). The percentage of increase in reaction time produced by the standard drug morphine (4 mg/kg) was 105.3% at 60 min.
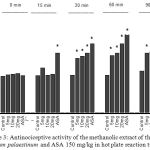 |
Figure 3: Antinociceptive activity of the methanolic extract of the leaves of Arum palaestinum and ASA 150 mg/kg in hot plate reaction time.
|
Control values indicate the animals injected with distilled water. * P < 0.05 compared with control group (n = 6)
Analgesy-Meter Induced Pain Study
Fig. 4 shows that the pretreatment with methanolic extract of Arum palaestinum (5–20 mg/kg) evoked a significant dose-dependent and time related (P < 0.05) antinociceptive effect when the extract compared to control group (Fig. 4). The maximal percentage increases in threshold of nociceptive reflex was 60.1, 109.7, and 120.7%, respectively, all at 15 min. The morphine (4 mg/kg), standard reference drug, produced significant (P < 0.05) effects compared to control group.
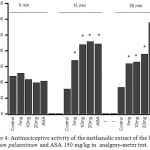 |
Figure 4: Antinociceptive activity of the methanolic extract of the leaves of Arum palaestinum and ASA 150 mg/kg in analgesy-meter test.
|
Control values indicate the animals injected with distilled water . * P < 0.05 compared with control group (n = 6)
Randall–Selitto Test
In the Randall Selitto test, the methanol extract of Arum palaestinum (5–20 mg/kg) significantly (P < 0.05) and in dose dependent manner increase the pain threshold in the inflamed and intact paws in comparision with control group over thirty minutes period (Table 1).
Evaluation of the Mechanism of Antinociceptive Action
Pretreatment of animals with antagonists naloxone significantly (P < 0.05) reversed the antinociceptive action of methanolic extract of Arum palaestinum (20 mg/kg) and morphine (4 mg/kg). The methanol extract dose used was chosen on the basis of hot plate test results (the dose that gives maximal response)
Discussion
In preliminary experiments it was found that the methanolic extract of Arum palaestinum was more active and easier to maintained than the aqueous extract and thus it was selected for this study. Furthermore, using methanol as a solvent may maximize the extraction of some polar solutes pharmacological investigations.14
The antinociceptive effect of the methanolic extract of Arum palaestinum has been investigated. The results of the present study showed that the methanolic extract elicited potent antinociceptive activities that assessed using different pain models. The present results also demonstrated that the methanolic extract of Arum palaestinum act both centrally and peripherally. The pain models used in this study were selected such that both centrally and peripherally mediated effects were measurable.15 Non-selective opioid receptor antagonist was used to anticipate the mechanism involved in methanolic extract of Arum palaestinum antinociceptive properties.
The writhing test is a chemical induced nociception indicating involvement of both central and peripheral mechanisms. In the writhing test, acetic acid injection activates the synthesis of prostaglandins which induce abdominal constrictions due to activation of peripheral nociceptors.16 Non-steroidal anti-inflammatory drugs like aspirin reduce the level of prostaglandin which reduce the sensitivity of the nociceptors to pain inducing agents such as bradykinin.17-20 Administration of methanolic extract of Arum palaestinum produced a significant dose-dependent decrease in the number of acetic acid-induced writhes. This observation points out that methanolic extract of Arum palaestinum possess peripherally-mediated antinociceptive property that may work via reducing the level of prostaglandin synthesis or other inflammatory mediators.
Formalin test produces a biphasic pain response: the first phase (0-15min) is supposed to be reflective of neurogenic pain (direct activation of C- fiber afferent nociceptors) while the second phase (15-30min) is supposed to be due to the release of inflammatory mediators.23-25 In formalin test, centrally acting analgesics reduce both phases almost equally15 whereas peripherally acting analgesics only reduce the late phase.21-22 Considering the inhibitory property of the methanolic extract of Arum palaestinum on both phases of pain response relative to controls, one can suggest that the methanolic extract of Arum palaestinum has both central and peripheral antinociceptive activities. This suggestion is in line with the significant effect seen in the hot-plate and acetic acid-induced abdominal writhing tests.
The the hot-plate test and analgesy-meter test has been used for evaluation of centrally mediated antinociceptive responses, which focuses mainly on changes above the spinal cord level. The dose dependent antinociceptive effect seen in the hot-plate test and analgesy meter test clearly indicates the central antinociceptive effect of the methanolic extract of Arum palaestinum.
Randall–Selitto test is used to measure analgesic activity based on the fact that inflammation increases pain sensitivity by reducing the pain threshold and this sensitivity is modifiable by analgesics.13,15 Peripherally acting analgesics, such as NSAIDs, increase only the threshold of the inflamed paw, whereas centrally acting analgesics, such as opiate, increase the threshold of both the inflamed and intact paws. In Randall-Selitto test, the methanolic extract was observed to increase the pain threshold in both the intact and inflamed paws. This result suggests the methanolic extract of Arum palaestinum acts via both central and peripheral mechanisms.
