I. Made Pande Dwipayana1, Prima Yogi1, Siswadi Semadi1, Suma Wirawan2 and Ketut Widiana3
1Department of Internal Medicine, Faculty of Medicine, Udayana University-Sanglah General Hospital Denpasar Bali.
2Faculty of Medicine, Udayana University Denpasar Bali.
3Department of Surgery, Faculty of Medicine, Udayana University-Sanglah General Hospital Denpasar Bali.
Corresponding Author E-mail: dwipayanapande@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1241
Abstract
Female, 55 years old with a chief complaint of shortness of breath and having a lump over her neck. Chest x-ray examination showed right colli mass with the impression of invading the thoracic inlet with trachea being compressed to the left. On neck and chest MSCT scan with contrast showed enlargement in the right thyroid gland with heterogenous lobulated solid mass, well demarcated border with irregular border, crossing the midline and isthmus, compressing the trachea to the left side and causing trachea narrowing. Histopathology anatomy examination consistent with anaplastic thyroid cancer, and concluded that the patient had anaplastic thyroid cancer stage IV B. Patient underwent chemotherapy using doxorubicin 80 mg as agent of choice and was given levothyroxin 100 mcg every 24 hours. Chemoterapy was done in 6 series in total for every 21 days. Patient was stable until the fifth chemotherapy. The patient dead due to respiratory failure. Anaplastic thyroid cancer is a very rare case and has a poor prognosis.
Keywords
Anaplastic; Chemotherapy; Thyroid;Thyroid cancer
Download this article as:| Copy the following to cite this article: Dwipayana I. M. P, Yogi P, Semadi S, Wirawan S, Widiana K. Diagnosis and Management of an Anaplastic Thyroid Cancer: Case Report. Biomed Pharmacol J 2017;10(3). |
| Copy the following to cite this URL: Dwipayana I. M. P, Yogi P, Semadi S, Wirawan S, Widiana K. Diagnosis and Management of an Anaplastic Thyroid Cancer: Case Report. Biomed Pharmacol J 2017;10(3). Available from: http://biomedpharmajournal.org/?p=16654 |
Introduction
Anaplastic thyroid cancer is a malignant tumor originated from a follicle cell with the highest incidence rate of sixth and seventh decade of life. Based on WHO classification, thyroid cancer is differentiated into a type of a well differentiated thyroid cancer such as papillary, follicular, and anaplastic. Anaplastic thyroid cancer is a type of undifferentiated thyroid cancer. This type of thyroid cancer is one of the most highest mortality rate of malignancy with a life expectancy less than 6 months.1
Anaplastic thyroid cancer is a case with a rare incidence. This malignancy happens 1-2% in all of thyroid malignancies. Even though, this cancer is the most aggresive type of maligancies in human, and frequently cause mortality. With the average survival rate only 6 months after the diagnosis has been made, and 5 years survival is only 0-10%. The etiology of this malignancy is currently unknown , but genetic and environment factors is known to be one of many causes contributed. One of the example is a evidence of an mutation gene p53, BRAF, and RAS that will cause cell proliferation, angiogenesis, and differentiation.2,3
Clinical manifestation of an anaplastic thyroid cancer is marked by a finding of a progressive tyroid mass enlargement. Local compression sign can also be found such as shortness of breath, dysphagia, and vocal cord paralysis. When the diagnosis is made, most of the patient has an regional lymph enlargement, and around 40% already has distant metastases especially to lung, bone, and brain. Until recently, there are no adequate therapy for this disease. Available therapies are include surgical approach, chemotherapy, and radiotherapy. Anaplastic tyroid cancer has a bad prognosis.4 Because all of the facts and reason above this case report is documented and reported as well.
Case
Female patient, age 55 years old, came with a chief complaint of shortness of breath. Shortness of breath felt 2 weeks prior to admission and worsen since 1 week before. Shortness of breath felt as if the patient has a difficulty in taking breath and there was a noticeable “snore” like sound. Shortness of breath did not improve with changing of body position. Shortness of breath is making the patient unable to do her daily activity. Patient also complaint of having a lump over her neck since 1 month ago. It was said the size of the lump was increasing progressively day by day. The lump has a hard consistency, fixed position, and there was no pain. Patient also complaint of having a hoarse sound since 1 month. There was no fever. A decrease of body weight 5 Kg in 1 month. History of diabetes mellitus, heart disease, lung disease, kidney disease and cancer was denied. There was no history of exposed to any radiation and smoking also was denied. There was no family history with the same complaint as above listed.