The central analgesic action of the methanolic extract of Arum palaestinum was confirmed using naloxone, a non-selective opioid antagonist receptors. Naloxone can block spinal and supraspinal opioid receptors in the central nervous system. Pretreatment with naloxone significantly (P<0.05) reverse the antinociceptive action of the methanolic extract of Arum palaestinum. This observation suggests that the antnociception induced by methanolic extract is associated with its interaction with opioid system.
In conclusion, the present findings indicate that the methanolic extract of Arum palaestinum has antinociceptive effect that involve central and peripheral pathways. The centrally mediated effect may be linked partly to activation opioid receptors, while peripherally effect may be due to the activation of lipoxygenase and /or cyclooxygenase. The effects of methanolic extract of Arum palaestinum on pain, explain the uses of lend Arum palaestinum in folk medicine in pain control.
Acknowledgements
The authors would like to thank the Hashemite University and The American University of Madaba for their funding of research and for providing the facilities to conduct the study. The author has no conflict of interest.
References
- Al-Eisawi D. Field Guide to Wild Flowers of Jordan and Neighbouring Countries. Commercial Press (Al Rai), Amman. 1998.
- Al-zweiri., Sarhan A. A., Mansi K., et al. Ethnopharmacological survey of medicinal herbs in Jordan the Northern Badia region. Journal of Ethnopharmacol. 20011;37:27-35.
- Saad B., Azaizeh H and Said O. Tradition and Perspectives of Arab Herbal Medicine: A Review. Evid Based Complement Alternat Med. 2005;2(4):475–47.
CrossRef - Afifi., Khalil E and Abdalla S. Effect of isoorientin isolated from Arum palaestinum on uterine smooth muscle of rats and guinea pigs. Journal of Ethnopharmacol. 1999;65:173-177.
CrossRef - El-Desouky S., Kim K., Ryu S., et al. A new pyrrole alkaloid isolated from Arum palaestinum and its biological activities. Arch Pharm Res. 2007;30(8):927-31.
CrossRef - Al-Mustafa A and Al-Thunibat O. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak J Biol Sci. 2008;1;11(3):351-8.
- Odebiyi O and Sofowora E. Phytochemical screening of Nigeria medicinal plants. Lloydia. 1978;41:234–237.
- Trease G. E and Evans M. C. Textbook of Pharmacognosy, 12th ed. Balliere, Tindall, London. 1989;343–383.
- Lorke D. A new approach to acute toxicity testing. Archives of Toxicology. 1983;54:275–287.
CrossRef - Koster R., Anderson M and De Beer E. J. Acetic acid for analgesic screening. Federation Proceedings. 1959;18:412.
- Dubuisson D and Dennis S. G. The formalin test: a quantitative study of the analgesic effect of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1997;4:161–174.
CrossRef - Turner R. R. A. (Ed.). In: Turner Screening Methods in Pharmacology. Academic Press, London. 1965:100.
CrossRef - Randall L and Selitto J. A method for measurement of analgesic activity on inflammed tissue . Archives of International Pharmacodynamics. 1957;111:409–419.
- Vogel H and Vogel W. Drug Discovery and Evaluation: Pharmacological Assays. Springer-Verlaag, Berlin, Heidelberg. 1997:22-24.
- Gene R., Segura L and Adzet T. Heterotheca inuloides: anti-inflammatory and analgesic effect. Journal of Ethnopharmacol. 1998;60:157–162.
CrossRef - Bentley G., Newton S and Starr J. Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. British Journal of Pharmacology. 1981;73:325–332.
CrossRef - Derardt R., Jougney S., Delevalcee F., et al. Release of prostaglandins E and F in an algogenic reaction and its inhibition. European Journal of Pharmacol. 1980;51:17–24.
CrossRef - Levini J., Lau W and Kwait G. Leukotriene B4 produces hyperalgesia that is dependent on the polymorphonuclear leucocytes. Science. 1984;225:743–745.
CrossRef - Dhara A., Suba V., Sen T. Preliminary studies on the anti-inflammatory and analgesic activity of the methanolic fraction of the root extract of Tragia involucrate. Journal of Ethnopharmacol. 2000;72:265–268.
CrossRef - Chen Y., Tsai H and Wu T. Anti-inflammatory and analgesic activity from roots of Angelica pubescens. Planta Medica. 1995;61:2–8.
CrossRef - Elisabetsky E., Ahmador T., Albuquerque R., et al. Analgesic activity of Pyschotria colorata (Wild.ex-R. and S.) Muell. Arg. Alkaloids. Journal of Ethnopharmacol. 1995;48:77–83.
CrossRef - Coderre T., Vacarino A., and Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Research. 1990;535:155–158.
CrossRef - Coderre T and Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue. Journal of Neuroscience. 1993;12:3665–3670.
CrossRef - Tjolsen A., Berge O., Hunskaar S., et al. The formalin test: an evaluation of the method. Pain. 1992;51:5–17.
CrossRef - Chan K., Islam M., Kamil M., et al. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. sativa (Haw.) Celak. Journal of Ethnopharmacol. 2000;73:445–451.
CrossRef