In physical examination we found a stable vital sign, with a general appearance of moderate illness, patient was fully alert (GCS :E4V5M6), blood pressure 140/90 mmHg, axilla temperature 36,80C, with pulse rate 108x/minute, respiration rate 24x/minute, oxygen saturation 98%. On eye examination there was no pale conjunctiva and normal pupillary reflex. Neck examination there was a thyroid gland enlargement with a size approximately 5-10 cm, solid, fixed, and no pain on palpation. There was a marked lymph node enlargement on the right side of the neck and right submandibular region. Heart and lung examination revealed a normal finding with a regular heart rate, no murmur or associated abnormal lung sounds. Abodminal examination revealed there was no abnormality, with a normal bowel sound, no mass revealed on palpation, liver and spleen was unpalpable. Warm on all four extrimities and no edema.
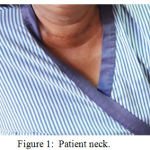 |
Figure 1: Patient neck.
|
On complete blood count examination revealed a white blood cell 9.91 x 10³/µL, haemoglobin 14,61 gram/dl, hematocrite 14.61 %, thrombocyte 231.1 x 10³/µL. Blood chemistry panel with result SGOT 23.7 U/L, SGPT 22.3 U/L, BUN 11.0 mg/dl, creatinine 0.75 mg/dl. On blood gas analysis of 16th February 2017 was found pH 7.46, PCO2 30.6 mmHg, PO2 151.2 mmHg, BEecf -2.8 mmol/L, HCO3– 21.1 mmol/L, SO2 99.1%, TCO2 22 mmol/L, Natrium 135 mmol/L, Kalium 3.33 mmol/L. Patient complained of having a worse shortness of breath, another blood gas analysis was being done and the result was pH 7.35, PCO2 55.5 mmHg, PO2 152.5 mmHg, BEecf 4.5 mmol/L, HCO3– 30.1 mmol/L, SO2 98.8%, TCO2 31.8 mmol/L. Patient had to undergo an emergency tracheostomy procedure but it was failed, instead the procedure isthmusectomy was done. Sepciment was taken during procedure for pathological anatomy evaluation. Thyroid fuction test revealed a hypothyroid condition with level of TSH 25.22 (0,27-4,7) µIU/mL.
ECG record showed sinus ryhtm with heart rate 100 times per minute. Thyroid ultrasound showed solid mass on right and left thyroid lobe, expanding into the isthmus, nodule, and hypervascular, compressing the trachea.
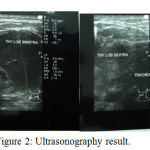 |
Figure 2: Ultrasonography result.
|
Chest x-ray examination showed right colli mass with the impression of invading the thoracic inlet with trachea being compressed to the left. Cervical AP/Lateral x-ray showed soft tissue mass on right neck region that compressed and narrowed the trachea.
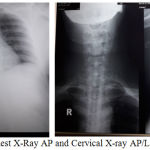 |
Figure 3: Chest X-Ray AP and Cervical X-ray AP/Lateral of patient
|
On neck and chest MSCT scan with contrast showed enlargement in the right thyroid gland with heterogenous lobulated solid mass, well demarcated border with irregular border, crossing the midline and isthmus, compressing the trachea to the left side and causing trachea narrowing. The mass is expanded onto the thyrohyoid muscle, right longus colli muscle, bulging until right anterior region of the neck, compressing the carotid artery and right jugular vein, and with contrast enhancement showed a marked compressed area. Right colli magnification and right submandibule showed no mass expansion to the lung area and no sign of nodule or even an infection of both side of the lung. Histopathology anatomy examination consistent with anaplastic thyroid cancer.
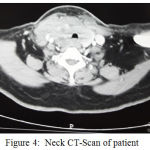 |
Figure 4: Neck CT-Scan of patient
|
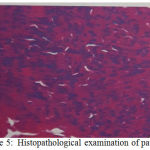 |
Figure 5: Histopathological examination of patient
|
With the clinical manifestation and also supported by the additional lab result, it was concluded that the patient had anaplastic thyroid cancer stage IV B. Patient underwent chemotherapy using doxorubicin 80 mg as agent of choice and was given levothyroxin 100 mcg every 24 hours. Chemoterapy was done in 6 series in total for every 21 days. Patient was stable until the fifth chemotherapy. On June 17th, 2017, patient felt shortness of breath and had a difficulty in breathing that makes the patient had to be admitted again to the hospital and patient finally underwent the 6th chemotherapy cycles.
On June 7th, 2017, a thorax photograph and CT cervical scan was performed with contrast after 5 series of doxorubicin chemotherapy. Thorax photo examination results showed small multiple nodules of perihilar and left-right parakardial suspected metastases, calcification, bronchus orthograde.
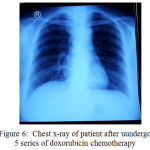 |
Figure 6: Chest x-ray of patient after uundergo 5 series of doxorubicin chemotherapy
|
Cervical CT scans with contrast after 5-series doxorubicin chemotherapy showed non-enhanced soft tissue mass in the lower neck area, with right-left infra-hyoid muscle thickening, and para-retrofaring area suppressing the trachea and esophagus to the left and anterior. The soft tissue enhancement area, thyroid rest observation or maligna focal area. Enlarged lymph nodes cervical area, infra- anterior, lateral jugular group right-left, retrofaring. Patient died on June 28, 2017 with the cause of death was respiratory failure.
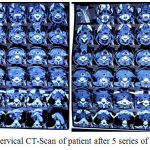 |
Figure 7: Cervical CT-Scan of patient after 5 series of doxorubicin
|
Discussion
Anaplastic thyroid cancer is one of the most aggressive solid tumors in humans, with an average survival of 3-5 months. The 1-year and 10-year survival rates are estimated to be 10-20% and less than 5%. Although only 1-3% of all thyroid cancers, but anaplastic thyroid cancer contributes to 14-50% of deaths from thyroid cancer. Anaplastic thyroid cancer is more common in women than in males5: 1 and peak occurs in the sixth and seventh decades of life.5,6
The exact etiology of anaplastic thyroid cancer remains unknown. But it is thought to be due to the interaction between genetic factors, and the environment. Risk factors for anaplastic thyroid cancer include goiter history, iodine deficiency, radiation, and female sex. Approximately 25-50% of patients with anaplastic thyroid cancer will have a history of well-differentiated thyroid cancer (papillary or follicular thyroid cancer). Anaplastic thyroid cancer cells is unable to perform the normal biological activity of follicular cells such as uptaking the iodine, thyroglobulin synthesis, and p53 mutation.3.7 In the case, the patient had no previous goiter history, nor family history of thyroid cancer, and never had previous radiation exposure.
There are two theories of etiopathogenesis of the occurrence of anaplastic thyroid cancer. The first theory suggests anaplastic thyroid cancer is due to the transformation of a well-differentiated thyroid tumor, while the second theory is de novo (derived first from follicle cells without via transformation from well-differentiated thyroid tumors). Anaplastic thyroid cancer results from the differentiation of differentiated thyroid cancers derived from follicular cells. The differentiation is characterized by loss of specific thyroid cell characteristics and function, including expression of thyroglobulin, thyroid peroxidase, thyroid stimulating hormone (TSH) receptor, and simporter sodium/iodide. Dedifferentiation is associated with addition, chromosomal deletion, and signal transduction disorders. Mutations are most common happen in p53, BRAF, and RAS genes.8,9
Approximately 17-80% of anaplastic thyroid cancers occur due to p53 gene mutations located on the 17p chromosome. P53 plays a role in nuclear transcription of factor production and regulation of cell cycle, DNA repair, and apoptosis. Mutation of p53 gene leads to growth, angiogenesis and de-differentiation. While BRAF mutations occur in 26% of anaplastic thyroid cancers. RAS is an important oncogen to regulate cell growth and contribute to thyroid differentiation. The most important signal transduction pathways for tumorigenesis are RAS-mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase / Akt (PI3 / Akt). RAS is important in setting PI3K / Akt path. Activation of RAS causes activation of protein kinase from rapidly accelerated fibrosarcoma (RAF) kinase, which causes phosphorylation and activates MAPK / extracellular signal regulated kinase (MEK). Mutation in one molecule causes cell proliferation.6,10
Clinical manifestations of anaplastic thyroid cancers can develop rapidly by invading surrounding local tissues and metastases into distant organs. Locally, anaplastic thyroid cancer shows rapid anterior neck enlargement accompanied by dysphagia (40%), sound change or hoarseness (40%), and stridor (24%). Regional symptoms include enlarged lymph nodes (54%), and neck pain (26%). Systemic symptoms include anorexia, weight loss and shortness of breath with pulmonary metastases. Thyroid cancer usually has an advanced stage when it was first diagnosed and often can not be resected. Approximately 20-50% of patients already have distant metastases (80% in lung, 6-16% in bone, 5-13% in brain).6,11 In the case, the patient complained of a rapidly growing mass in the thyroid within 1 month, the patient also experienced hoarseness, stridor, spasms and weight loss of 5 kg in 1 month. On physical examination, the thyroid enlargement measured 5-10 cm in size, palpable with solid consistency, accompanied by enlarged lymph nodes in the colli dextra and submandibular.
In patients with thyroid nodules, serum TSH levels should be examined. If serum TSH is low, then a radioiodine examination is performed. If serum TSH is normal or elevated, continued with ultrasound (ultrasound) ultrasonography and fine needle aspiration based on clinical and ultrasound results. High serum TSH levels are associated with an increased risk of malignancy from thyroid nodules.13,14 In the case, the thyroid function check results indicate the patient has hypothyroidism in which the patient’s serum TSH level increases.
The presence of suspicion of a thyroid cancer is obtained based on anamnesis then confirmed by ultrasound examination, CT scan and histopathology. Ultrasound colli is one of the supporting tools to diagnose thyroid disease. Ultrasound provides us with information about extensions, nodular structures and invasive thyroid disease. Ultrasound finding with a hypoechoic nodule with irregular margins, microcalcification, and central hypervascularization increases the likelihood of nodules in the direction of malignancy up to more than 80%.14 In the case, thyroid ultrasound (ultrasonography) examination shows solid mass in the right and left lobe thyroid, extending to istmus, noduler, and hypervascular, suppressing the trachea.
Routine preoperative imaging of all patients should be performed to evaluate the spread of the disease locally and to exclude the presence of distant metastasis. Anaplastic thyroid cancer may invade structures in the middle, lateral, and mediastinal neck by direct tumor invasion or by lymphatic invasion. Locoregional invasion into internal jugular vein, carotid artery, nerve (eg recurrent laryngeal, vagus, spinal accessory, and phrenic), sternocleidomastoid muscle, esophagus, trachea, and superior vena cava are not uncommon and need to be evaluated to determine whether the tumor is resectable or not.15 In the case of CT Scan Colli and Thorax examination showed tumor invasion into thyrohyoid muscle, right longus colli muscle, protruding to the right anterior colli region, urging carotid artery and right jugular vein. In the case, we did not find any distant metastasis.
On histopalogical examination of anaplastic thyroid cancer, we can find a squamoid, spindle cells and giant cells. Often encountered with high mitotic activity, extensive necrosis, invasive thyroid and extratiroid structures.16 In the case we encountered malignant round cells and invasive short spindles in the muscle and around the thyroid follicle. Histopathological examination results concluded small cell anaplastic thyroid carcinoma.
Determination of the stage of thyroid cancer depends on the size of the primary tumor, lymph node involvement, or presence of distant metastases. All of the anaplastic thyroid cancers are included in stage IV because they are very aggressive. The American Joint Committee on Cancer divides anaplastic thyroid cancer into 3 stages: IVA is characterized by an intratiroid tumor, IVB primary tumor with eccentric spreading, and IVC with distant metastases.13,17 In the case, the patient included stage IVB because on the basis of CT scans the thyroid mass spread to extratiroid to urgent carotid artery (T4b), there was enlargement of regional lymph nodes (N1), and no metastasis.
Table 1: TNM Classification of Anaplastic Thyroid Cancer (13,17)
| Primary Tumor (T) |
| T4a: Any size of tumor that has extended throught out the thyroid capsule and invaded soft tissue structure subcutaneous, larynx, trachea, oesophagus, and recurrent laryngeal nerve |
| T4b: Tumor ivade prevertebrae fasciae, mediastinal blood vessels or carotid arteriy |
| Regional Lymph Node (N) |
| Nx: Lymph node cannot be assessed |
| N0: No lymph node invlovement |
| N1: Regional lymph node metastases |
| Distant Metastases (M) |
| M0: No Metastases |
| M1: Distant Metastases |
| Stage IVA: T4a, Any N, M0 |
| Stage IVB: T4b, Any N, M0 |
| Stage IVC: Any T, Any N, M1 |
Most patients with anaplastic thyroid cancer do not require pneumatic airway intervention unless the patient has acute respiratory disturbance or severe stridor. Tracheostomy in anaplastic thyroid cancer patients is performed in the event of life-threatening asphyxia. Tracheostomy should be avoided where possible. However, if the patient has severe respiratory problems, the patient should be tracheostomized. In addition, some patients will require isthmusectomy or pretracheal tumor debulking to gain adequate access to tracheostomy.15
In this case, the patient experiences an episode of airway obstruction with a narrowing of the tracheal lumen due to a tumor causing the patient to tightness and stridor. Patients was planned to do a tracheostomy procedure, but unfortunately it was failed to do. It is because of the difficult to identify trachea due to large mass in front of trachea, although isthmusectomy is done. Therefore, it was decided not to continue the tracheostomy and instead little thyroid tissue was taken for histopathological examination.
Anaplastic thyroid cancer therapy is still controversial and non-specific, including surgery, radiation, chemotherapy. Surgery in anaplastic thyroid cancers is usually difficult because it is often diagnosed at an advanced stage so curative resection is not possible. There are two criteria used to determine whether the tumor can be resected for curative purposes ie (i) distinguishing between locoregional disease and distant metastatic disease, and (ii) the extent of local invasion and structure involved. In patients with locoregional disease (stage IVA / IVB), tumor determination can be resected based on the structure involved, whether satisfactory resection is achievable (R0 / R1), and whether the structural resection involved results in significant morbidity or mortality.15,16
The majority of tumors are detected at stage IV B in the presence of extrathyroid spread, often causing the tumor to not resect. Factors that cause a resection cannot be done including invasion of fascia prevertebral, carotid artery or involvement of other large blood vessels, and spread to thorax.7 In the case, the patient is diagnosed with stage IV B anaplastic thyroid cancer so that the tumor resection could not be performed. This is because the tumor has spread to the extiroid and urged the carotid artery, and the right jugular vein.
Treatment options in anaplastic thyroid cancer patients that can not be resected is in the form of radiotherapy or chemotherapy. External radiotherapy is given at a dose between 45-85 Gy. Before starting patient therapy should be evaluated the upper airway by using flexible fiberoptic laryngoscopy to evaluate vocal cord function and degree of laryngeal, compression or invasion edema. If the patient is not tracheostomized before radiation, the airway becomes unstable due to inflammation of the laryngotrachea, edema, mucus thickening causing respiratory disturbance associated with the direct effects of tumor.19,20 In this case, tracheostomy with flexible fiberoptic laryngoscopy can not be performed without radiotherapy. Therefore, chemotherapy treatment with doxorubicin dosage of 60 mg / m2 (80 mg) of intravenous doses every 3 weeks was selected.
Table 2: Regiment and Doses of Chemotherapies (15)
| Regiment and Doses of Chemotherapies | Frequency |
| Paclitaxel 50 mg/m2, carboplatin AUC 2 mg/m2 IV | Every 1 week |
| Docetaxel 60 mg/m2 IV, doxorubicin 60 mg/m2 IV
or Docetaxel 20 mg/m2 IV, doxorubicin 20 mg/m2 IV |
Every 3-4 week
Every 1 week |
| Paclitaxel 30-60 mg/m2 IV | Every 1 week |
| Cisplastin 25 mg/m2 IV | Every 1 week |
| Doxorubicin 60 mg/m2 IV | Every 3 week |
| Doxorubicin 20 mg/m2 IV | Every 1 week |
Doxorubicin is an anthracycline and is the most commonly used drug for chemotherapy of anaplastic thyroid cancer. These drugs are rapidly eliminated from plasma and metabolized in the liver. Doxorubicin can cause myelosuppressive, and gastrointestinal toxicity. Administration of doses exceeding 500-550 mg/m2 may lead to cardiomyopathy complication. Doxorubicin is contraindicated to be given to patients with heart disease and liver function impairment. Partial responses are seen in some patients, but little evidence indicates a complete response. In a study conducted by Gottlieb et al, there were two partial remissions of 9 patients with cancer anaplastic carcinoma treated with doxorubicin 75 mg/m2 chemotherapy for 3 weeks. It was reported that not more than 20% cases of anaplastic thyroid cancer is responsive to doxorubucin chemotherapy.21
Based on the results of a study conducted by Lowe N and colleagues on 20 patients with anaplastic thyroid cancer, the prognosis for this cancer was poor with a survival time of 59 days and a 1 year survival time of only 5%. Patients with chemotherapy and radiotherapy have the longest survival of 220 days. In chemotherapy, the survival time was 137 days, while the radiotherapy was 58.5 days. This suggests anaplastic thyroid cancer has a poor prognosis with a survival time of no more than 6 months.22
Table 3: Survival rate on each of treatment modality (22)
| Therapy | Number of patient | Median Survival (days) |
| No therapy | 1 | 32 |
| Surgical | 3 | 38 |
| Radiotherapy | 6 | 58,5 |
| Chemotherapy | 3 | 137 |
| Surgery and chemotherapy | 4 | 176 |
| Chemotherapy and radiotherapy | 3 | 220 |
Some factors such as age, sex, tumor size, disease spread, resectable or not, affects the disease course and prognosis. The patient’s survival rate is usually less than 6 months, and death occurs due to uncontrolled local invasion or distant metastases. Younger female patients (<65 years), small (<5 cm or intra thyroid) cancer size, and no distant metastases at diagnosis, have a better prognosis (6.23). In this case, the younger patient is 55 years old, the tumor can not be resected, and there is no metastasis at diagnosis.
In the case, the patient was performed a 5-series doxorubicin chemotherapy evaluation obtained by evaluating multiple small nodules of the perihilar and right-left paracardials suspected metastases, calcification, orthograde bronchus in the thorax photo. CT scans with contrast showed a cervical tissue enhancement areas, thyroid resting observations or malignant focal areas, there was tracheal and esophageal suppression to the left and anterior. Enlarged lymph nodes cervical area, infra-anterior, lateral jugular group right-left, and retrofaring. Small nodules mutipel perihilar and parakardial right-left obtained from the examination of thorax photographs require further evaluation for metastasis. This is because in a case of anaplastic thyroid cancers is very common to metastasis to the lungs. The patient died although 5 series doxorubicin had been given with a cause of dead respiratory failure.
Conclusion
A case of a 55 year old woman diagnosed with anaplastic thyroid cancer has been reported. Anaplastic thyroid cancer is a very rare case and has a poor prognosis. The important information such as clinical manifestations, physical examination, and support is necessary to diagnose and administer proper management of anaplastic thyroid cancer patients.
References
- Dumke A., Pelz T., Vordemark D. Long Term Results of Radiotherapy in Anaplastic Thyroid Cancer. Bio. Med Central. 2014;9:1-7.
CrossRef - Liu T., Xiao Z., Xu H., Wei F., Zhuang S., Sun X., et al. Treatment and Prognosis of Anaplastic Thyroid Carcinoma: A Clinical Study of 50 Cases. Plos One. 2016;11:1-15.
CrossRef - Brown R. F., Ducic Y. Aggressive Surgical Resection of Anaplastic Thyroid Carcinoma May Provide Long-Term Survival in Selected Patients. American Academy of Otolaryngology Head and Neck Surgery Foundation. 2013;564-571.
- Koussis H., Maruzzo M., Scola A., Casal E., Fassina A., Marioni G., et al. A Case of Anaplastic Thyroid Cancer with Long Term Survival. Anti Cancer Research. 2010;1273-1278.
- Nagalah G., Hossain A., Mooney C., Parmentier J., Remick S. Anaplastic Thyroid Cancer: A Review of Epidemiology, Pathogenesis and Treatment. Journal of Oncology. 2011;1-13.
CrossRef - Taccaliti A., Silvetti F., Palmonella G., Boscaro M. Anaplastic Thyroid Carcinoma. Fountiers in Endrocinology. 2012;3:1-7.
CrossRef - Sayer G., Sidell D., Sercarz J. Long Term Survival in A Patient with Anaplastic Thyroid Carciona Treated with Cricotracheal Resection. International Jounal of Otolaryngology, Head and Neck Surgery. 2012;1:39-43.
CrossRef - Gunes P., Vardar F., Erkan M., Demirturk P., Dulundu E. Incidental Anaplastic Thyroid Carciona: A Case Report. Turkish Journal of Pathology. 2008;24(1):54-58.
- Piromchai P., Ratanaanekchai T., Kasemsiri P. Diagnosis and Treatment of Anaplastic Thyroid Carcinoma. Scientific Research. 2012;3:69-73.
CrossRef - Reddi H., Kumar A., Kulstad R. Anaplastic Thyroid Cancer – An Overview of Genetic Variations and Treatment Modalitas. Advances in Genomic and Genetics. 2015;44-52.
- Keutgen X., Sadowski S. M., Kebebew E. Management of Anaplastic Thyroid Cancer. Gland Surgery. 2015;4(1):44-51.
- Haugen B. R., Alexander E. K., Bible K. C., Doherty G. M., Mandel S. J., Nikiforov Y. E., et al. Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. American Thyroid Association. 2016;26(1):1-133.
CrossRef - Haddad R., Lydiatt W., Ball D., Busaidy N., Byrd D., Callender G., et al. Guidelines Thyroid Carcinoma. National Comprehensive Cancer Network. 2015;1-135.
- Dralle H., Musholt T. J., Schabram J., Steinmuller T., Frilling A., Simon D., et al. German Association of Endocrine Surgeons Practice Guideline for Surgical Management of Malignant Thyroid Tumors. Langenbecks Arch Surg. 2013;398:347-375.
CrossRef - Smallridge R. C., Ain K. B., Asa S. L., Bibie K. C., Brierley J. D., Burman K. D., et al. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2012;11:1104-1139.
CrossRef - Scopa C. Histopathology of Thyroid Tumor An Overview. Hormones. 2004;3(2):100-110.
CrossRef - Pinto N., Black M., Patel K., Yoo J., Mymryk J., Barret J., et al. Genomically Driven Precision Medicine to Improve Outcomes in Anaplastic Thyroid Cancer. Journal of Oncology. 2014;1-7.
CrossRef - How S., Hadi I. A. The Aggressive Anaplastic Thyroid Carcinoma: A Case Report and Institutional Review. World J Endocr Surg. 2012;4(1):20-22.
- Denaro N., Nigro C. L., Russi E. G., Merlano M. C. The Role of Chemotherapy and Latest Emerging Target Therapies in Anaplastic Thyroid Cancer. Dovepress. 2013;3(6):1231-1241.
CrossRef - Cabanillas M. E., Zafereo M., Gunn B., Ferrarotto R. Anaplastic Thyroid Carcinoma: Treatment in The Age of Molecular Targeted Therapy. American Society of Clinical Oncology. 2016;511-517.
- Saller B., Biersack H., Grunwald F., eds. Treatment with Cytotoxic Drugs. In: Thyroid Cancer. New York: Springer. 2005;171-183.
CrossRef - Lowe N., Loughran S., Slevin N., Yap B. K. Anaplastic Thyroid Cancer: The Addition of Systemic Chemotherapy to Radiotherapy Led to an Observed Improvement in Survival. Scientific World Journal. 2014;1-8.
CrossRef - Perri F., Lorenzo G., Scarpati G., Buonerba C. Anaplastic Thyroid Carcinoma: A Comprehensive Review of Current and Future Therapeutic Options. World J Clin Oncol. 2011;2(3):150-157.
CrossRef








